生物来源的固体颗粒制备Pickering乳液的研究进展
2015-08-02孙翠霞刘夫国高彦祥
孙翠霞,刘夫国,杨 伟,袁 芳,高彦祥
(中国农业大学食品科学与营养工程学院,北京 100083)
生物来源的固体颗粒制备Pickering乳液的研究进展
孙翠霞,刘夫国,杨 伟,袁 芳,高彦祥*
(中国农业大学食品科学与营养工程学院,北京 100083)
天然来源、可再生和可生物降解的固体颗粒用于制备Pickering乳液已成为研究热点。本文综述了固体颗粒的种类、制备方法与性质表征,重点介绍了生物来源的有机颗粒如多糖和蛋白质对Pickering乳液的稳定作用,并总结了影响Pickering乳液稳定性的因素,同时阐述了Pickering乳液在生命科学领域的潜在应用。
固体颗粒,Pickering乳液,稳定性
乳液(emulsion)是由一种或几种液体以小液滴的形式分散于另一种与其互不相溶的液体中形成的多相分散体系,通常分为水包油型(O/W)和油包水型(W/O)[1]。由于油-水界面积较大,乳液是一种热力学不稳定体系,必须向体系中加入乳化剂,通过减少两相之间的界面张力,才能获得稳定的乳液[2]。然而,过量的非食品级乳化剂必须从样品中去除,否则会对人体产生伤害,影响和破坏乳液后续的应用,如乳化剂会诱导组织发炎甚至造成细胞损伤,这使得由乳化剂制备的传统乳液在医药制剂方面的应用受到限制[3]。
20世纪初,Ramsden等人最早发现将不溶性固体细粉与水和一些油性溶剂进行混合分散时,固体细粉包裹在分散相液滴的表面,形成一个固体壳层;当分散相液滴相互碰撞时,固体壳层对液滴的变形和聚集起到阻碍作用,可以形成较为稳定的乳液[4]。随后,Pickering对该乳液体系进行了较为深入和系统的研究[5]。因此,由固体颗粒代替乳化剂而制备的乳液被命名为Pickering乳液,如图1所示[6]。另外,由固体颗粒通过界面作用阻止乳液液滴聚结的机制称为Pickering稳定[7]。Binks研究指出,固体颗粒在油-水界面产生不可逆的吸附,而表面活性剂在一定的时间范围内其吸附和解吸速度相对较快[8]。因此,与传统表面活性剂稳定的乳液相比,Pickering乳液具有更高的稳定性,可广泛应用于医药、农业、食品和化妆品等领域。
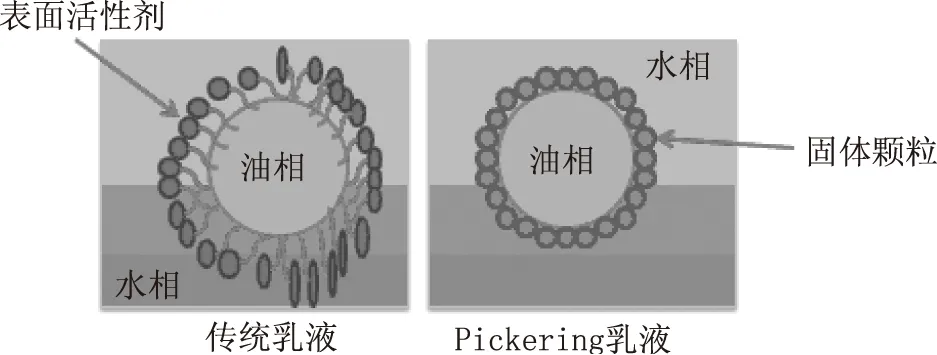
图1 O/W型传统乳液和Pickering乳液的结构图示[3]Fig.1 Sketches of the Pickering emulsionand a classical emulsion[3]
研究表明,Pickering乳液的稳定性在很大程度上取决于固体颗粒的性质,因此,本文综述了固体颗粒的种类、制备方法与性质表征,重点介绍了生物来源的有机颗粒如多糖和蛋白质对Pickering乳液的稳定机理,并总结了影响Pickering乳液稳定性的因素,同时阐述了Pickering乳液的应用前景。
1 用于制备Pickering乳液的固体颗粒
目前对于固体颗粒稳定Pickering乳液的研究集中于无机或合成粒子,如二氧化硅[9]、二氧化钛[10]、锂皂石粘土[11]、磁铁矿[12]、氧化锌[13]、氧化石墨烯[14]、各向异性的Janus颗粒[15]等。虽然由这些无机或合成粒子制备的Pickering乳液稳定性较好,但生物相容性较差,这极大限制了Pickering乳液在医药、农业、食品和化妆品等领域的应用。因此,寻求环境友好型、天然来源、可再生和可生物降解的固体颗粒用于稳定Pickering乳液已成为目前研究的热点。
在现有的研究中,生物来源的胶体颗粒已用于稳定Pickering乳液,如多糖类,包括淀粉纳米晶[16-18]、经化学修饰的淀粉纳米颗粒[19]、纤维素纳米晶[20-24]、甲壳素纳米晶[25-26];蛋白质类,包括大豆分离蛋白[27-28]、豌豆分离蛋白[29]、乳铁蛋白[30]、乳清蛋白[31]、玉米醇溶蛋白[32-33];另外,还有具有生物活性的小分子物质如黄酮[34-35]和植物甾醇[36]。
1.1 多糖
多糖是多分散性大分子,亲水性强。一些多糖能够吸附在油-水界面,通过界面相互作用,作为乳液的稳定剂。Li等[37]选用大米淀粉、小麦淀粉和蜡质玉米淀粉制备出稳定的乳液,其中大米淀粉(颗粒直径为5.2 μm)制备的乳液稳定性最好。Rayner等[38]采用藜麦淀粉(颗粒直径为2.5 μm)制备稳定的乳液,由于藜麦淀粉不含谷朊蛋白,无致敏性,颗粒直径相对较小(直径为0.5~3 μm)且分布均匀,使藜麦淀粉成为稳定Pickering乳液的研究热点。
多糖胶体表面活性源于两种途径,一是某些非极性的化学基团吸附在亲水多糖的骨架上,二是蛋白质与碳水化合物(如阿拉伯胶、甜菜果胶)发生部分共价结合[39-40]。即使多糖不具有表面活性,只要能与预先吸附的蛋白质通过静电络合形成次级空间保护层,也能够促进乳液的界面稳定[41]。另外,多糖组分可通过诱导与蛋白质共价结合形成永久的结合物。最常用的方法是干热处理,即美拉德反应。所得的具有表面活性的蛋白质-多糖结合物可以用于制备Pickering乳液[42-43]。
1.2 蛋白质
大多数蛋白质分子具有吸附在油-水界面、伸展并且聚集形成二维网络的能力,可作为乳液有效的稳定剂[44]。用于吸附的主要热力学驱动力使蛋白质的非极性侧链远离水溶液的不利环境,使界面区域的水分子浓度降低。次级驱动力与吸附的蛋白质分子的伸展有关。这会进一步导致蛋白质与蛋白质、蛋白质与水之间相互作用的平衡发生变化[45]。一旦吸附,蛋白质通常不能轻易地从界面解吸。因此,蛋白质的吸附通常认为是“不可逆”的。吸附在界面的蛋白质对分散颗粒和液滴的稳定作用与蛋白质的分子结构、水溶液的离子强度有关,可以结合空间作用和静电作用进行解释[46]。如无序的蛋白质如酪蛋白,是一种优良的空间稳定剂[47]。一旦蛋白质吸附在单个分散的油滴上,在油-水界面形成单吸附层,会产生较强的斥力屏障,可阻止相邻液滴紧密靠近。球形蛋白质(乳蛋白)吸附层的界面结构有很大不同,可以看作是一个二维排列的紧凑颗粒层。蛋白质聚集体的吸附或蛋白质与多糖相互作用后的混合吸附会产生不均匀的吸附层或多重吸附层,具有更复杂的界面结构。蛋白质吸附层的结构区别可通过蛋白质的表面剪切流变性差异进行表征。酪蛋白的吸附层呈液体状,容易移动,球状蛋白的单吸附层刚性更强,具有二维凝胶或玻璃态的性质[44-45]。
1.3 蛋白质与多糖复合颗粒的协同稳定作用
通过对胶体颗粒的表面进行疏水改性,可改善颗粒的润湿性,增强乳液的稳定性。疏水改性可通过加入小分子的表面活性剂,如十二烷基磺酸钠(SDS)、吐温系列(Tween 20、40、60、80)的乳化剂等[48-49]。为避免加入小分子表面活性剂而改善颗粒润湿性的方法是,加入两种带相反电荷的纳米颗粒,通过异种电荷的吸引聚合改善颗粒的润湿性,颗粒混合物呈絮凝状分散在水介质中,静电荷足够低,充分疏水吸附在油滴表面使其稳定,从而稳定乳液[50-51]。
2 固体颗粒的制备方法与性质表征
2.1 反溶剂沉淀法
最常用的制备固体颗粒的方法为反溶剂沉淀法,又称为液液分散法或相分离法。Patel等[52]采用反溶剂沉淀法制备玉米醇溶蛋白—姜黄素复合胶体颗粒,透射电镜结果表明颗粒呈球形,平均粒径为100~150 nm;姜黄素在颗粒中的包埋率为86.8%,负载能力4.1%;通过差示扫描量热法和X-射线衍射对固态胶体颗粒的研究表明,被包埋的姜黄素表现出非晶性质;紫外全波长扫描结果表明,胶体颗粒对姜黄素的包埋作用增强了姜黄素的耐光性;另外,Patel等[52]还发现,该颗粒在广泛的pH范围内(1.2,4.5,6.7和7.4)和在模拟肠道条件下具有良好的胶体稳定性;Patel等[52]通过体外粘膜吸附研究表明,姜黄素在粘膜中150 min的保留率仍在60%以上,Caco-2细胞粘蛋白的研究进一步证实了玉米醇溶蛋白-姜黄素复合纳米颗粒的粘膜吸附特性。de Folter等[32]采用反溶剂沉淀法制备玉米醇溶蛋白胶体颗粒(ZCP),并首次采用ZCP制备出稳定的O/W型Pickering乳液,乳液稳定性与颗粒浓度、pH、离子强度有关。研究表明,未经改性的ZCP同时具有疏水性和亲水性,在高于或低于玉米醇溶蛋白等电点(pH6.5)时,在10 mol/L的离子强度下,ZCP在O/W乳液中的三相接触角在90°左右,可强力吸附于油-水界面,可以制备稳定的、不含表面活性剂的乳液,该乳液经高速剪切分散器13500 r/min,2 min的混合分散后,乳液的液滴粒径范围为10~200 μm。Zou等[53]采用液液分散法制备玉米醇溶蛋白-蔓越莓原花青素(Z-CPs)复合纳米颗粒,结果表明,随着CPs在Z-CPs中的比例由1∶8增加至1∶2,Z-CPs纳米颗粒的直径由392 nm增加至447 nm,包埋率由10%增加至86%。扫描电镜结果表明,Z-CPs纳米颗粒呈球形;傅里叶变换红外光谱研究表明,Z与CPs之间的交互作用是氢键和疏水相互作用;通过人类前骨髓性白血病HL-60细胞的细胞培养研究表明,与CPs相比,包封在纳米颗粒中的CPs降低了细胞毒性。
2.2 超临界流体技术
Hu等[54]采用超临界流体技术(SEDS)通过增强溶液的分散性能用于制备玉米醇溶蛋白-叶黄素(Z-L)纳米颗粒。SEDS的压力、温度、叶黄素与玉米醇溶蛋白的比率、溶液流速等对负载量、包封率、平均粒径、颗粒形态产生显著影响。较低的温度和流速结合高的压力,能制备粒径更小,更规则的球形颗粒。当工艺参数为压力10 MPa,L与Z的质量比为1∶18,45 ℃下,控制溶液流速1.0 mL/min,能够获得粒径尺寸范围较小、具有缓释能力的Z-L纳米颗粒。
2.3 热处理结合静电络合作用
最近的研究表明,球状蛋白和带电多糖的复合纳米颗粒可通过热处理结合静电络合作用制备。有两种制备方法:一是在高于其热变性温度的条件下加热球状蛋白溶液形成蛋白颗粒,然后加入离子多糖,调整溶液条件,以促进多糖与蛋白质颗粒的静电络合[55];二是蛋白质-多糖先在室温下通过静电作用形成复合物,然后在高于其热变性温度的条件下加热此复合物,以促进蛋白质聚集和微粒形成。这两种方法都能形成非常小的生物聚合物颗粒,具有良好的稳定性,不易沉淀,不易受pH和离子强度的影响[56-57]。
Peinado等[58]采用热处理的乳铁蛋白(LF)与阴离子多糖(海藻酸盐、卡拉胶、果胶)通过静电络合作用制备生物聚合物复合胶体颗粒,并研究了颗粒稳定性和形态特征。在高于LF的变性温度下(91 ℃,20 min)加热LF溶液(0.2% LF,pH7),可促进蛋白质的伸展,抑制蛋白质聚集,形成蛋白质颗粒,粒径范围为200~400 nm,等电点约为8.5。将该蛋白质颗粒与阴离子多糖在pH8时混合,然后降低pH,通过静电作用促进多糖沉积在蛋白颗粒表面形成复合胶体颗粒。使用浊度仪、动态光散射和电泳研究不同pH(2~11)和离子强度(0~200 mmol/L NaCl)对颗粒稳定性的影响。在pH5~pH8的范围内颗粒相对稳定,但在较低pH时颗粒发生明显的聚集,这是因为蛋白质与多糖发生了静电中和和桥连絮凝。LF-果胶复合胶体颗粒,在所研究的盐浓度范围内都比较稳定,但LF-卡拉胶和LF-海藻酸盐络合物在较高盐浓度时发生聚集。
3 影响Pickering乳液稳定性的因素
3.1 固体颗粒的润湿性
Pickering乳液的类型和传统乳液类似,包括O/W、W/O、O/W/O、W/O/W等多种乳液[59]。Finkle[60]指出,Pickering乳液的稳定性很大程度上取决于固体颗粒的部分润湿性,即要求颗粒具有亲水和亲油两亲特性,如果颗粒完全溶解于水或油,它们就会分散在两相中的一相中,不能吸附在油-水界面,在这种情况下,不能制备稳定的乳状液。Finkle还首次描述了颗粒的润湿性及其稳定乳液能力之间的相关性,即根据油—颗粒—水界面处的接触角度θ判断乳液的类型和稳定性(图2)。固体颗粒在油-颗粒-水界面的接触角θ<90°时为O/W型乳液,而θ>90°时为W/O型乳液,θ=90°时乳液稳定性最好。
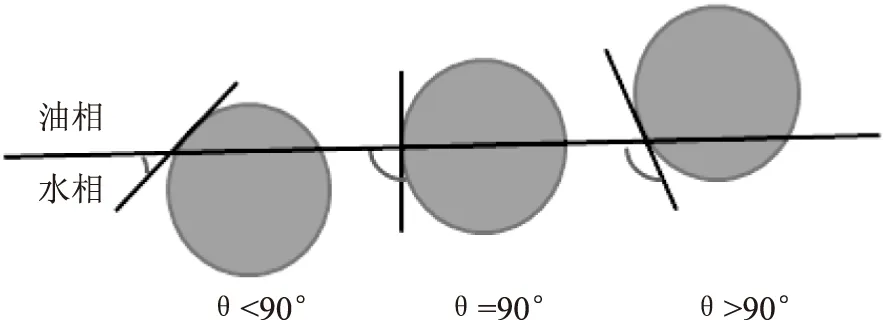
图2 油—颗粒—水界面处的接触角图示[60]Fig.2 Position of Pickering-type stabilizers ata planar o/w interface for contact angles[60]
3.2 固体颗粒在油—水界面的吸附形式
固体颗粒与界面强烈的吸附作用形成了具有刚性的界面,而界面的刚性也决定了液滴抗絮凝的能力。吸附在界面的颗粒之间横向的交互作用也是调节界面性质与维持乳液稳定的非常重要的因素。当两个被颗粒吸附的液滴相互靠近时,颗粒在界面可能会产生不同的分布,从而影响乳液的稳定性。两个在界面紧密排列的单颗粒层将相邻分散相分割(图3A),这是球形颗粒均一、单层吸附在界面用于稳定Pickering乳液的理想模型,但是即便通过高压均质或高强度超声处理也很难实现,因为颗粒通常是多分散性的[61-62]。已有研究表明,液滴即使不被颗粒完全包裹也能制备稳定的Pickering乳液,这就说明颗粒在界面的分布还存在其他的形式,如单层颗粒紧密排列,在相邻液滴间形成桥连的致密吸附层,单个颗粒同时与两个液滴吸附,但每个颗粒的主要部分仍处于连续的水相中(图3B)[63]。这种单层排列的颗粒层能够稳定Pickering乳液,是因为颗粒层产生空间阻碍作用,阻止大的颗粒脱离液滴或进入液滴内部。大多数固体粒子具有比较均匀的表面,这意味着颗粒吸附的液滴表面具有类似颗粒表面的性质。接触角有利于强烈吸附在油-水界面,这也意味着颗粒在乳液中以弱聚集的状态存在。因此,颗粒在界面的分布存在第三种形式即颗粒聚集体吸附在液滴表面用于稳定Pickering乳液(图3C)[64-65]。在这种分布形式下,液滴表面不是简单的单层或双层吸附,也不是紧密堆积吸附。相反,颗粒形成的刚性吸附层是无序的,形成网络结构吸附在油-水界面上,能够通过分子间引力使整个聚集结构保持在一起从而阻止液滴聚集。
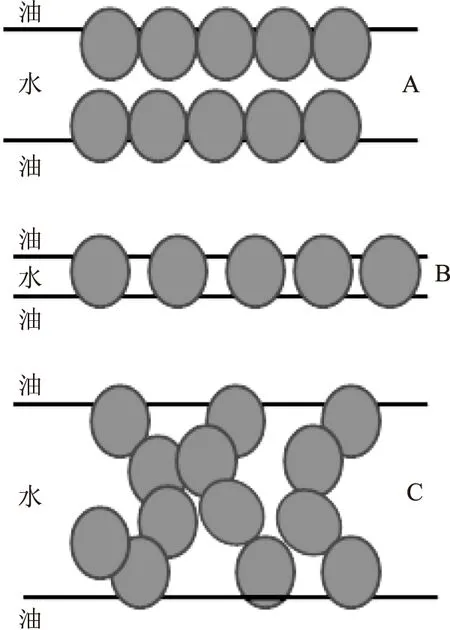
图3 固体颗粒在油—水界面的分布形式示意图Fig.3 a schematic distribution diagram of thesolid particles in the oil-water interface
3.3 固体颗粒的形状
颗粒的形状对颗粒在液滴表面的覆盖率及与形状有关的界面交互作用产生影响。Madivala等[66]研究了颗粒形状对Pickering乳液聚集稳定性的影响。对于O/W或W/O乳液,作者发现乳液的稳定性很大程度上决定于颗粒的长宽比。当具有相似润湿性的球形颗粒或较短的细长颗粒不能有效稳定乳液时,足够长的颗粒是非常有效的乳化剂。颗粒的长宽比对乳液稳定性的影响可通过测定油-水界面的剪切流变性质进行表征。
3.4 固体颗粒的浓度
当固体颗粒浓度较低时,静电相互作用在很大程度上受到抑制,由于颗粒较少,相邻液滴间共享吸附的颗粒或大分子,起初形成的液滴迅速结合,通常发生某种程度的絮凝,直到液滴表面有足够的颗粒或分子吸附形成空间保护层[67]。当颗粒浓度较高时,乳液稳定性主要由界面张力决定。随着颗粒浓度的提高,颗粒在油-水界面吸附能力增强,引起界面张力降低,从而使液滴粒径逐渐减小。继续增加颗粒浓度,颗粒不再吸附于界面,而是进入连续相中,液滴粒径基本保持不变,但水相黏度逐渐增加,乳液呈凝胶状,阻止乳液分层、絮凝或聚集,乳液稳定性明显增强[68]。
3.5 pH
固体颗粒可能在不同pH的产品中使用,在通过人体胃肠道时pH也不同,因此,探讨pH对乳液的影响是非常重要的。pH的变化能够改变颗粒的润湿性和静电性质,从而影响颗粒的界面吸附。Luo等[34]研究了pH对黄酮类化合物(山奈酚,芦丁和柚皮苷)制备的O/W型Pickering乳液的影响。研究表明,在较高的pH时芦丁稳定的乳液液滴的平均尺寸最小(5 μm)。芦丁和山奈酚在油-水界面的表面活性在pH8时比pH2时略有增加。在pH较高时乳液的稳定性增加,这是因为黄酮类化合物颗粒的ζ-电势显著增加,改善了颗粒分散性能,增加了液滴的表面电荷。Liang和Tang[36]研究指出,豌豆分离蛋白(PPI)在pH3.0时以纳米颗粒形式存在,粒径为134~165 nm可用于制备稳定的O/W型Pickering乳液。Wen等[69]研究了不同pH(4.2,4.8,5.5,6.2,7.0和7.8)对纤维素纳米晶(CNCs)制备的柠檬烯Pickering乳液的影响,结果表明随着pH的增加,液滴表面的ζ-电位由-42.9 mV增加到-54.5 mV,液滴间静电斥力增加,增强了乳液的稳定性。
3.6 离子强度
NaCl溶液加入主要影响颗粒的表面电位,NaCl溶液浓度较低时,会降低颗粒表面的电位,有助于颗粒在油-水界面的吸附。当NaCl浓度过高时,颗粒表面发生静电屏蔽,颗粒聚集,粒径增加,影响乳液的稳定性。de Folter等[32]研究了不同浓度NaCl溶液对玉米醇溶蛋白胶体颗粒(ZNP)制备的Pickering乳液的影响,结果表明,ZNP在1 mmol/L的NaCl溶液中亲水性较强,三相接触角为98°;1 mmol/L的NaCl溶液使Z疏水性增加,三相接触角为87°,有利于ZNP在油-水界面的吸附;然而,0.1 mol/L的NaCl溶液使液滴聚集,发生分层,严重降低了乳液的稳定性。
4 Pickering乳液的应用
目前还没有Pickering乳液制备的市售产品或材料。原因之一是Pickering乳液最近几年才引起大家的关注。与传统表面活性剂稳定的乳液相比,Pickering乳液由于不含表面活性剂,能有效避免表面活性剂可能引发的刺激性、毒性、溶血行为等不利影响,在生命科学领域有很多潜在的应用[70-71]。例如,研究表明固体颗粒在细胞表面有较强的粘附作用,因此Pickering乳液可作为药物和具有生物活性物质的包埋和传递系统,并且可达到缓释和靶向的效果[72-74];另外,由于固体颗粒吸附在液滴表面产生具有刚性的吸附层,经干燥的Pickering乳液可以用于制备固体剂型的生物材料[75-77]。
5 展望
近年来Pickering乳液的研究成果促进了乳液基础理论的发展,并且拓展了乳液的实际应用范围。随着人们对Pickering乳液研究兴趣的增加,寻求新型、安全、可降解的固体纳米颗粒作为乳液的有效稳定剂应用于食品行业是食品领域面临的挑战。同时,固体颗粒的制备技术仍需突破,Pickering乳液的稳定机理有待进一步研究。
[1]McClements D J. Nanoemulsions versus microemulsions:terminology,differences,and similarities[J]. Soft Matter,2012,8(6):1719-1729.
[2]Zeeb B,Herz E,McClements D J,et al. Impact of alcohols on the formation and stability of protein-stabilized nanoemulsions[J].Journal of Colloid and Interface Science,2014,433(10):196-203.
[3]Schrade A,Landfester K,Ziener U. Pickering-type stabilized nanoparticles by heterophase polymerization[J]. Chemical Society Reviews,2013,42(16):6823-6839.
[4]Ramsden W. Separation of solids in the surface-layers of solutions and ‘suspensions’(observations on surface-membranes,bubbles,emulsions,and mechanical coagulation).-preliminary account[J]. Proceedings of the royal Society of London,1903,43(5):156-164.
[5]Pickering S U M. CXCVI.-Emulsions[J]. Journal of Chemistry Society Trans,1907,91(2),2001-2021.
[6]Chevalier Y,Bolzinger M A. Emulsions stabilized with solid nanoparticles:Pickering emulsions[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2013,439(11):23-34.
[7]Binks B P,Horozov T S. Colloidal particles at liquid interfaces:an introduction[J]. Colloidal Particles at Liquid Interfaces,Chapt,2006,83(6):1-74.
[8]Binks B P. Particles as surfactants-similarities and differences[J]. Current Opinion in Colloid & Interface Science,2002,7(1):21-41.
[9]Yin Y,Zhou S,You B,et al. Facile fabrication and self-assembly of polystyrene-silica asymmetric colloid spheres[J]. Journal of Polymer Science Part A:Polymer Chemistry,2011,49(15):3272-3279.
[10]Teixeira R F A,McKenzie H S,Boyd A A,et al. Pickering emulsion polymerization using Laponite clay as stabilizer to prepare armored “soft” polymer latexes[J]. Macromolecules,2011,44(18):7415-7422.
[11]Chen J H,Cheng C Y,Chiu W Y,et al. Synthesis of ZnO/polystyrene composites particles by Pickering emulsion polymerization[J]. European Polymer Journal,2008,44(10):3271-3279.
[12]Song X,Yin G,Zhao Y,et al. Effect of an anionic monomer on the Pickering emulsion polymerization stabilized by titania hydrosol[J]. Journal of Polymer Science Part A:Polymer Chemistry,2009,47(21):5728-5736.
[13]Zhao Y,Yin G,Zheng Z,et al. Preparation of polymer hollow microspheres covered by polymer solid particles via two polymerization steps[J]. Journal of Polymer Science Part A:Polymer Chemistry,2011,49(24):5257-5269.
[14]Yin G,Zheng Z,Wang H,et al. Preparation of graphene oxide coated polystyrene microspheres by Pickering emulsion polymerization[J]. Journal of Colloid and Interface Science,2013,394(7):192-198.
[15]Wu C H,Chiu W Y,Don T M. Conductive composite particles synthesized via Pickering emulsion polymerization using conductive latex of poly(3,4-ethylenedioxythiophene)(PEDOT)as stabilizer[J]. Polymer,2012,53(5):1086-1092.
[16]Li C,Sun P,Yang C. Emulsion stabilized by starch nanocrystals[J]. Starch-Stärke,2012,64(6):497-502.
[17]Miao M,Li R,Jiang B,et al. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch[J]. Food Chemistry,2014,151(9):154-160.
[18]Li C,Li Y,Sun P,et al. Starch nanocrystals as particle stabilisers of oil-in-water emulsions[J]. Journal of the Science of Food and Agriculture,2013,132(6):186-192.
[19]Tan Y,Xu K,Liu C,et al. Fabrication of starch-based nanospheres to stabilize pickering emulsion[J]. Carbohydrate Polymers,2012,88(4):1358-1363.
[20]Capron I,Cathala B. Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals[J]. Biomacromolecules,2013,14(2):291-296.
[21]Kalashnikova I,Bizot H,Bertoncini P,et al. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions[J]. Soft Matter,2013,9(3):952-959.
[22]Kalashnikova I,Bizot H,Cathala B,et al. New Pickering emulsions stabilized by bacterial cellulose nanocrystals[J]. Langmuir,2011,27(12):7471-7479.
[23]Tasset S,Cathala B,Bizot H,et al. Versatile cellular foams derived from CNC-stabilized Pickering emulsions[J]. RSC Advances,2014,4(2):893-898.
[24]Kalashnikova I,Bizot H,Cathala B,et al. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface[J]. Biomacromolecules,2011,13(1):267-275.
[25]Tzoumaki M V,Moschakis T,Kiosseoglou V,et al. Oil-in-water emulsions stabilized by chitin nanocrystal particles[J]. Food Hydrocolloids,2011,25(6):1521-1529.
[26]Tzoumaki M V,Moschakis T,Scholten E,et al.Invitrolipid digestion of chitin nanocrystal stabilized o/w emulsions[J]. Food & function,2013,4(1):121-129.
[27]Paunov V N,Cayre O J,Noble P F,et al. Emulsions stabilised by food colloid particles:Role of particle adsorption and wettability at the liquid interface[J]. Journal of Colloid and Interface Science,2007,312(2):381-389.
[28]Liu F,Tang C H. Soy protein nanoparticle aggregates as Pickering stabilizers for oil-in-water emulsions[J]. Journal of Agricultural and Food Chemistry,2013,61(37):8888-8898.
[29]Liang H N,Tang C. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH3.0[J]. LWT-Food Science and Technology,2014,58(2):463-469.
[30]Shimoni G,Shani Levi C,Levi Tal S,et al. Emulsions stabilization by lactoferrin nano-particles underinvitrodigestion conditions[J]. Food Hydrocolloids,2013,33(2):264-272.
[31]Destribats M,Rouvet M,Gehin-Delval C,et al. Emulsions stabilised by whey protein microgel particles:towards food-grade Pickering emulsions[J]. Soft matter,2014,10(2):6941-6949.
[32]de Folter J W J,van Ruijven M W M,Velikov K P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein[J]. Soft Matter,2012,8(25):6807-6815.
[33]Gao Z M,Yang X Q,Wu N N,et al. Protein-Based Pickering Emulsion and Oil Gel Prepared by Complexes of Zein Colloidal Particles and Stearate[J]. Journal of Agricultural and Food Chemistry,2014,62(12):2672-2678.
[34]Luo Z,Murray B S,Ross A L,et al. Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers[J]. Colloids and Surfaces B:Biointerfaces,2012,92(4):84-90.
[35]Luo Z,Murray B S,Yusoff A,et al. Particle-stabilizing effects of flavonoids at the oil-water interface[J]. Journal of Agricultural and Food Chemistry,2011,59(6):2636-2645.
[36]Liu F,Tang C H. Phytosterol Colloidal Particles as Pickering Stabilizers for Emulsions[J]. Journal of Agricultural and Food Chemistry,2014,62(9):5133-5141.
[37]Li C,Li Y,Sun P,et al. Pickering emulsions stabilized by native starch granules[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects,2013,431(10):142-149.
[38]Rayner M,Timgren A,Sjöö M,et al. Quinoa starch granules:a candidate for stabilising food-grade Pickering emulsions[J]. Journal of the Science of Food and Agriculture,2012,92(9):1841-1847.
[39]Dickinson E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems[J]. Food hydrocolloids,2003,17(1):25-39.
[40]Dickinson E. Hydrocolloids as emulsifiers and emulsion stabilizers[J]. Food Hydrocolloids,2009,23(6):1473-1482.
[41]Dickinson E,Euston S R. Stability of food emulsions containing both protein and polysaccharide[M]. Royal Society of Chemistry:Cambridge,UK,1991.
[42]Jourdain L,Leser M E,Schmitt C,et al. Stability of emulsions containing sodium caseinate and dextran sulfate:relationship to complexation in solution[J]. Food Hydrocolloids,2008,22(4):647-659.
[43]Akhtar M,Dickinson E. Whey protein-maltodextrin conjugates as emulsifying agents:an alternative to gum arabic[J]. Food Hydrocolloids,2007,21(4):607-616.
[44]McClements D J. Protein-stabilized emulsions[J]. Current opinion in colloid & interface science,2004,9(5):305-313.
[45]Dickinson E. Adsorbed protein layers at fluid interfaces:interactions,structure and surface rheology[J]. Colloids and surfaces B:Biointerfaces,1999,15(2):161-176.
[46]Semenova M G,Dickinson E. Biopolymers in food colloids:Thermodynamics and molecular interactions[M]. Brill,2010,141(7):124-133.
[47]Dickinson E. Caseins in emulsions:interfacial properties and interactions[J]. International Dairy Journal,1999,9(3):305-312.
[48]Binks B P,Rodrigues J A. Enhanced stabilization of emulsions due to surfactant-induced nanoparticle flocculation[J].Langmuir,2007,23(14):7436-7439.
[49]Whitby C P,Fornasiero D,Ralston J. Effect of adding anionic surfactant on the stability of Pickering emulsions[J]. Journal of Colloid and Interface Science,2009,329(1):173-181.
[50]Pichot R,Spyropoulos F,Norton I T. Mixed-emulsifier stabilised emulsions:investigation of the effect of monoolein and hydrophilic silica particle mixtures on the stability against coalescence[J]. Journal of Colloid and Interface Science,2009,329(2):284-291.
[51]Binks B P,Liu W,Rodrigues J A. Novel stabilization of emulsions via the heteroaggregation of nanoparticles[J]. Langmuir,2008,24(9):4443-4446.
[52]Patel A,Hu Y,Tiwari J K,et al. Synthesis and characterisation of zein-curcumin colloidal particles[J]. Soft Matter,2010,6(24):6192-6199.
[53]Zou T,Li Z,Percival S S,et al. Fabrication,characterization,and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles[J]. Food hydrocolloids,2012,27(2):293-300.
[54]Hu D,Lin C,Liu L,et al. Preparation,characterization,andinvitrorelease investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids[J]. Journal of Food Engineering,2012,109(3):545-552.
[55]Jones O G,McClements D J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes[J]. Advances in Colloid and Interface Science,2011,167(1):49-62.
[56]Hong Y H,McClements D J. Formation of hydrogel particles by thermal treatment of β-lactoglobulin-chitosan complexes[J]. Journal of Agricultural and Food Chemistry,2007,55(14):5653-5660.
[57]Jones O G,Decker E A,McClements D J. Formation of biopolymer particles by thermal treatment of β-lactoglobulin-pectin complexes[J]. Food Hydrocolloids,2009,23(5):1312-1321.
[58]Peinado I,Lesmes U,Andrés A,et al. Fabrication and morphological characterization of biopolymer particles formed by electrostatic complexation of heat treated lactoferrin and anionic polysaccharides[J]. Langmuir,2010,26(12):9827-9834.
[59]Aveyard R,Binks B P,Clint J H. Emulsions stabilised solely by colloidal particles[J]. Advances in Colloid and Interface Science,2003,100(10):503-546.
[60]Finkle P,Draper H D,Hildebrand J H. The theory of emulsification[J]. Journal of the American Chemical Society,1923,45(12):2780-2788.
[61]Binks B P,Clint J H,Mackenzie G,et al. Naturally occurring spore particles at planar fluid interfaces and in emulsions[J]. Langmuir,2005,21(18):8161-8167.
[62]Horozov T S,Binks B P. Particle-Stabilized Emulsions:A Bilayer or a Bridging Monolayer?[J]. Angewandte Chemie,2006,118(5):787-790.
[63]Pawar A B,Caggioni M,Ergun R,et al. Arrested coalescence in Pickering emulsions[J]. Soft Matter,2011,7(17):7710-7716.
[64]Gautier F,Destribats M,Perrier-Cornet R,et al. Pickering emulsions with stimulable particles:from highly-to weakly-covered interfaces[J]. Physical Chemistry Chemical Physics,2007,9(48):6455-6462.
[65]Arditty S,Schmitt V,Lequeux F,et al. Interfacial properties in solid-stabilized emulsions[J]. The European Physical Journal B-Condensed Matter and Complex Systems,2005,44(3):381-393.
[66]Madivala B,Vandebril S,Fransaer J,et al. Exploiting particle shape in solid stabilized emulsions[J]. Soft Matter,2009,5(8):1717-1727.
[67]Tcholakova S,Denkov N D,Lips A. Comparison of solid particles,globular proteins and surfactants as emulsifiers[J]. Physical Chemistry Chemical Physics,2008,10(12):1608-1627.
[68]Binks B P. Particles as surfactants—similarities and differences[J]. Current Opinion in Colloid & Interface Science,2002,7(1):21-41.
[69]Wen C,Yuan Q,Liang H,et al. Preparation and stabilization ofd-limonene Pickering emulsions by cellulose nanocrystals[J]. Carbohydrate polymers,2014,112(4):695-700.
[70]Tan A,Simovic S,Davey A K,et al. Silica-lipid hybrid(SLH)microcapsules:a novel oral delivery system for poorly soluble drugs[J]. Journal of controlled release,2009,134(1):62-70.
[71]Prestidge C A,Simovic S. Nanoparticle encapsulation of emulsion droplets[J]. International journal of pharmaceutics,2006,324(1):92-100.
[72]Simovic S,Prestidge C A. Nanoparticle layers controlling drug release from emulsions[J]. European journal of pharmaceutics and biopharmaceutics,2007,67(1):39-47.
[73]Frelichowska J,Bolzinger M A,Valour J P,et al. Pickering w/o emulsions:drug release and topical delivery[J]. International journal of pharmaceutics,2009,368(1):7-15.
[74]Simovic S,Hui H,Song Y,et al. An oral delivery system for indomethicin engineered from cationic lipid emulsions and silica nanoparticles[J]. Journal of Controlled Release,2010,143(3):367-373.
[75]Fujii S,Okada M,Sawa H,et al. Hydroxyapatite nanoparticles as particulate emulsifier:fabrication of hydroxyapatite-coated biodegradable microspheres[J]. Langmuir,2009,25(17):9759-9766.
[76]Maeda H,Okada M,Fujii S,et al. Pickering-type water-in-oil-in-water multiple emulsions toward multihollow nanocomposite microspheres[J]. Langmuir,2010,26(17):13727-13731.
[77]Aranberri I,Binks B P,Clint J H,et al. Synthesis of macroporous silica from solid-stabilised emulsion templates[J]. Journal of Porous Materials,2009,16(4):429-437.
Research progress in Pickering emulsions stabilized with biomass-based solid nanoparticles
SUN Cui-xia,LIU Fu-guo,YANG Wei,YUAN Fang,GAO Yan-xiang*
(College of Food Science and Nutritional Engineering,China Agricultural University,Beijing 100083,China)
Pickering emulsions prepared by natural,renewable and biodegradable solid particles have become a hot topic. Present studies have indicated that the stability of Pickering emulsions depended largely on the nature of the solid particles. Therefore,the types,fabrication and characterization of solid particles were reviewed in this paper,focusing on the stabilization of biological organic particles such as polysaccharides and proteins. The factors affecting the Pickering emulsion stability were summarized and potential applications of Pickering emulsions in life science were also illustrated.
solid particles;Pickering emulsions;characterization stability
2014-12-15
孙翠霞(1987-),女,博士,研究方向:功能食品研发,E-mail:scx0728@126.com。
*通讯作者:高彦祥(1961-),男,博士,教授,研究方向:超临界加工技术,E-mail:gyxcau@126.com。
国家自然基金(31371835)。
TS202.3
A
1002-0306(2015)15-0370-07
10.13386/j.issn1002-0306.2015.15.070
