Toxic Effects of Three Heavy Metallic Ions on Rana zhenhaiensis Tadpoles
2015-10-31LiWEIGuohuaDINGSainanGUOMeilingTONGWenjunCHENJonFLANDERSWeiweiSHAOandZhihuaLIN
Li WEI, Guohua DING, Sainan GUO, Meiling TONG, Wenjun CHEN, Jon FLANDERS, Weiwei SHAOand Zhihua LIN*
1College of Ecology, Lishui University, Lishui 323000, Zhejiang, China
2School of Biological Sciences, Bristol Life Sciences Building, University of Bristol, 24 Tyndall Avenue, Bristol,BS8 1TQ. UK
Toxic Effects of Three Heavy Metallic Ions on Rana zhenhaiensis Tadpoles
Li WEI1, Guohua DING1, Sainan GUO1, Meiling TONG1, Wenjun CHEN1, Jon FLANDERS2, Weiwei SHAO1and Zhihua LIN1*
1College of Ecology, Lishui University, Lishui 323000, Zhejiang, China
2School of Biological Sciences, Bristol Life Sciences Building, University of Bristol, 24 Tyndall Avenue, Bristol,BS8 1TQ. UK
Heavy metal pollution is widespread in some areas of China and results in contamination of land, water,and air with which all living organisms interact. In this study, we used three heavy metallic ions (Cu2+, Pb2+and Zn2+) to assess their toxicity effects on mortality, blood biomarker and growth traits (body length and body mass) of Rana zhenhaiensis tadpoles. The results showed that the toxicity levels of the three metallic ions were different when conducted with different experiment designs. For acute toxicity tests, Cu2+was the most toxic with the highest tadpole mortality. The mortalities of tadpoles showed signifi cant differences among the treatments at the same exposure time endpoints (24, 48, 72 and 96h). Results from repeated measures ANOVA indicated that metallic ion concentration,exposure time and their interactions signifi cantly affected the mortalities of R. zhenhaiensis tadpoles. Also, the toxicity effects of all binary combinations of the three metallic ion treatments showed synergism. The half lethal concentrations(LC50) decreased with increasing exposure time during the experimental period, and the safe concentration (SC) values of Cu2+, Pb2+and Zn2+were different from each other. Combined and compared LC50values with previous data reported,it is suggestes that the toxicity levels of metal pollution to anuran tadpoles should be species-and age-related. For blood biomarker tests, Zn2+was the most toxic with the highest total frequencies of abnormal erythrocytic nucleus. All three metallic ions caused higher abnormal erythrocytic nucleus compared with control groups. In a chronic toxicity test,Pb2+was the most toxic with lowest growth traits. Survival rate (except for 18 days), total body length and body mass showed signifi cant differences among the treatments. These fi ndings indicated that tadpoles of R. zhenhaiensis should be as a bioindicator of heavy metals pollution.
Acute toxicity; micronucleus; chronic toxicity; growth; metal pollution; Rana zhenhaiensis
1. Introduction
The rapid and unprecedented decline of global biodiversity is of great concern and highlights the need to research the many different factors that can impact a species and its ecosystem. Amphibians play an important role in many ecological communities, ranging from helping nutrient cycles to serving as high quality prey items for predators, and as such their decline will impact on the ecosystems they are part of (deMaynadier andHunter, 1995; Vertucci and Corn, 1996; Stuart et al.,2004; Natale et al., 2006; Hussain and Pandit, 2012).
Among amphibians, they have a biphasic life cycle comprising of an aquatic and terrestrial phase. They are highly sensitive to water pollution due to their association with aquatic habitats and permeable skin and are widely used in the monitoring of water contamination(Ezemonye and Tongo, 2009; Xia et al., 2012). Indeed,the pollution of anuran habitats is considered to be one of the major factors in their decline (Hussain and Pandit,2012) with heavy metals considered as one of the worst chemical stressors due to their high toxicity at very low concentrations (Shuhaimi-Othma et al., 2012a). There have been many studies documenting the toxicity of exposure to metal compounds in different aquaticspecies. For example, in fish exposure metals such as copper (Cu2+), cadmiun (Cd2+) and Chromium (Cr6+)is known to affect key parameters, including survival,growth and development and has been shown to interfere with the octavolateral system (Johnson et al., 2007). Freshwater insects, such as Nais elinguis were found to be more sensitive to exposure to Cu2+, Cd2+, iron (Fe3+),manganese (Mn2+), lead (Pb2+), nickel (Ni2+), zinc (Zn2+)and aluminum (Al3+) than freshwater worms (Shuhaimi-Othman et al., 2012b). In amphibians, identifying what effects being exposed to metals such as Cd2+, Cu2+, Pb2+and Zn2+have been carried out on a number of species including Hypsiboas pulchellus (Natale et al., 2006),Duttaphrynus melanostictus (Shuhaimi-Othma et al.,2012a), Bufo bufo gargarizans (Yang and Jia, 2006),Rana chensinensis (Shi et al., 2007) and R. catesbeiana(Li and Tian, 2010). These studies show that although increases in metallic ion concentration and time of exposure leads to higher rates of mortality there is considerable species-specifi c variation in their sensitivity to different metallic ions. Moreover, in studies focusing on the sub-lethal/chronic effects of heavy metallic ions,such as R. chensinensis exposed to Pb2+(Wang and Wang, 2008) and Cu2+(Shi et al., 2007), Pelophylax nigromaculatus exposed to Pb2+, Cu2+and Hg2+(Zhang,2009; Huang et al., 2014) and B. raddei exposed to Cd2+and Pb2+(Zhang et al., 2007), found that individuals exhibited abnormal growth, development, behavior and erythrocytic nuclear abnormalities, which increased their susceptibility to predation and competition and overall decrease reproductive success.
Rana zhenhaiensis (previously Rana japonica) is common in Southeast China. This species is mainly found in rural areas with tadpoles living in low-lying, temporary water bodies and ditches (Zhou et al., 2005). Previous studies on this species have focused on their vulnerability to pesticides such as Triazophos (Zhong et al., 2011)and emamectin benzoate (Chen et al., 2011), and they found the tadpoles were highly sensitive to agricultural pesticides. In south and east China metals such as Cu,Cd, Zn, Pb, Cr, Fe are widely used in industry and are common water pollutants. As the full impact of these metallic ions on the aquatic habitats and species is still unknown. In this study, we examine the acute and chronic toxicity effects of three heavy metallic ions (Cu2+, Pb2+and Zn2+) on tadpoles of R. zhenhaiensis, an important bio-indicator for water quality. The results of this work will provide a fundamental platform for establishing regulatory limits for metal loads in aquatic environments.
2. Materials and Methods
We collected Zhenhai brown frog (R. zhenhaiensis) eggs from a fi eld in a suburb of Lishui City, Zhejiang Province,China, in March 2014. Eggs were then incubated within opaque plastic cages (60 cm length × 40 cm width × 30 cm height) with 20cm depth of dechlorinated tap water. Prior to experimentation the tadpoles were reared with commercial fi sh food (Shanghai Tech-bank feed industry Co. LTD). Tadpoles that were considered to be in good health (swimming freely, with good reflexes; average body weight = 0.04±0.001g; average body length = 1.54±0.11cm) were selected for toxicity treatment.
A standard stock solution of Cu2+, Pb2+and Zn2+(100 mg/ L) were prepared from analytical grade metallic salts of CuSO4·5H2O, ZnSO4·7H2O and (CH3COO)2Pb·3H2O. The stock solutions were prepared with deinoized water in 1L volumetric flask and then kept for subsequent concentration dilutions.
2.1 Pre-experiment A wide range of metal solution concentrations were used in the pre-experiment; seven Cu2+(0.2, 0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 mg/L), fi ve Pb2+(10.0, 30.0, 50.0, 70.0 and 90.0 mg/L) and six Zn2+(5.0, 10.0, 20.0, 30.0, 40.0 and 50.0 mg/L). Each metaltreated concentration group consisted of 2 replicates of 10 randomly allocated tadpoles in a round 1000mL plastic container with 500 mL of the appropriate solution. The numbers of dead tadpoles in each container were counted 48h later. By observing tadpole mortality the lethal concentration of no mortality (LC0) and maximum mortality (LC100) were used to obtain the range concentration of LC0to LC100for the following experiments (Wei et al., 2014).
2.2 Acute toxicity Based on the pre-experiment results static-water tests were used in the toxicity experiments(Zhou and Zhang, 1989). Six Cu2+(0.3, 0.4, 0.5, 0.6, 0.7 and 0.8 mg/L), fi ve Pb2+(30.0, 40.0, 50.0, 60.0 and 70.0 mg/L) and Zn2+(10.0, 20.0, 30.0, 35.0 and 45.0 mg/L)were chosen for the acute toxicity experiments with each having their own control (0.0 mg/L). 15 experimental tadpoles were randomly allocated to each metal-treated concentration in a 1000 mL plastic container with 800 mL metal solution. Each concentration treatment was conducted in triplicate at room condition. Mortality was recorded every 24 hours for 4 days (96 hours) for each treatment. Tadpoles were recorded as dead when they turned upside down and sank to the bottom of the container or when their tail showed no form of movement even when prodded with a glass rod (Mgbaeruhu, 2002).During the acute toxicity test, the tadpoles were not fed.
2.3 Joint toxicity To examine the joint toxicity of the three metallic ions, pairwise combinations were performed based on the results of the acute toxicity tests. The half lethal concentration (LC50) acute toxicity test at 48h was then taken as 1 toxic unit (U). For the following joint toxicity testing, test concentrations of the three metallic ions combined are listed in table 1. Ten experimental tadpoles were randomly allocated to each joint concentration following the method of acute toxicity tests. The mortalities of tadpoles exposed to combined metals at 48h were recorded following the methods of Chen et al. (2007).
2.4 Blood biomarkers To further investigate the toxic effects of the three metallic ions on R. zhenhaiensis tadpoles, blood parameter measurements were conducted. Five tadpoles were randomly exposed to three new concentrations of each metallic ion (0.10, 0.20 and 0.33 mg/L) with 4 replications respectively over a period of 4 days (96h). One more treatment (0 mg/L) was set up for control. Every 24 hours genotoxicity of each treatment was tested using the measuring erythrocytic nuclear assay (ENA), carried out in mature peripheral erythrocytes according to the procedures of Guilherme et al (2008). The blood smear of the live tadpoles were fixed with methanol for 10 min and stained with 10% Giemsa for 15-20 min. For each smear, 500 erythrocytes were observed and scored under 1000× magnification to determine the frequency of the following nuclear lesions categories: mitotic (M), binucleated (BN),micronuclei (MN), 8 shape nuclei (8SN), karyopyknosis(K), anucleated (AN) and unequal division (UD). The control group was only carried out after the 48h exposed. The results were expressed as the mean value (%) of the sum (M+BN+MN+8SN+K+AN+UD) for all the lesions observed (Guilherme et al., 2008).
2.5 Chronic toxicity Chronic toxicity tests were carried out in a similar manner as the acute tests. Only one low concentration (1/10 toxic U) of each metallic ion was used. Thus the treatment concentrations of Cu2+, Pb2+and Zn2+were 0.055, 4.44 and 2.40 mg/L, respectively. Tests were done using three replications per metallic ion and one control group. Ten tadpoles were randomly allocated to each container. Exposure lasted for 18 days, and growth traits including survival rate, body mass and total body length (the length from snout to tail tip) of tadpoles were collected every 6 days. Tadpoles were reared with commercial fi sh food (Shanghai Tech-bank feed industry Co. LTD). A new stock solution for each metallic ion was made up every 3 days immediately before each water change.
2.6 Data analysis Prior to any statistical tests all variables were tested for normality and homogeneity. For the acute tests, One-way ANOVA and Tukey's post hoc multiple comparisons test were used to evaluate the effects of each metal on the mortalities of tadpoles under different concentrations and different exposure times. To examine the correlated effects of both concentration and exposure on tadpole mortality repeated measures ANOVA was used. For comparisons of the growth data among the three metallic ions in the chronic toxicity tests, Oneway ANOVAs were mainly used. Statistical analyses were conducted via Statistica 6.0, with α=0.05 taken as statistically signifi cant.
Half lethal concentration (LC50) for each metallic ion was determined using probit analyses and straight line interpolations (Chen et al., 2007), while the corresponding safe concentrations (SC) were carried out with two typical equations:
SC I = (48h-LC50×0.3)/(24h-LC50/48h-LC50)2(Zhang et al.,2011)
SC II = 96h-LC50×0.1 (Ezemonye and Tongo, 2009)
The evaluation of joint toxicity for each binary metallic ions combined was conducted using characteristic of mortality-concentration curves based on data recorded at 48h exposure. When the mortality was > 50%, the co-effects were taken as synergistic; in turn, when the mortality < 50%, the coeffects were taken as antagonistic(Chen et al., 2007).
3. Results
3.1 Acute toxicity and joint toxicity When R. zhenhaiensis tadpoles were exposed to the three different metallic ion solutions over the same exposuretimes tadpole mortality was significantly different between treatments (P<0.001, Table 2) with half lethal concentrations (LC50) decreasing with increasing exposure time. Variation between metallic ion types was also observed in the safe concentration (SC) values (Table 3)with overall toxicity levels going from Cu2+> Zn2+> Pb2+. The results of repeated measures ANOVA indicated that metallic ion concentration and exposure time signifi cantly affected R. zhenhaiensis tadpole mortality (Table 4).

Table 1 The proportion of binary combined concentrations of the three metallic ions.
For pairwise ions combined tests, the toxicity effects of all the three treatment groups were similar (Figure 1). However, tadpole mortality was not significant different between the various concentrations within each joint treatments (Cu-Pb: F4,10=2.000, P=0.171; Zn-Pb:F4,10=0.657, P=0.635; Cu-Pb: F4,10=1.000, P=0.452, Oneway ANOVA).
3.2 Blood biomarker All tadpoles in 0.33 mg/L of Cu2+treatment died before the end of their 12 hour exposure. Overall the three metallic ions produced sev en different types of erythrocyte abnormalities: mitotic, binucleated,micronuclei, 8 shape nuclei, karyopyknosis, anucleated and unequal division (Figure 2). The results showed that the total frequencies of abnormal erythrocytic nuclei(TFAEN) were all significantly higher than the control group (Table 5). Moreover, during the same exposure concentration, frequencies of abnormal erythrocytic nuclei observed (FAEN) were also different among various exposure times (Table 5).
3.3 Chronic toxicity Tadpoles exposed to the three metallic ions at low concentrations showed differences in growth pattern compared to the control group (Figure 3). The percentage survival of the exposed tadpoles was reduced compared to that of the control group, and signifi cant differences were found at day 6 (F3,11=9.200,P=0.006, One-way ANOVA) and at day 12 (F3,11=4.133,P=0.048, One-way ANOVA) but not on day 18 (Figure 3A). The Pb2+treatment recorded the lowest survival rate,body mass and smallest total body length across all time periods. Cu2+treatment caused significantly lower body lengths across all time periods compared to Zn2+treatment and also when compared to the control for body mass and body length (all P < 0.05, One-way ANOVA, Figure 3B and 3C).
4. Discussion
4.1 Acute and joint toxicity Our study shows that tadpole mortality was positively correlated with heavy metallic ion concentration. However, mortality rates were not uniform across the different metal types with copper being more toxic than zinc which was more toxic than lead (Cu2+> Zn2+> Pb2+; Table 2). These results correspond with previous studies by Khangarot et al.(1985) and Yang and Jia (2006) who identifi ed the level of toxicity of different metallic ions on R. hexadactyla and B. bufo gargarizans tadpoles to be Cu2+> Zn2+> Fe3+> Pb2+and Cu2+> Cd2+> Zn2+, respectively. In contrast,Shuhaimi-Othman et al. (2012b) found that Duttaphrynus melanostictus tadpoles were more sensitive to Cu2+>Cd2+> Fe3+> Al3+> Pb2+> Zn2+> Ni2+> Mn2+indicatingthat some species are more susceptible to certain metallic elements more than others.
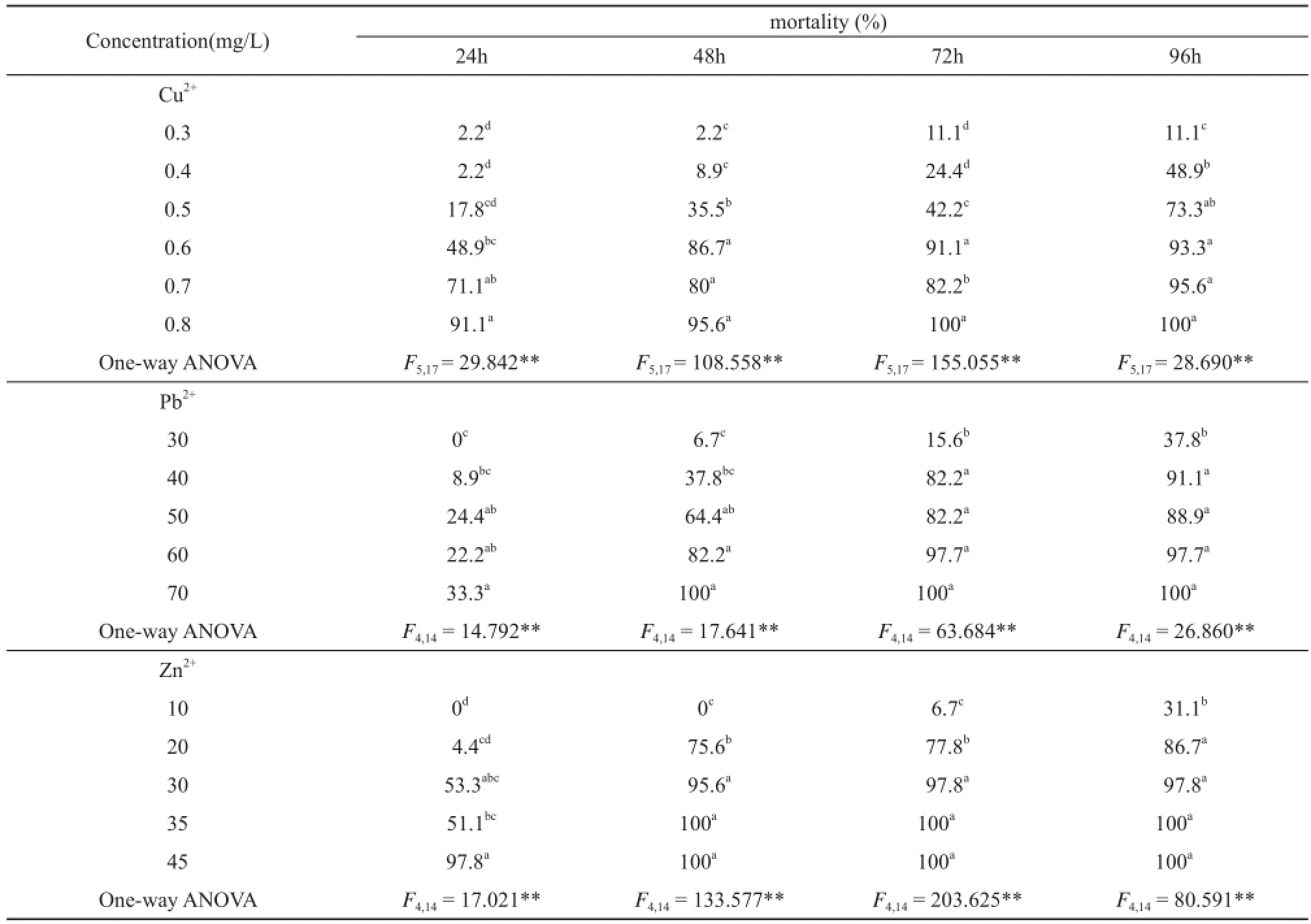
Table 2 The acute toxicity test results of the three metallic ions alone to R. zhenhaiensis tadpoles (n = 15).
This study showed that LC50values for 24, 48, 72 and 96h of Cu2+, Pb2+and Zn2+were 0.62, 67.08 and 32.90;0.55, 44.39 and 23.98; 0.48, 31.72 and 16.88, and 0.43,31.72 and 12.21 mg/L, respectively (Table 3). Compared to other studies that have investigated the toxicity of the three heavy metallic ions on anuran species at their different life stages, especially for tadpoles, we found different LC50values produced by different study species(Table 3, Table 6). One possible reason for this is because the experimental methods conducted in each study, such as body size/developmental stage, body mass of tadpoles and experimental water (soft or hard) were different. For example, Rao and Madhyastha (1987) conducted a study on the toxicity of heavy metallic ions (Hg2+, Cd2+, Cu2+,Mn2+, and Zn2+) on different ages (1- and 4-week old) of tadpoles of M. ornata and found that 4-week old tadpoles were more sensitive toward heavy metallic ions than were l-week old ones. Also, Harris et al. (2000) conducted toxicity testing with R. pipiens and B. americanus to pesticides, and found that the former was more sensitive to pesticides than the latter. This is probably due to species and age related differences in susceptibility to pesticides and heavy metallic ions.
In this study we found that Cu2+was approximately 100 times more toxic to R. zhenhaiensis tadpoles than Pb2+and 50 times more toxic than Zn2+. Typically, aquatic organisms sensitivity to trace metals follows the trend:Hg2+> Ag+> Cu2+> Cd2+> Zn2+> Ni2+> Pb2+> Cr6+>Sn4+(Luoma and Rainbow, 2008). However, some toxicity studies with other species found that Pb2+was more toxic than Zn2+, such as with D. melanostictus (Shuhaimi-Othman et al., 2012a), R. hexadactyla (Khangarot et al.,1985), Nais slinguis (Shuhaimi-Othman et al., 2012b). Therefore, It is by no means the case that all essential metals are more toxic than all nonessential metals.
Comparisons of the pairwise joint toxicity treatments were highly similar across treatments (Figure 1). This corresponds to a study on R. limnocharis tadpoles by Jia et al. (2005) who found similar results when testing the joint toxicity of Cu-Zn. Compared to previous studies reporting on the toxicity of metallic ions to aquatic organisms, we found that different results produced by different species. For example, the joint toxicity of Cu-Zn conducted by Yang and Jia (2006) with B. bufo gargarizans showed antagonistic. Likewise, the joint toxicity of Cu-Zn, Pb-Zn, and Cu-Pb conducted by Chen et al. (2007) with Hydra sp, and Pb-Zn conducted by Zhang et al. (2011) with Carassius auratus showed antagonistic. This is probably because the co-effects of metallic ions are complicated, which the way they act on cells are different (Eaton, 1973).
4.2 Blood biomarker The toxic effects of Cu2+, Pb2+and Zn2+on the tadpoles could be observed in their blood tests(Table 5). The total frequency of abnormal erythrocytic nuclei (TFAEN) increased by increasing exposure concentration for all the three metallic ions (Table 5).Zn2+was the most toxic to the tadpoles blood red cells followed by Pb2+and then Cu2+at 0.10 mg/L treatments,Zn2+>Cu2+>Pb2+at 0.20 mg/L treatments and Zn2+>Pb2+with 0.33 mg/L treatment (Table 5). This is in agreement with the results of Rosenberg et al. (1998) conducted with B. arenarum exposed to Pb2+and Jiang et al. (2008)conducted with B. melanostictus exposed to Cu2+, Pb2+and Hg2+, which showed that there was a micronuclei response to heavy metallic ions. Interestingly, Jiang et al. (2008)and Zhang (2009) also found that TFAEN produced by B. melanostictus and Pelophylax nigromaculatus were higher in Pb2+treatments than in Cu2+treatments. However,other micronuclei studies with other species found that Cu2+was more toxic than Pb2+, such as B. gargarizans(Zhou et al., 2008). Although these contrasting results may be due to different methodologies, they also show that amphibian hematological parameters will be affected by the interactions between various combinations of nutrients, metals and pesticides (Ilizaliturri-Hernandez et al., 2013). Thus, erythrocytic nuclear abnormalities(ENA) should be recognized as one type of biomarker to assess water quality and the genotoxicity of contaminants on organisms (Costa et al., 2011).
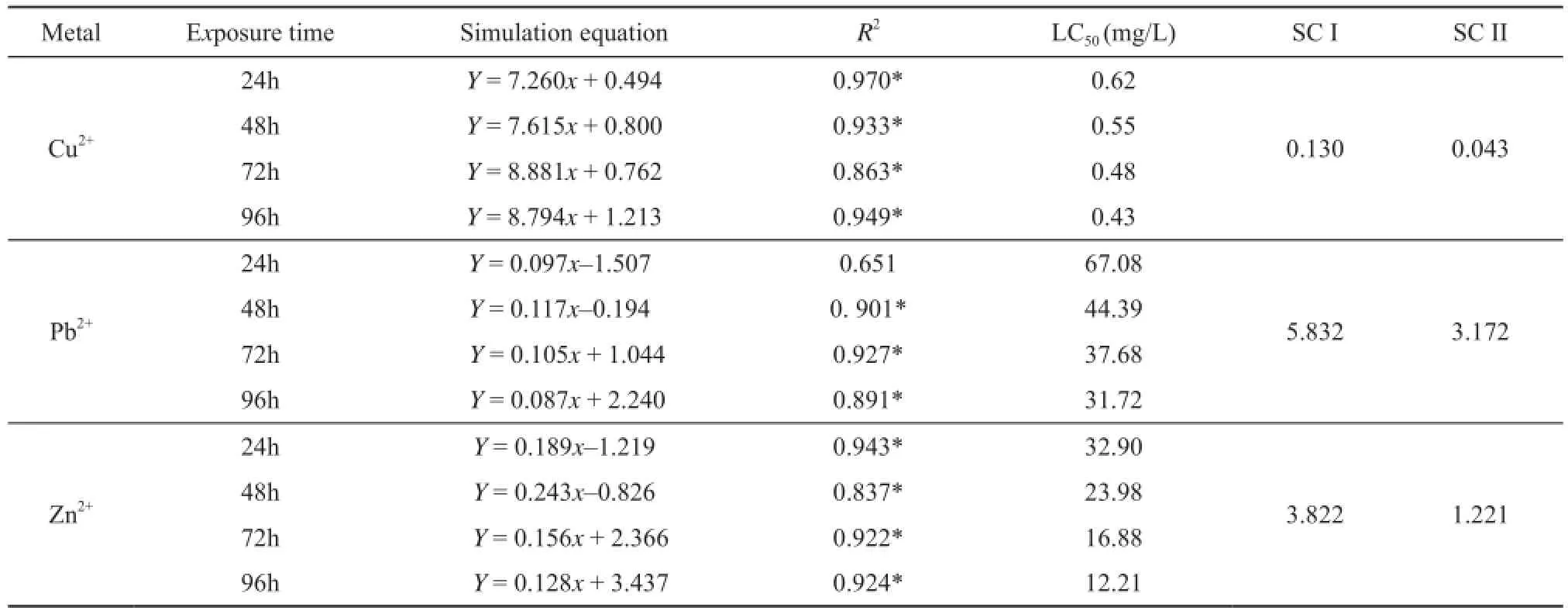
Table 3 The half lethal concentrations (LC50) and safe concentrations (SC) of the three metallic ions, mg/L.
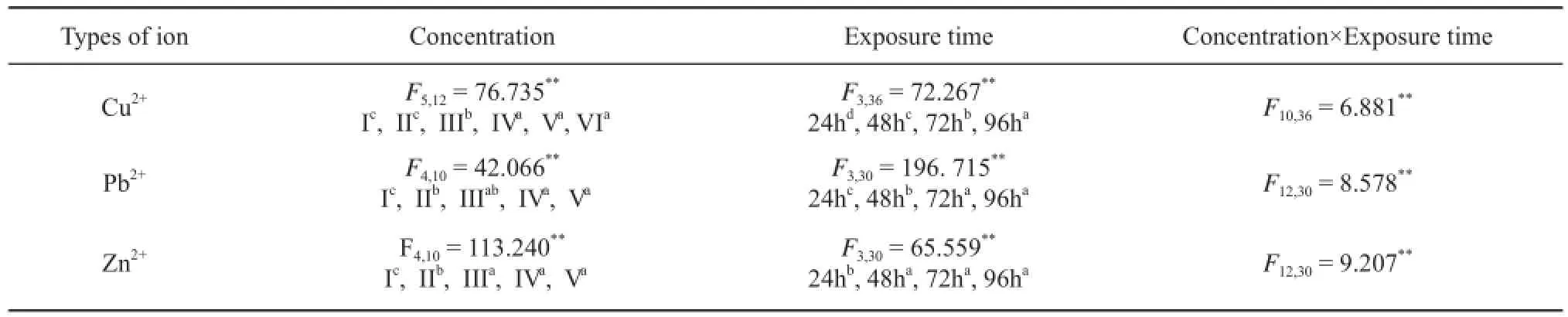
Table 4 The effects of ion concentration, exposure time and their interactions on mortality of R. zhenhaiensis tadpoles.
4.3 Chronic toxicity This study revealed the chronic toxicity of Cu2+, Pb2+and Zn2+to R. zhenhaiensis tadpoles over an 18-day period. The tadpoles were exposed to 1/10 LC50concentration of metallic ions with the toxic effects were recorded over several time points. The results showed that the three metallic ions affected the growth of the tadpoles compared to the control group. In the Pb2+treatment survival rate, body length and body mass were all lower than those in Cu2+and Zn2+treatments (Figure 3)showing that Pb2+was the most toxic to the development of R. zhenhaiensis tadpoles. Similar results were seen in a study by Jiang et al (2008). on B. melanostictus, although Jackson et al. (2005) found that Pb2+was less toxic than other metallic ions in Callianassa kraussi.
In conclusion, we predict that Cu2+, Pb2+and Zn2+could significantly affect the mortality, blood biomarker and growth traits of R. zhenhaiensis tadpoles. It is indicatedthat different heavy metallic ions should produced various toxic effects to organisms. Therefore, R. zhenhaiensis tadpoles should be a potential objective in toxicity testing and as a bioindicator of heavy metals pollution.

Table 5 Effects of the three metallic ions on micronuclei of red blood cells of R. zhenhaiensis tadpoles. N = the numbers of trial tadpoles, n = erythrocytic cells from smear observed, TNAEN = total numbers of abnormal erythrocytic nucleus observed, FAEN = frequency of abnormal erythrocytic nucleus observed, TFAEN = total frequency of abnormal erythrocytic nucleus observed
Acknowledgements This work was funded by the National Natural Science Foundation of China(31270443) and Natural Science Foundation of Zhejiang Province (LY13C030004). We thank Mengsha Xu for herassistance with laboratory work.

Table 6 Comparisons of LC50values of different larval amphibian species tadpoles tested with the three heavy metallic ions.
Chen N., Hao J. S., Wang Y., Su C. Y., Wu B. F. 2007. Single and binary-combined acute toxicity of heavy metal iron Hg2+, Cu2+,Cd2+, Ag+, Zn2+and Pb2+to Hydra. J Biol, 24(3): 32-35
Chen Z. X., Fang X. Q., Lin L., Geng B. R. 2011. Acute toxicity of emamectin benzoate on Rana zhenhaiensis tadpoles. J Ningde Teach Coll (Nat Sci Edit), 23(1): 21-23
Costa P. M., Neuparth T. S., Caeiro S., Lobo J., Martins M., Ferreira A. M., Caetano M., Vale C., Del Valls T. A., Costa M. H. 2011. Assessment of the genotoxic potential of contaminated estuarine sediments in fi sh peripheral blood: Laboratory versus in situ studies. Environ Res, 111(1): 25-36
Eaton J. G. 1973. Chronic toxicity of a copper, cadmium and zinc mixture to the fithead minnow (Pimcobales promelas rafi nesque). Wat Res, 7(11): 1723-1736
Ezemonye L. I. N., Tongo I. 2009. Lethal and sublethal effects of atrazine to amphibian larvae. Jordan J Biol Sci, 2(1): 29-36
Guilherme S., Válega M., Pereira M. E., Santos M. A., Pacheco M. 2008. Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol Environ Saf, 70(3): 411-421
Huang M. Y., Duan R. Y., Ji X. 2014. Chronic effects of environmentally-relevant concentrations of lead in Pelophylax nigromaculata tadpoles: Threshold dose and adverse effects. Ecotoxicol Environ Saf, 104: 310-316
Hussain Q. A., Pandit A. K. 2012. Global amphibian declines: A review. Inter J Biodiv Conserv, 4(10): 348-357
Ilizaliturri-Hernández C. A., González-Mille D. J., Mejía-Saavedra J., Espinosa-Reyes G., Torres-Dosal A., Pérez-Maldonado I. 2013. Blood lead levels, δ-ALAD inhibition, and hemoglobin content in blood of giant toad (Rhinella marina)to asses lead exposure in three areas surrounding an industrial complex in Coatzacoalcos, Veracruz, Mexico. Environ Monit Assess, 185(2): 1685-1698
Jackson R. N., Baird D., Eis S. 2005. The effect of the heavy metals lead (Pb2+) and zinc (Zn2+) on the brood and larval development of the burrowing crustacean, Callianassa kraussi. Water SA, 31(1): 107-116
Jia X. Y., Dong A. H., Yang Y. Q. 2005. Acute and joint toxicities of copper, zinc and triazophos to Rana limnocharis Boie tadpole. Res Environ Sci, 18(5): 26-30
Jiang B. Q., Chen W. T., Li D. F. 2008. Effect of heavy metal iron on growth of tadpole of toad (Bufo melanostictus Schneider). J South Chin Nor Univ (Nat Sci Edit), 2: 100-105
Johnson A., Carew E., Sloman K. A. 2007. The effects of copper on the morphological and functional development of zebrafi sh embryos. Aquat Toxicol, 84(4): 431-438
Khangarot B. S., Sehgal A., Bhasin M. K. 1985. ‘‘Man and Biosphere''-Studies on the Sikkim Himalayas. Part 5: Acute toxicity of selected heavy metals on the tadpoles of Rana hexadactyla. Acta Hydrochim Hydrobiol, 13(2): 259-63
Li H. M., Tian X. J. 2010. The effects of nine chemicals factors on the subsistence of Rana catesbeiana tadpoles. J Anhui Agricult Sci, 38(2): 769-771
Mgbaeruhu J. E. 2002. The influence of pH on the toxicity domestic detergents against tadpoles of Rana rana and fi ngerlings of Tilapia niloticus. MSc thesis University of Lagos. 67p
Natale G. S., Ammassari L. L., Basso N. G., Ronco A. E. 2006. Acute and chronic effects of Cr(VI) on Hypsiboas pulchellus embryos and tadpoles. Dis Aquat Organ, 72(3): 261-267
Rao I. J., Madhyastha M. N. 1987. Toxicities of some heavy metals to the tadpoles of frog, Microhyla ornata (Dumeril & Bibron). Toxicol Lett, 36(2): 205-208
Rosenberg C. E., Perí S. I., Arrieta M. A., Fink N. E., Salibián A. 1998. Red blood cell osmotic fragility in Bufo arenarum exposed to lead. Arch Physiol Biochem, 106(1): 19-24
Shi G., Wang J. X., Wang R. X. 2007. Toxic Effects of Cu2+on Rana Chensinensis tadpole growth and development. J Jilin Nor Univ (Nat Sci Edit), 3: 71-73
Shuhaimi-Othman M., Nadzifah N. S., Umirah N. S., Ahmad A. K. 2012a. Toxicity of metals to tadpoles of the common Sunda toad, Duttaphrynus melanostictus. Toxicol Environ Chem, 94(2):364-376
Shuhaimi-Othman M., Nadzifah Y., Umirah N. S., Ahmad A. K. 2012b. Toxicity of metals to an aquatic worm, Nais slinguis(Oligochaeta, Naididae). Res J Environ Toxicol, 6(4): 122-132
Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S., Fischman D. L., Waller R. W. 2004. Status and trends of amphibian declines and extinctions worldwide. Science,306(5702): 1783-1786
Vertucci F. A., Corn P. S. 1996. Evaluation of episodol acidifi cation and amphibian declines in the rocky mountains. Ecol Appl, 6(2):449-457
Wang J. X., Wang R. X. 2008. Toxic effects of Pb2+on growth and development of Rana chensinensis tadpole. Acta Agricult Zhejiangensis, 20(3): 203-207
Wang X. Y., Lu X. Y., Li C. M., Gao W. P., Gao M. 2001. Toxicity of heavy metal irons to embryos and larvae of Rana nigromaculata. Sichuan J Zool, 21(2): 59-61
Wei L., Shao W. W., Ding G. H., Fan X. L., Yu M. L., Lin Z. H. 2014. Acute and joint toxicity of three agrochemicals to Chinese tiger frog (Hoplobatrachus chinensis) tadpoles. Zool Res, 35(4):272-279
Xia K., Zhao H. F., Wu M. Y., Wang H. Y. 2012. Chronic toxicity of copper on embryo development in Chinese toad, Bufo gargarizans. Chemosphere, 87(11): 1395-1402
Yang Y. Q., Jia X. Y. 2006. Joint toxicity of Cu2+, Zn2+and Cd2+to tadpole of Bufo bufo gargarizans. Chin J Appl Environ Biol,12(3): 356-359
Yao D., Wan L. Y., Geng B. R., Huang H., Zhang Q. J. 2004. Acute toxicity of Cu2+to Rana japonica tadpoles. J Fujian Nor Univ (Nat Sci Edit), 20(4): 117-120
Zhang M. H. 2009. The toxic effect analysis of heavy metal ions on tadpoles of Pelophylax nigromaculatus. J Guiyang Coll (Nat Sci Edit), 4(1): 19-23
Zhang Y. L., Yuan J., Chen L. P., Shao H. 2011. Joint toxicity experiment of three heavy metal on fry of Carassius auratus. Heibei Fish, 39(2): 24-27
Zhang Y. M., Huang D. J., Zhao D. Q., Long J., Song G., Li A. N. 2007. Long-term toxicity effects of cadmium and lead on Bufo raddei tadpoles. Bull Environ Contam Toxicol, 79(2):178-183
Zhong B. J., Geng Y., Geng B. R. 2011. Acute toxicity of triazophos to Rana japonica tadpoles and its effects on growth. Herpetol Sinica, 11: 209-215
Zhou F., Jiang A.W., Lu Z. 2005. A new record of amphibian species in Guangxi-Rana zhenhaiensis. J Guangxi Agricult Biol Sci, 24(3): 248
Zhou X. R., Shen H. F., Pan Z. J., Wang L. J. 2008. Study on micronuclear and nuclear abnormalities induced by Pb2+and Cu2+in tadpoles erythrocyte. J Anhui Agricult Sci, 36(14): 5842-5844
Zhou Y. X., Zhang Z. S. 1989. Toxicity testing methods of aquatic organism. Beijing: Chinese Agricultural Press
Prof. Zhihua LIN, from Lishui University,Zhejiang, China, with his research focusing on physiological ecology of amphibians and reptiles.
E-mail: zhlin1015@126.com
14 December 2014 Accepted: 21 May 2015
杂志排行
Asian Herpetological Research的其它文章
- Isolation and Characterization of 17 Microsatellite DNA Loci for Odorrana margaretae (Anura: Ranidae)
- Genetic Analysis of Multiple Paternity in an Endangered Ovoviviparous Lizard Shinisaurus crocodilurus
- Is Habitat Preference Associated with Locomotor Performance in Multiocellated Racerunners (Eremias multiocellata) from a Desert Steppe?
- Herpetological Diversity of Timor-Leste: Updates and a Review of Species Distributions
