Genetic Analysis of Multiple Paternity in an Endangered Ovoviviparous Lizard Shinisaurus crocodilurus
2015-10-31HuayuanHUANGDanLUOCongGuoZhuoTANGZhengjunWUandJinpingCHEN
Huayuan HUANG, Dan LUO, Cong Guo, Zhuo TANG, Zhengjun WUand Jinping CHEN
1College of Life Science, Sichuan University, Chengdu 610065, Sichuan, China
2Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
3Key Laboratory of Rare and Endangered Animal Ecology, College of Life Science, Guangxi Normal University, Guilin 541004, Guangxi, China
4Guangdong Key Laboratory of Integrated Pest Management in Agriculture, Guangdong public Laboratory of Wild Animal Conservation and Utilization, Guangdong Entomological Institute, Guangzhou 510260, China
Genetic Analysis of Multiple Paternity in an Endangered Ovoviviparous Lizard Shinisaurus crocodilurus
Huayuan HUANG1,2, Dan LUO3,4, Cong Guo1, Zhuo TANG2, Zhengjun WU3*and Jinping CHEN4*
1College of Life Science, Sichuan University, Chengdu 610065, Sichuan, China
2Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
3Key Laboratory of Rare and Endangered Animal Ecology, College of Life Science, Guangxi Normal University, Guilin 541004, Guangxi, China
4Guangdong Key Laboratory of Integrated Pest Management in Agriculture, Guangdong public Laboratory of Wild Animal Conservation and Utilization, Guangdong Entomological Institute, Guangzhou 510260, China
The crocodile lizard (Shinisaurus crocodilurus) is an ovoviviparous lizard belonging to a monotypic family that originated during the end of the quaternary ice age. A rare species in the wild, the crocodile lizard was listed in CITES Appendix II . Knowledge of the reproductive biology and mating system of this species is important for designing conservation strategies and improving genetic variation. To investigate the paternity of the crocodile lizards and to interpret their reproductive behaviour, we collected saliva from females, potential fathers and offspring in a seminatural enclosure experiment and analyzed the paternity of the crocodile lizard using 12 microsatellite genetic loci. The overall observed incidence of multiple paternity was 42.9% (6 of 14 clutches) and Fis was 0.089 ± 0.056. These results indicate that the primary mating mode of the crocodile lizard is that males are polygynous while with females are polyandrous, and there is multiple paternity among offspring of the same mother.
Shinisaurus crocodilurus, Mating system, Paternity assessment, Saliva sample, Microsatellite, Polygyny,Polyandry
1. Introduction
Investigations on the mating behaviour of reptiles have advanced substantially over the past decade (Davis et al.,2001; Laloi et al., 2004; Pearse et al., 2001). A number of studies using molecular genetic methods indicate that multiple paternity, mate choice and sperm competition are important components of the reproductive strategies in reptiles (Gullberg et al., 1997; Lebas, 2001; Olsson et al., 2011). Knowledge of the reproductive biology and mating system of endangered species is important fordesigning effective conservation strategies and improving genetic variation (Joseph and Shaw, 2011). Promiscuous mating systems and multiple paternity schemes have been reported in major reptile groups. For example, a promiscuous mating system has been observed in the sand lizard, Lacerta agilis, and the adder snake, Vipera berus(Olsson and Madsen, 2001), whereas multiple paternity has been detected in many species, including for instance the painted turtle, Chrysemys picta (Pearse et al., 2002),hawksbill turtle, Eretmochelys imbricata (Joseph and Shaw, 2011) and the fi ve-lined skink, Plestiodon fasciatus(Bateson et al., 2011).
The Chinese crocodile lizard, Shinisaurus crocodilurus,is an endangered lizard and was listed in CITES Appendix II (Zhang and Tang, 1985). S. crocodilurus(adult females snout-vent length(SVL) =147.0 ± 2.3 mm;adult males SVL =143.6 ±1.5 mm, the SVL between the males and females were not significantly different(n = 69, P = 0.193)) thrive in areas between 200 and 1500 m above sea level along densely vegetated karst streams or ponds (He et al., 2011;Huang et al., 2009)exclusively in the eastern part of the Guangxi (Kwangsi)Zhuang Autonomous Region, the western and northern parts of Guangdong province in southern China and in the mountainous areas of northern Vietnam (Huang et al.,2014a). Several aspects of S. crocodilurus biology have been investigated in thermal ecology (Wang et al.,2008, 2009), the wild population survey (Huang et al.,2009), ecology (Wu et al., 2007; Zhang, 2002; Zhao et al., 2006) and conservation genetics (Huang et al.,2014a). Additionally, several studies have evaluated mating behaviour and reproduction (Ysu et al., 2006; Yu et al., 2009). Although a previous study that combined behavioural observations and video-camera recordings on individuals in a semi-natural enclosure showed that both sexes are promiscuous, it remains unclear whether multiple matings lead to effective multiple paternity. Such observations have led to the supposition that a promiscuous mating system is employed by S. crocodilurus (Yu et al., 2009).
Allozyme, DNA fingerprinting, amplified fragment length polymorphism and microsatellites are currently available to investigate relatedness of individuals in wild populations (Parker et al., 1998). Since Bei et al. (2012) have described polymorphic microsatellite loci in S. crocodilurus, high-resolution assays can now be performed to answer questions regarding genetic relatedness in this lizard. To identify evidence for multiple paternity in S. crocodilurus, the current study employed 12 microsatellite DNA loci in assessing 14 clutches from from two experimental populations maintained in seminatural enclosures, established in 2011 and 2013.
2. Materials and Methods
2.1 Collection of samples in the semi-natural enclosed population A semi-natural enclosure experiment for the Chinese crocodile lizard was performed at the Luokeng Nature Reserve in Guangdong Province,China. The reserve is located north of Guangdong province (24°36′-24°9′N, 113°13′-113°22′E) (Wan,2009), at an altitude ranging from 200 to 1587 m. From May to July in 2010 and 2012, prior to the beginning of the mating season, 44 adults (28 males and 16 females) were randomly distributed into a pond with damp soil and supplied with water and Pyralis larvae. The animals were allowed to move freely around the pond. To obtain clutches, gravid females were captured from March to May in 2011 and 2013 and maintained in the laboratory until they gave birth. Rearing conditions were similar to those applied for the study of wild populations(water temperature = 20.1 ± 0.5°C)(Wang et al., 2008). Regular monitoring allowed us to collect oral swab samples from all individuals, including the parents and offspring. The method of collecting samples was similar to that previously described, the cotton swabs used to collect saliva samples were stored in 1.5 ml centrifugal tubes containing 100% ethanol. After sampling, individuals were immediately returned to the pond where they were captured (Huang et al., 2014). A total of 129 individuals were collected, including the 44 introduced adults (28 males and 16 females that had just given birth) and 85 offspring.
Sampling was approved by the Forestry Administration of Guangdong province, Luokeng Nature Reserve. All lizards were immediately released after the saliva was collected. Buccal swabbing is a noninvasive method. The Committee on the Ethics of Animal Experiments of the Guangxi Normal University and the Guangdong Entomological Institute Administrative Panel on Laboratory Animal Care approved the protocol.
2.2 Genomic DNA extraction Total genomic DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen,Germany), according to the instructions with slight modifications. In brief, samples were dried with waterfilter paper to allow ethanol to evaporate. Furthermore,the samples were transferred into a 1.5 mL Eppendorf tube, thoroughly mixed with 480 μL buffer solution and 20 μL proteinase K and then incubated at 56°C for 3 h. The mixture was isolated after centrifuging at 8000 rpm for 1 min, following which 500 μL of buffer solution AW1 was added to the supernatant. After discarding the waste liquid, 500 μL of buffer solution AW2 was added,and the mixture was centrifuged at 14000 rpm for 3 min. Finally, genomic DNA was dissolved in 200 μL of TE buffer and was stored at -20°C until analysis.
2.3 Microsatellite genotyping We amplified 12 microsatellite loci (GenBank Accession Numbers JQ411749-JQ411760) from nuclear DNA using 5′-fl uorolabelled forward primers (Bei et al., 2012). Polymerase chain reaction (PCR) amplifications were performed using the following conditions: an initial denaturing step of 3 min at 94°C, followed by 35 cycles of 35 s at 94°C and a Ta (an annealing step) (55°C-61°C) for 35 s (Bei et al., 2012), 30 s at 72°C and a final extension step at 72°C for 10 min. The total volume of the PCR reaction mixture was 15 μL, which consisted of 1 μL of templateDNA, 1 μL of forward primers and 1 μL of reverse primers, 7.5 μL of premixed Taq DNA and 4.5 μL of H2O. Fragment analysis was conducted on an ABI3700 sequencer (Applied Biosystems), and alleles were sized using the programs GENESCAN version 2.1 and GENOTYPER version 2.5 (Applied Biosystems).
2.4 Detection of microsatellite DNA polymorphism The microsatellite data were analysed using web-based Genepop software, with Markov chain parameters of 1000 dememorisation, 100 batches and 1000 iterations per batch to determine whether each locus deviated from the Hardy-Weinberg equilibrium. The GeneALEx software was employed to calculate the number of alleles (Na),average number of alleles (A), observed heterozygosity(Ho), expected heterozygosity (He), and Fis of each locus. 2.5 Paternity analysis Paternity analysis was performed(95% confidence) using the software package CERVUS 3.0.3 (Marshall et al., 1998). For each considered pair of individuals, the average number of shared alleles at each microsatellite locus was calculated. To prevent false identifi cation of the father due to genotyping or reading errors, any individuals without a specific genotype as generated by GeneMapper were subjected to another round of PCR amplifi cation.
3. Results
3.1 Genetic variation of parents and offspring The genetic diversity of the 28 males, 16 females and 85 offspring based on the 12 microsatellite loci are listed in Table 1. The A of the offspring was higher than that of their parents. The He of both parent and offspring exceeded Ho.
3.2 Paternity relationships In the present study, a total of 16 clutches were analysed, which included 11 clutches collected during 2011 and five gathered during 2013(Table 2). Females collected during 2011 and 2013 had broods that ranged in size from 2 to 7 offspring (Table 2);one clutch with only two hatchlings was excluded from the estimation of multiple paternity because it was not possible to detect more than two paternal alleles in such a situation (Laloi et al., 2004). Thus, the observed incidence of multiple paternity was 40.0% (4 of 10 clutches). Among the fi ve clutches collected during 2013, multiple paternity was detected in five clutches (50.0%, two of four clutches), which were sired by at least three males. The overall observed incidence of multiple paternity was 42.9% (6 of 14 clutches; Table 2), and the average number of genotypes per clutch was 5.2 (range: 2-7).
4. Discussion
Our data represent the first genetic tests of paternity in S. crocodilurus. The incidence of multiple paternity was high in S. crocodilurus (42.9% of clutches). Although the number of clutches tested is small and from a limited semi-natural enclosed population, which limits the conclusions that can be drawn with regard to the species as a whole, some important initial observations on the mating system of S. crocodilurus can be made. The genetic data supported the assumption generatedfrom behavioural observations that at least some female S. crocodilurus mate with and produce offspring clutches sired by multiple males (Yu et al., 2009). This is a much higher incidence of multiple paternity than found in Ameiva exsul (9.1%) and Vipera berus (16.7%) (Höggren,1995; Lewis et al., 2000) and lower than that reported for Eulamprus heatwolei (65%-82%) (Morrison et al., 2002)but similar to that of the Grand skink, Oligosoma grande(46.7%) (Berry, 2006).
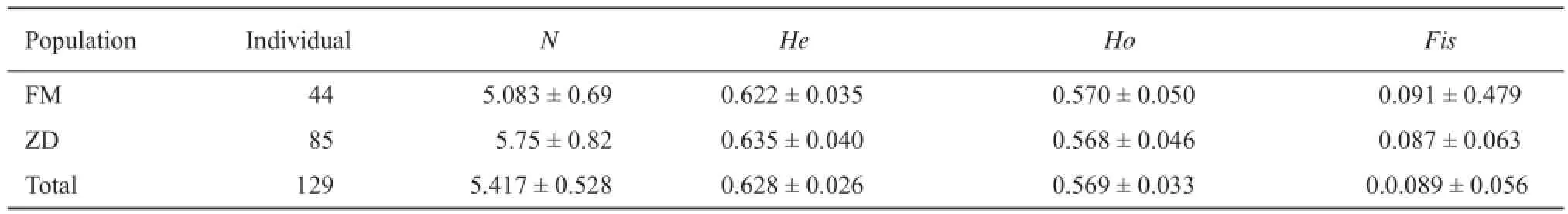
Table 1 Genetic diversity of studied parents and offspring at 12 microsatellite loci.

Table 2 Summary of the characteristics of each clutch.
In lacertids, multiple paternity is often related to the coexistence of conflicting male mating strategies (Laloi et al., 2004). The question of whether territorial behaviour affects the level of multiple paternity is pertinent. There are two species showing strong territoriality that also have high levels of multiple paternity. One is Sceloporus virgatus (62% of clutches are multiple sired) (Abell,1997), whereas the other is E. heatwolei (65%-82%)(Morrison et al., 2002). However, some territorial species are associated with notably low incidence of multiple paternity, for example A. exsul (9.1%) and V. berus (16.7%) (Höggren, 1995; Lewis et al., 2000). Furthermore, the sand lizards are not territorial, but levels of multiple paternity remain high (Gullberg et al., 1997). There are currently insufficient data to allow a rigorous phylogenetically controlled test of the relationship between categories, such as pair bonding and territorial and nonterritorial lizards (Uller and Olsson,2008). In S. crocodilurus, adult males show the strongest territoriality (Wan, 2009); however, it remains diffi cult to determine whether territorial behaviour affects the level of multiple paternity.
Sperm storage plays an important role in reptile reproduction, particularly when male and female cycles do not coincide (Joseph and Shaw, 2011). In addition,sperm storage has been well documented in many snake, lizard and turtle species (Schuett and Gillingham,1986; Valenzuela, 2000; Villaverde and Zucker, 1998);S. crocodilurus anatomy supports the presence of a sperm storage structure (Zhang, 2002). However, many of the arguments for the adaptive signifi cance of sperm storage in these taxa may not apply to S. crocodilurus. For example, it has been proposed that in turtles producing multiple clutches per season, eggs moving down the oviduct may ‘sweep' away sperm moving upwards that would have been used to fertilise subsequent clutches(Gist and Jones, 1989). However, S. crocodilurus produces only a single clutch per season (Yu et al., 2006) and may not require stored sperm for subsequent clutches in the same year. Another argument for sperm storage in turtles and some snakes is that many species show asynchrony in gonadal cycles between the sexes (Galbraith 1993;Halpert et al. 1982). Studies of S. crocodilurus hormonal cycles have shown that male testosterone levels increase significantly during June, while there are no significant differences in female estradiol levels during the breeding season (Huang et al., 2014b). According to the previous study, we cannot determine whether the gonadal cycles of S. crocodilurus develop asynchronously. Therefore,multiple paternity in S. crocodilurusis is most likely due to within season multiple matings, and the utilisation of sperm storage structures remains unknown, further research on the possibility of switching out males in the enclosure to look into the adult female still reproduce with sperm store will be carried out in the future.
In addition, we noticed that some females produce multiply sired clutches, while others do not, and some fathers could not be identifi ed (Table 2). For singly sired clutches, some females may mate either only once or multiple times with the same male. It is also possible that females mate with multiple males, but only one male employing the best timing manages to fertilise the eggs. Alternately, sperm competition and sperm selection may also account for singly sired clutches by females which have mated multiply (Davis et al., 2001). The missing fathers may have been dead or may have been released back into the wild.
The average expected heterozygosity (He = 0.628)of the semi-natural population is similar to that of the wild groups (He = 0.61) (Huang et al., 2014a). The previous study showed low genetic diversity in wild population (Huang et al., 2014a), whereas the seminatural population did not show changes in the level of genetic diversity. It is possible that the genetic difference in parents is low, resulting in inbreeding and leading to the defi cit in heterozygosity.
Although these data reveal multiple paternity of S. crocodilurus, a number of questions remain unanswered. These include whether multiple paternity is a strategy employed by S. crocodilurus in wild populations,whether the mating order of males affects offspring genotypes and the degree to which multiple mating affects offspring genotypes. Undoubtedly, further investigations of the S. crocodilurus mating system are required to uncover complex interactions as well as genetic and environmental determinants of offspring genotypes.
Acknowledgements This study was supported by the National Natural Science Foundation of China (No. 31360522). We would like to thank all the staff of the Luokeng Nature Reserve who helped us in collectingfarm samples, in particular Nan HE and Haiyang LIU.
Abell A. J. 1997. Estimating paternity with spatial behaviour and DNA fingerprinting in the striped plateau lizard, Sceloporus virgatus (Phrynosomatidae). Behav Ecol Sociobiol, 41: 217-226
Bateson Z. W., Krenz J. D., Sorensen R. E. 2011. Multiple paternity in the common fi ve-lined skink (Plestiodon fasciatus). J Herpetol, 45: 504-510
Bei R. B., Chen J. P., Liu H. Y., Huang J. L., Yu H., Wu Z. J. 2012. Isolation and characterization of 12 microsatellite loci in the Chinese crocodile lizard (Shinisaurus crocodilurus). Conserv Genet Resour, 4: 743-745
Berry O. F. 2006. Inbreeding and promiscuity in the endangered grand skink. Conserv Genet, 7: 427-437
Davis L. M., Glenn T. C., Elsey R. M., Dessauer H. C., Sawyer R. H. 2001. Multiple paternity and mating patterns in the American alligator, Alligator mississippiensis. Mol Ecol, 10:1011-1024
Galbraith D. A. 1993. Multiple paternity and sperm storage in turtles. Herpetol J, 3: 117-123
Gist D. H., Jones J. M. 1989. Sperm storage within the oviduct of turtles. J Morphol, 199: 379-384
Gullberg A., Olsson M., Tegelström H. 1997. Male mating success, reproductive success and multiple paternity in a natural population of sand lizards: Behavioural and molecular genetics data. Mol Ecol, 6: 105-112
Halpert A. P., Garstka W. R., Crews D. 1982. Sperm transport and storage and its relation to the annual sexual cycle of the female red-sided garter snake, Thamnophis sirtalis parietalis. J Morphol, 174: 149-159
Höggren M. 1995. Mating strategies and sperm competition in the adder (Vipera berus). PhD Thesis. Uppsala University, Uppsala
He N., Wu Z. J.,Cai F.J., Wang Z.X., Yu H., Huang C. M. 2011. Sexual dimorphism of Shinisaurus crocodilurus. Chin J Ecol,30:7-11(In Chinese)
Huang C. M., Yu H., Wu Z. J., Li, Y., Wei, F., Gong, M. H. 2009. Population and conservation strategies for the Chinese crocodile lizard (Shinisaurus crocodilurus) in China. Anim Biodiv Conserv, 31: 63-70
Huang H. Y., Wang H., Li L., Wu Z. J., Chen J. P. 2014a. Genetic diversity and population demography of the Chinese crocodile lizard (Shinisaurus crocodilurus) in China. PLoS ONE, 9(3):e91570.
Huang H. Y., Liu H. Y., He N., Wu Z. J. 2014b. Preliminary noninvasive analysis study of fecal hormone in the Chinese Crocodile Lizard (Shinisaurus crocodilurus, Ahl). J Guangxi Normal Univ (Nat Sci Ed), 4: 123-127(In Chinese)
Joseph J., Shaw P. W. 2011. Multiple paternity in egg clutches of hawksbill turtles (Eretmochelys imbricata). Conserv Genet, 12:601-605
Laloi D., Richard M., Lecomte J., Massot M., Clobert J. 2004. Multiple paternity in clutches of common lizard Lacerta vivipara: Data from microsatellite markers. Mol Ecol, 13: 719-723
Lebas N. R. 2001. Microsatellite determination of male reproductive success in a natural population of the territorial ornate dragon lizard, Ctenophorus ornatus. Mol Ecol, 10: 193-203
Lewis A. R., Tirado G., Sepulveda J. 2000. Body size and paternity in a teiid lizard (Ameiva exsul). J Herpetol, 34, 110-120
Marshall T. C., Slate J., Kruuk L. E., Pemberton J. M. 1998. Statistical confi dence for likelihood-based paternity inference in natural populations. Mol Ecol, 7: 639-655
Morrison S. F., Keogh J. S., Scott I. A. 2002. Molecular determination of paternity in a natural population of the multiply mating polygynous lizard Eulamprus heatwolei. Mol Ecol, 11:535-545
Olsson M., Madsen T. 2001. Promiscuity in sand lizards(Lacerta agilis) and adder snakes (Vipera berus): Causes and consequences. J Hered, 92: 190-197
Olsson M., Wapstra E., Schwartz T., Madsen T., Ujvari B., Uller T. 2011. In hot pursuit: fluctuating mating system and sexual selection in sand lizards. Evolution, 65: 574-583
Parker P. G., Snow A. A., Schug M. P., Booton G. C., Fuerst P. A. 1998. What molecules can tell us about populations: choosing and usinga molecular marker. Ecology, 79: 361-382
Pearse D., Janzen F., Avise J. 2002. Multiple paternity, sperm storage, and reproductive success of female and male painted turtles (Chrysemys picta) in nature. Behav Ecol Sociobiol, 51:164-171
Pearse D. E., Janzen, F. J., Avise J. C. 2001. Genetic markers substantiate long-term storage and utilization of sperm by female painted turtles. Heredity, 86: 378-384
Schuett G. W, Gillingham J. C. 1986. Sperm storage and multiple paternity in the copperhead Agkistrodon contortrix. Copeia,1986: 807-811
Uller T., Olsson M. 2008. Multiple paternity in reptiles: patterns and processes. Mol Ecol, 17: 2566-2580
Valenzuela N. 2000. Multiple paternity in side-necked turtles Podocnemis expansa: evidence from microsatellite data. Mol Ecol, 9: 99-105
Villaverde G. A., Zucker N. 1998. Sperm storage resulting in viable offspring in the tree lizard Urosaurus ornatus (Sauria:Phrynosomatidae). Southwest Nat, 43: 92-95
Wan J. J. 2009. Study on diurnal activity rhythms and territoriality of Chinese crocodile lizard (Shinisaurus crocodilurus) in Luokeng Nature Reserve, Guangdong. Ph. D. Thesis. Sichuan University, 23-28 pp (In Chinese)
Wang Z. X., Wu Z. J., Chen L., Yu S., Yu H., Huang C. M., Jiang J. 2009. Effect s of pregnancy and ages on tempera ture selection and resting metabolicrates in chinese crocodile lizard,Shinisaurus crocodilurus. J Guangxi Normal Univ (Nat Sci Ed),2: 80-84 (In Chinese)
Wang Z. X., Wu Z. J., Yu H., Huang C. M. 2008. Thermoregulatory and thermal dependence of resting metabolicrates in the Chinese crocodile lizard (Shinisaurus crocodilurus) in the Luokeng Nature Reserve, Guangdong. Acta Zool Sin, 54: 964-971 (In Chinese)
Wu Z. J., Dai D. L., Huang C. Yu H. M., Ning J. J., Zhong Y. M. 2007. Selection of Shinisaurus crocodilurus forest type in mountain streams in Luokeng Nature Reserve of Guangdong Province. Chin J Ecol, 26: 1777-1781 (In Chinese)
Yu H., Huan C. M., Wu Z. J., Ning J. J., Dai, D. L. 2006. Observation on habit of Chinese crocodilian lizard. Sichuan J Zool, 25: 364-366 (In Chinese)
Yu S., Wu Z. J., Wang Z. X., Chen L., Huang C. M., Yu H. 2009. Courtship and mating behaviour of Shinisaurus crocodilurus bred in Luokeng Nature Reserve, Guangdong. Chin J Zool, 44:38-44 (In Chinese)
Zhang Y. X. 2002. The biology in crocodilian lizard. Guilin, China: Guangxi Normal University Press (In Chinese)
Zhang Y. X., Tang Z. J. 1985. The biology of Shinisaurus crocodilurus. Acta Herpetological Rearch, Vol.11 2008 Guenther. Acta Herpetol Sin, 1985, 4: 337-340 (In Chinese)
Zhao J. Y., Zhang Y. X., Lang D. Y. 2006. Ecology of Chinese crocodilian lizard in Burrow. Sichuan J Zool, 12: 261-263(In Chinese)
Dr. Zhengjun WU, from Guangxi Normal University, China, with his research focusing on the ecology of amphibians and reptiles; and Dr. Jinping CHEN, from Guangdong Entomological Institute, China, with his research focusing on molecular ecology.
E-mail: 2581572507@qq.com (Zhengjun WU); chenjp@gdei.gd.cn(Jinping CHEN)
24 September 2014 Accepted: 11 February 2015
杂志排行
Asian Herpetological Research的其它文章
- Isolation and Characterization of 17 Microsatellite DNA Loci for Odorrana margaretae (Anura: Ranidae)
- Is Habitat Preference Associated with Locomotor Performance in Multiocellated Racerunners (Eremias multiocellata) from a Desert Steppe?
- Toxic Effects of Three Heavy Metallic Ions on Rana zhenhaiensis Tadpoles
- Herpetological Diversity of Timor-Leste: Updates and a Review of Species Distributions
