Preparation and Luminescence Properties of ZnAl2O4/SiO2:RE3+(RE=Eu,Tb,Ce)Glass Ceramics by Sol-gel Method
2015-10-28CUIXiangshuiCHENWenzhe
CUI Xiang-shui,CHEN Wen-zhe,2*
(1.College of Materials Science and Engineering,Fuzhou University,Fuzhou 350116,China;2.College of Materials Science and Engineering,Xiamen University of Technology,Xiamen 361024,China)*Corresponding Author,E-mail:chenwz@fzu.edu.cn
Article ID:1000-7032(2015)04-0400-08
Preparation and Luminescence Properties of ZnAl2O4/SiO2:RE3+(RE=Eu,Tb,Ce)Glass Ceramics by Sol-gel Method
CUI Xiang-shui1,CHEN Wen-zhe1,2*
(1.College of Materials Science and Engineering,Fuzhou University,Fuzhou 350116,China;2.College of Materials Science and Engineering,Xiamen University of Technology,Xiamen 361024,China)
*Corresponding Author,E-mail:chenwz@fzu.edu.cn
Transparent bulk glass ceramics of 4.5ZnO-5.5Al2O3-90SiO2(ZAS)and single doped ZAS:RE3+(RE=Eu,Tb,Ce)were synthesized by sol-gel method.X-ray diffraction(XRD),transmission electron microscopy(TEM)and photoluminescence(PL)spectra were employed to study the influence of doping rare-earth ions concentration on the structure and luminescence properties of ZAS glass-ceramics.XRD results show that ZAS:RE3+(RE= Eu,Tb,Ce)glass ceramics heat-treated at 900℃consist of ZnAl2O4phase with average grain size of 30 nm and amorphous SiO2.TEM results show that ZnAl2O4were precipitated from ZAS system when heat-treated at 900℃,and doping ions have not changed the original structure.PL spectra indicate that ZAS:Eu3+emits intense 611 nm red light originating from5D0→7F2transition of Eu3+ions,ZAS:Tb3+shows 541 nm green emission attributed to5D4→7F5transition of Tb3+ions,and ZAS:Ce3+gives 381 nm blue light corresponding to the 5d to 4f transition of Ce3+ions.Furthermore,the concentration quenching phenomena are observed while the single doping mole fraction of Eu3+,Tb3+and Ce3+was 20%,20%and 3%,respectively.The excellent Commission International de I'Eclairage(CIE)chromaticity coordinates of ZAS:RE3+(RE=Eu,Tb,Ce)glass-ceramics indicate that they are good candidates for the application of white LEDs.
ZAS glass ceramics;sol-gel method;rare-earth ions doped;luminescence properties
1 Introduction
Solid-state lighting(SSL)that uses white lightemitting diodes(w-LEDs)for illumination has been a subject of considerable interest[1-3].The presently commercial white LED is encapsulated by the combination of a blue-emitting LED chip,InGaN-GaN and a yellow-emitting phosphor,YAG:Ce3+[4].This white light,produced by mixing blue and yellow light,offers high luminescence efficiency but a poor color rendering index(CRI)[5].To meet the optimum requirements of w-LEDs,the white light materials with improved properties are in great demand.
Nowadays,some researchers have investigated on the potential application of rare-earth ions doped phosphors and glasses in the fabrication of w-LEDs owing to their excellent light output,color rendering properties and superior stability under near-ultraviolet light[6-11].For examples,Zhu[3]studied the full color and tunable white emitting behaviors of Sm/Tb/Ce co-actived CaO-B2O3-SiO2glasses.Fan[12]reported Ce/Dy/Eu doped CaF2-Al2O3-SiO2glass emits warm white light with tunable CIE coordination.Tshabalala[13]proved the existence of energy transfer from Ce3+to Tb3+in Ce3+and Tb3+co-actived ZnAl2O4phosphor.It is interesting that ZAS contains ZnAl2O4crystalline phase and SiO2amorphous phase.Zinc aluminate(ZnAl2O4)is a well-known wide-band gap semiconductor with good optical properties[14-15],and SiO2provides a good transparency environment,in addition,ZnAl2O4uniformly dispersed in SiO2host improves the mechanical properties of the whole materials.So ZAS can be a good material for w-LEDs with good mechanical and optical properties.
Until now,there are many methods to prepare ZAS nanocrystalline materials,for example,solid-state reaction[12],hydrothermal and combustion methods[15],sol-gel method[16-17],and so on.Incomparison with conventional solid-state reaction,the sol-gel method has been chosen due to advantages from the listed techniques,such as good chemical homogeneity and high purity as well as good properties of the prepared nanocrystals at relatively low temperature[17].
In this paper,transparent bulk ZAS,single doped ZAS:RE3+(RE=Eu,Tb,Ce)glass-ceramics were synthesized by sol-gel method.The crystal structure and the photoluminescence properties of these samples are discussed,and they can be expected to become good candidates for research in optical and optoelectronic devices.
2 Experiments
Transparentglass-ceramicswithcomposition 4.5ZnO-5.5Al2O3-90SiO2(ZAS),single doped ZAS: RE3+(RE=Eu,Tb,Ce)were synthesized by sol-gel method,respectively.The starting materials were tetraethoxy silane(TEOS),Zn(NO3)2·6H2O,Al(NO3)3·9H2O,Eu(NO3)3·6H2O,Tb(NO3)3· 6H2O and Ce(NO3)3·6H2O,and here,all the reagents were analytical-grade and were used without further purification.Firstly,a mixture of TEOS,ethanol and water in a molar ratio 1:4:1 was stirred for 1 h at room temperature.After this hydrolysis,requisite amounts of Zn(NO3)2·6H2O,Al(NO3)3· 9H2O,which had been dissolved in water,were added to give a H2O/TEOS ratio of 10.In the process,some amount of nitric acid was employed as a catalyst to control the pH of the solution,ensuring TEOS was hydrolyzed adequately.Meanwhile,a small amount of N,N-dimethylformamide(DMF)as surface drying control agent was added into the solution to avoid the cracking of gels.This solution wasstirred normally for 3 h at room temperature,poured into petri dishes of 60 mm diameter every 16 mL and allowed to gel at room temperature for 6 d.After gelation,the samples were aged from 40℃to 60℃,and then dried at 60℃for about 4 d.The preparation process of single doped ZAS:RE3+(RE=Eu,Tb,Ce)is similar to this.The dried gels were then heat treated in air at 900℃for 3 h.
Powder X-ray diffraction(XRD)patterns of the samples were carried out with a powder diffractometer(DAMX2500),using a Cu-target tube(λ=0.154 18 nm).Transmission electron microscopy(TEM)images were recorded with a JEM-2010 transmission electron microscope.The emission and excitation spectra were recorded on a Fluormax-4 spectrofluorometer(HORIBA Scientific)equipped with a xenon source.
3 Results and Discussion
3.1 Formation Process of Gels
The digital images of the samples were displayed in Fig.1,in which the ZAS gels keep complete and transparent when they were dried at 60℃for 4 d as shown in Fig.1(a),after heat-treated at 900℃in Fig.1(b).It shows from Fig.1 that all the samples keep transparent and colorless when big ZAS gels were broken into some little blocks.
The results show that when the gels were heattreated over 100℃,water and ethanol were removed,and then,when heated above 550℃,nitrates and organics were eliminated thoroughly.Furthermore, the gels transited to ZAS glasses when heated at 800℃,and especially,ZnAl2O4nanocrystals were precipitated from ZAS glass system,when the gels were further heated to 900℃for 3 h.Finally,the glass transition is due to forming the oxide network[18].

Fig.1 (a)Photograph of dried ZAS gel at 60℃.(b)Different samples heat-treated at 900℃for 3 h.
3.2 XRD
Fig.2 shows the XRD patterns of ZAS and ZAS: xRE3+(RE=Eu,x=0.20;RE=Tb,x=0.20;RE=Ce,x=0.03)samples heat-treated at 900℃for 3 h.It is clear,from the Fig.2,that all the samples have one broad reflection centered at 2θ=22°,which is characteristic of diffraction of amorphous SiO2matrix glass.The diffraction peaks are marked at 31.2°,36.8°,55.7°,59.3°,65.2°,respectively.WithreferencetoZnAl2O4spinelcard(ICCD No.05-0669),all of the diffraction peaks are totally consistent with the typical ZnAl2O4crystal structure peaks.These peaks are indexed as(220),(311),(422),(511)and(440),which correspond to some specific crystal faces.No characteristic peaks of impurity phase(such as willemite)were detected in the heated samples.In addition,the well-agreeing XRD results indicate that the limited rare-earth doping ions do not cause any significant change in the host structure.It should be noted that there is a character of residual amorphous phase-SiO2in the samples,which means the materials consist of ZnAl2O4nanocrystals and amorphous SiO2-based glass.In pure ZAS samples,the average ZnAl2O4grain size is calculated to be 30 nm,by the strongest peak(311)at 2θ=36.8°.While in ZAS: xRE3+(RE=Eu,x=0.20;RE=Tb,x=0.20;RE=Ce,x=0.03)samples,the average ZnAl2O4grain size is calculated to be 40-45 nm,which means some rare earth ions are partly incorporated into ZnAl2O4lattice,rare earth ions(Eu3+ionic radius 0.095 0 nm,Tb3+ionic radius 0.092 3 nm,Ce3+ionic radius 0.103 4 nm)are supposed to replace Al3+ions(ionic radius 0.068 nm)according to their ionic radius and valence states.Here,the crystalline size was calculated using Scheerer's equation:D= 0.9λ/(βcosθ),where λ is the X-ray wavelength(0.154 18 nm),and β is the full-width at half-maximum(FWHM)intensity of the diffraction line.

Fig.2 XRD patterns of different samples heat-treated at 900℃for 3 h
3.3 TEM
TEM images were conducted to investigate the morphology and size of nanocrystals in the samples. Fig.3 shows different magnification TEM images of ZAS nanocomposites heated at 900℃for 3 h.It is observed that the samples have a flake morphology in the TEM image and the dark and flocculent particles are ZnAl2O4nanocrystals.The samples consist of crystalline phase and amorphous phase,as can be seen,flocculent ZnAl2O4nanocrystallines are precipitated homogeneously from the SiO2glass matrix,which confirm the XRD results.The average size is estimated as 25-35 nm.

Fig.3 Different magnification TEM images of ZAS samples heat-treated at 900℃for 3 h
3.4 PL Spectra

Fig.4 PL excitation(a)and emission(b)spectra of ZAS: xEu3+(x=0.01-0.30)samples heated at 900℃
Fig.4 depicts the excitation and emission spectra of ZAS:Eu3+glass ceramics with different europium concentrations heated at 900℃for 3 h in air. Luminescence of the Eu3+is a consequence of electron transitions inside the f shell.This shell is well shielded from the surrounding environment,which results in characteristic narrow band on the emission spectra.Eu3+ions can be excited by either direct or indirect manner.The former one,through the ground stateabsorption,is relativelyinefficient process[19].The excitation spectrum(Fig.4(a))obtained by monitoring the emission of Eu3+at 611 nm(5D0→7F2)consists of several intense and sharp peaks,that corresponds to the direct excitation of the europium ground states into the higher excited states of the europium f-electrons.The position of these peaks is practically identical to the characteristic absorption bands for f-f transitions in trivalent europium[8].The little weak bands at 317 nm are due to the Eu3+-O2-charge transfer band(CTB),which exists in the excitation spectrum of ZnAl2O4: Mn2+[20],thus we regard this band as the host absorption.Peaks centered at 361,381,393,413and 464 nm correspond to the direct excitation of the Eu3+ions from the ground state7F0to different excited levels(5D4,5G4,5L6,5D3,and5D2),respectively[21].Thestrongestexcitationintothe7F0→5L6transition at 393 nm yields the emission spectrum of ZAS:Eu3+glass ceramics,as exhibited in Fig.4(b),and the emission spectrum consists of several intense bands in the range of 550-720 nm for red emission,which is from5D0excited state to7FJground states of Eu3+,i.e.,5D0→7F0(576 nm),5D0→7F1(595 nm),5D0→7F2(611 nm),5D0→7F3(652 nm),5D0→7F4(701 nm).When the doping Eu3+ions concentration is low,the Eu3+emission lines show low and broadband behavior,indicating that Eu3+ions might be located at the surface or close to the surface sites of ZnAl2O4phase,and with increased Eu3+ions,its emission lines become sharp and intense,which is the typical transitions lines of Eu3+in a crystal lattice of ZnAl2O4[6].
It is well known that Eu3+ions are sensitive to the surrounding environment and their luminescence property can be used for structural investigations[22]. In addition,the analysis of the non-degenerated5D0→7F0transition may provide information about impurities and sites occupied by Eu3+ions.The relative intensities of5D0→7F1and5D0→7F2emission,which are typical magnetic and electronic dipoledipole transitions,respectively,depend strongly on the local symmetry of the Eu3+ions.In a site with inversion symmetry the5D0→7F1magnetic dipole transition is dominating,while in a site without inversion symmetry5D0→7F2electric dipole transition is the strongest[8].Therefore,the intensity ratio of5D0→7F2transition to5D0→7F1transition is a good measure of the Eu3+ion site symmetry.As shown in Fig.5,the integrated intensity ratio of5D0→7F2/5D0→7F1(I2/I1)and5D0→7F4/5D0→7F1(I4/I1)for ZAS:Eu3+increases with increasing of Eu3+ions concentration,which suggests the incorporation of Eu3+ions into ZnAl2O4phase[23].The strongest emission centered at 611 nm corresponds to5D0→7F2electric dipole transition indicates that Eu3+locates at sites without inversion symmetry.

Fig.5 Asymmetry parameter R5D0→7F2/5D0→7F1(I2/I1)and5D0→7F4/5D0→7F1(I4/I1)of ZAS:Eu3+vs. Eu3+mole fraction

Fig.6 PL excitation(a)and emission(b)spectra of ZAS: xTb3+(x=0.01-0.30)samples heated at 900℃
The PL excitation and emission spectra of Tb3+doped ZAS glass ceramics heated at 900℃,3 h in air are shown in Fig.6.The excitation spectrum monitored at 541 nm consists of the excitation peak characteristics of Tb3+ions,which corresponds to transitions from7F6(4f8)ground state to higher energy states of 4f8configuration,i.e.,7F6→5L9(317 nm),7F6→5L6(339 nm),7F6→5G5(350 nm),7F6→5L10(367nm),and7F6→5G6(377 nm)[10,24].Under 377 nm excitation,the emission spectra of Tb3+doped ZAS displays the characteristic emission of Tb3+ions,and the strongest emission peak appears at 541 nm(5D4→7F5),indicating theprominent green emission of Tb3+ions.
The above results suggest that in order to get a white emitting material using ZAS as host,a blue emission is demanded to achieve an ideal RGB combination.Here,Ce3+ions were successfully induced in ZAS matrix as a blue element.Fig.7 gives the PL excitation and emission spectra of ZAS:xCe3+(x=0.01-0.15)samples heated at 900℃,3 h in air.The excitation spectrum when monitored at 381 nm presents a relative broad band with the maximum at 280 nm,which corresponds to the transitions from the ground state2F5/2of Ce3+to the excited Ce3+5d states[25].The emission spectrum(Fig.7(b))of Ce3+doped ZAS glass-ceramics includes a broad band ranging from 379 to 383 nm and peaking at 381 nm,originating from the 5d-4f transition of Ce3+[8]. In the case of rare earth actived white LEDs,the wider the emission band of the samples,the higher the color rendering index of white LEDs[2].Therefore,Ce3+doped ZAS glass-ceramics allow good color rendering of illuminations for white LEDs.

Fig.7 PL excitation(a)and emission(b)spectra of ZAS: xCe3+(x=0.01-0.15)samples heated at 900℃
In order to evaluate the influences of dopant mole fraction(x)on emission intensity of ZAS: xRE3+(RE=Eu,Tb,Ce),the emission intensity as a function of doping concentration(x)is presented in Fig.8.It can be seen that relative intensity increases with the increasing of the doping mole fraction,and reaches the maximum at 20%,20%and 3%for Eu3+,Tb3+and Ce3+,respectively,and then the emission intensity decreases with the doping concentration beyond the critical concentration due to the increase of intra ionic nonradiative relaxation between adjacent rare earth ions[26].
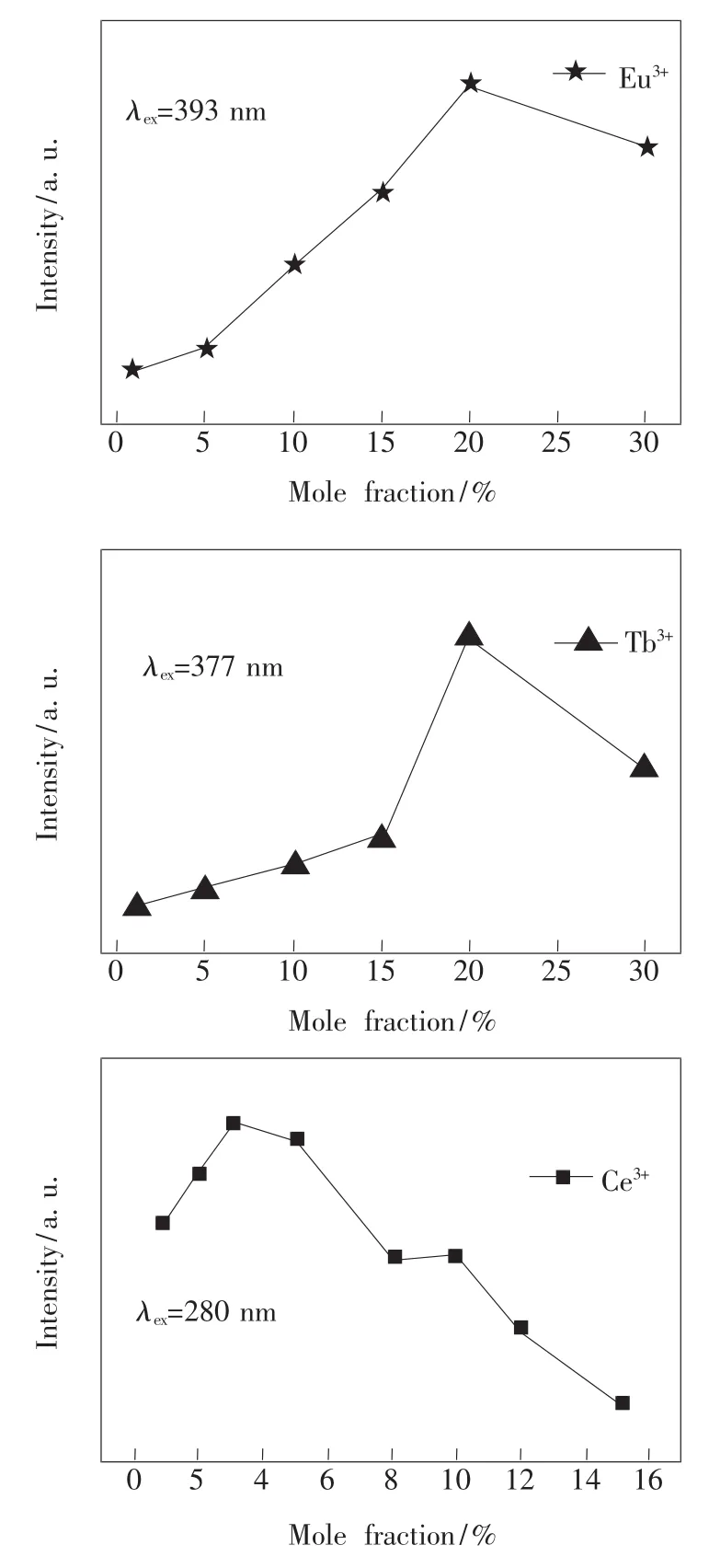
Fig.8 Emission intensity of ZAS:xRE3+(RE=Eu,Tb,Ce)samples as a function of the doping mole fraction
3.4 CIE Chromaticity Coordinates
Most lighting specifications refer to color in terms of the 1931 CIE(Commission International de L'Eclairage)chromatic color coordinates.In general,the color of any light source can be represented as an(x,y)coordinate in this color space[27].Fig.9shows the CIE chromaticity coordinates of ZAS: xRE3+(RE=Eu,Tb and Ce)glass ceramics,which were calculated based on the corresponding PL spectra.The CIE coordinates(x,y)of ZAS: 0.20Eu3+(λex=393 nm),ZAS:0.20Tb3+(λex= 377 nm)and ZAS:0.03Ce3+(λex=280 nm)are(0.64,0.35),(0.29,0.69)and(0.17,0.006),which are located in red,green and blue region,respectively.The chromatic coordinates,dominant wavelength,color purity and color temperature for the red,green,and blue glass ceramics are listed in Table 1.Totally,white light region is in the center region(0.333,0.333)encircled by the high lighted triangle,indicating that white emission can be obtained by ZAS:RE3+(RE=Eu,Tb and Ce)tricolor emission glass-ceramics in a suitable ratio.

Table1 Chromatic coordinates,dominant wavelength,color purity and color temperature of red,green,and blue emitting glass-ceramics

Fig.9 CIE chromaticity coordinates of ZAS:xRE3+(RE= Eu,Tb and Ce)glass ceramics
4 Conclusion
In summary,transparent bulk glass ceramics of 4.5ZnO-5.5Al2O3-90SiO2(ZAS)and ZAS:RE3+(RE=Eu,Tb,Ce)were successfully prepared by the sol-gel method and heat treated in air at 900℃for 3 h.ZAS:RE3+(RE=Eu,Tb,Ce)glass ceramics exhibit precipitated ZnAl2O4phase with an average grain size of 30 nm,while doping ions have not changed the original structure.The optimal doping mole fraction of Eu3+,Tb3+and Ce3+was 20%,20%and 3%,respectively.ZAS:RE3+(RE=Eu,Tb,Ce)glass ceramics single doped Eu3+,Tb3+,and Ce3+shows strong red,green and blue emissions with peak wavelength centered at 611,541 and 381 nm,respectively.The excellent luminescence of ZAS:RE3+(RE=Eu,Tb,Ce)nanocomposites indicates its potential application for white LEDs.
[1]Nakamura S,Fasol G.The Blue Laser Diode:GaN Based Light Emitters and Lasers[M].Berlin:Springer,1997:7-8.
[2]Xiao F,Xue Y N,Zhang Q Y.Y4MgSi3O13:RE3+(RE3+=Ce,Tb and Eu)nanophosphors for a full-color display[J]. Physica B,2010,21(405):4445-4449.
[3]Zhu Z F,Zhang Y B,Qiao Y P,et al.Full color and tunable white emitting in ternary Ce/Tb/Sm co-doped CaO-B2O3-SiO2glasses[J].Non-Cryst.Solids,2012,358(12-13):1550-1553.
[4]Daldosso M,Sokolnicki J,Kepinski L,et al.Preparation and optical properties of nanocrystalline Lu2O3:Eu3+phosphors[J].J.Lumin.,2007,122:858-861.
[5]Piao X Q,Machida K,Horikawa T,et al.Preparation of CaAlSiN3:Eu2+phosphors by the self-propagating high-temperature synthesis and their luminescent properties[J].Chem.Mater.,2007,19(18):4592-4599.
[6]Peng C,Li G G,Geng D L,et al.Fabrication and luminescence properties of one-dimensional ZnAl2O4and ZnAl2O4:A3+(A=Cr,Eu,Tb)microfibers by electrospinning method[J].Mater.Res.Bull.,2012,47(11):3592-3599.
[7]Mao Z Y,Zhu Y C,Gan L,et al.Tricolor emission Ca3Si2O7:Ln(Ln=Ce,Tb,Eu)phosphors for near-UV white light-emitting-diode[J].J.Lumin.,2013,134(2):148-153.
[8]Zhu L,Liu Y J,Fan X Z,et al.Facile synthesis and luminescence properties of uniform and monodisperse KY3F10:Ln3+(Ln=Eu,Ce,Tb)nanospheres[J].J.Lumin.,2011,131(7):1380-1385.
[9]Sokolnicki J.Rare earths(Ce,Eu,Tb)doped Y2Si2O7phosphors for white LED[J].J.Lumin.,2013,134:600-606.
[10]Mukherjee S,Dutta D P.Rear earth doped hydrated and anhydrous zinc phosphate nanophosphors:A promising white light emitter[J].J.Lumin.,2013,134:880-887.
[11]Ren L J,Lei X H,Du X Q,et al.Effect of Eu2O3concentration on luminescent properties of Ce/Tb/Eu co-doped calcium borosilicate glass for white LED[J].J.Lumin.,2013,142:150-154.
[12]Fan X P,Qiao X S,Zhao D L,et al.Nanocrystallization and photoluminescence of Ce/Dy/Eu-doped fluorosilicate glass ceramics[J].J.Alloys Compd.,2012,511(1):232-236.
[13]Tshabalala K G,Cho S H,Park J K,et al.Luminescence properties of Ce3+and Tb3+co-actived ZnAl2O4phosphor[J]. Physica B,2012,407(10):1489-1492.
[14]Kumar M,Mohapatra M,Natarajian V.Luminescence characteristics of blue emitting ZnAl2O4:Ce nanophosphors[J]. J.Lumin.,2014,149:118-124.
[15]Farhadi S,Jahanara K.ZnAl2O4/SiO2nanocomposite catalyst for theacetylation of alcohols,phenols and amines with acetic anhydride under solvent-free conditions[J].Chin.J.Catal.(催化学报),2014,35(3):368-375(in English).
[16]Duan X L,Yuan D R,Cheng X F,et al.Synthesis and optical properties of transparent ZnO-Ga2O3-SiO2glass-ceramics embedded with cobalt-doped nanocrystals[J].Nanotechnology,2007,18(17):175609-1-5.
[17]Duan X L,Wu Y C,Wang X Q,et al.Synthesis,structure and optical properties of co-doped MgGa2O4/SiO2nano-glassceramic composites[J].Appl.Surf.Sci.,2013,276:613-619.
[18]Duan X L,Yuan D R,Sun Z H,et al.Synthesis and characterization of ZnAl2O4/SiO2nanocomposites by sol-gel method[J].J.Cryst.Growth,2003,252(1-3):4-8.
[19]Wiglusz R J,Grzyb T,Lukowiak A,et al.Tuning luminescence properties of Eu3+doped CaAl2O4nanophosphors with Na+co-doping[J].J.Lumin.,2013,133:102-109.
[20]Matsui H,Xu C N,Tateyama H.Stress-stimulated luminescence from ZnAl2O4:Mn2+[J].Appl.Phys.Lett.,2001,78(8):1068-1070.
[21]Liu L,Xie R J,Hirosaki N,et al.Crystal structure and photoluminescence properties of red-emitting Ca9La1-x(VO4)7:xEu3+phosphors for white light-emitting diodes[J].Am.Ceram.Soc.,2010,93(12):4081-4086.
[22]Saurel D,Tikhomirov V K,Moshchalkov V V,et al.Effect of confinement on the Eu3+emission band5D0→7F0in Eu3+-doped nano-glass-ceramics[J].J.Lumin.,2009,129(12):1575-1577.
[23]Deng W,Cheng J S.New transparent glass-ceramics containing large grain Eu3+:CaF2nanocrystals[J].Mater.Lett.,2012,73:112-114.
[24]Li L,Lei X H,Ren L J,et al.Effect of glass matrix on luminescent properties of Tb3+doped luminescence glass[J]. Chin.J.Lumin.(发光学报),2014,35(4):420-424(in Chinese).
[25]Liu X H,Xiao S G,Xiang Z F,et al.Enhanced NIR emission in Ce3+/Er3+-doped YAG induced by Bi3+doping[J]. Chin.Opt.Lett.,2013,12:76-79.
[26]Zhang L,Xia H P,Qiu Y,et al.Preparation and characterization of Y3Al5O12:Ln(Ln=Eu,Ce)phosphor powders by ultrasonic atomization and co-precipitation process[J].J.Rare Earths,2010,28(1):236-240.
[27]Lou Z D,Hao J H.Cathodoluminescence of rare-earth-doped zinc aluminate films[J].Thin Solid Films,2004,450(2):334-340.

崔祥水(1989-),男,江苏徐州人,硕士研究生,2012年于江苏科技大学获得学士学位,主要从事稀土掺杂发光材料方面的研究。
E-mail:cuixiangshui@163.com

陈文哲(1957-),男,福建泉州人,教授,博士生导师,1997年于西安交通大学获得博士学位,主要从事先进结构材料、稀土发光材料方面的研究。
E-mail:chenwz@fzu.edu.cn
稀土离子(Eu3+,Tb3+,Ce3+)掺杂ZnAl2O4/SiO2微晶玻璃的制备与发光性能
崔祥水1,陈文哲1,2*
(1.福州大学材料科学与工程学院,福建福州 350116; 2.厦门理工学院材料科学与工程学院,福建厦门 361024)
采用凝胶法分别制备出4.5ZnO-5.5Al2O3-90SiO2(ZAS)以及ZAS:RE3+(RE=Eu,Tb,Ce)透明微晶玻璃。利用X射线衍射仪(XRD)、透射电子显微镜(TEM)和荧光光谱仪(PL)等测试手段,研究了稀土离子掺杂浓度对ZAS微晶玻璃的结构和发光性能的影响。XRD结果表明,ZAS:RE3+(RE=Eu,Tb,Ce)微晶玻璃包含ZnAl2O4晶相和SiO2非晶相,ZnAl2O4平均晶粒尺寸约为30 nm,稀土离子的掺杂没有显著改变原来的ZnAl2O4晶体结构。TEM结果表明,900℃时ZnAl2O4从ZAS体系中析出。PL光谱显示,Eu3+存在5D0→7F2跃迁,ZAS:Eu3+在611 nm处发出强烈的红色光;由于Tb3+的5D4→7F5跃迁,ZAS:Tb3+在541 nm处发出明亮的绿色光;ZAS:Ce3+在381 nm处显示了蓝光发射,对应于Ce3+的5d→4f轨道跃迁。ZAS:RE3+(RE=Eu,Tb,Ce)的PL发射光谱存在着浓度猝灭现象,Eu3+、Tb3+和Ce3+的最佳单掺杂摩尔分数分别为20%、20%和3%。CIE色度图表明,ZAS:RE3+(RE=Eu,Tb,Ce)的色坐标分别位于红光、绿光和蓝光区域。实验结果表明,ZAS:RE3+(RE=Eu,Tb,Ce)微晶玻璃是一种良好的可用于全色显示的白光LED材料。
ZAS微晶玻璃;溶胶-凝胶法;稀土离子掺杂;发光性能
2015-01-07;
2015-03-03
国家自然科学基金(61108056);福建省教育厅基金(JA14223)资助项目
O482.31 Document code:A DOI:10.3788/fgxb20153604.0400
