肩峰下间隙药物注射治疗肩关节撞击综合征疗效
2015-06-27郁凯杨剑刘敏杨杰杨人军张殿英
郁凯 杨剑 刘敏 杨杰 杨人军 张殿英,3
肩峰下间隙药物注射治疗肩关节撞击综合征疗效
郁凯1杨剑1刘敏2杨杰1杨人军1张殿英1,3
目的 比较曲安奈德、派瑞昔布钠肩峰下间隙注射治疗肩关节撞击综合征的疗效。方法 59例确诊为肩关节撞击综合征患者随机分为激素组和非甾体消炎药(NSAID)组,激素组注射曲安奈德40 mg, NSAID组注射派瑞昔布钠40 mg,每位患者注射前后分别进行疼痛(VAS评分)和肩关节功能评价(HSS评分、外展度)。结果 37例最终获得随访,3周后随访,两组患者活动度与疼痛均明显改善。肩关节HSS评分NSAID组和激素组注射后30 min均明显优于注射前,3周随访时NSAID组优于注射前,激素组与注射前比较差异无统计学意义。肩关节外展,NSAID组和激素组注射后30 min均明显优于注射前,3周随访时NSAID组优于激素组,差异有统计学意义。结论 肩峰下注射激素和NSAID药物均有助于减轻肩关节撞击综合征患者的疼痛,NSAID组肩关节活动度和HSS评分优于激素组。
肩关节撞击综合征;保守治疗;非甾体消炎药;激素
肩关节撞击综合征常见的症状是肩部疼痛和活动受限,其中疼痛为大多数患者的主诉[1]。引起疼痛的原因很多,常见的如肩峰下滑囊及肩袖在肩部外展活动时肱骨与肩峰发生撞击导致的滑囊炎及肌腱退变。肩袖损伤导致的肱骨头与肩胛骨活动异常也可以导致肩关节发生撞击而出现症状[2]。为了达到减轻疼痛和增加活动度的目的,非手术治疗肩关节撞击综合征目前常常采用无负重休息、冷敷、理疗、口服非甾体消炎药(nonsteroidal anti-inflammatory drugs,NSAID)及注射皮质激素等方法。国际的多中心研究表明,对采取休息等方法均无效的患者注射皮质激素是一种有效手段,但其显效的机制尚不明确[3-4],其中广为接受的一种解释就是激素可以减轻无菌性炎症反应。但多次应用激素的副作用是明显的,常见的有冈上肌腱自发断裂,注射局部皮肤萎缩及肱骨头软骨退变等。
局部注射激素对肩关节撞击综合征有效的原因是因为减轻了局部的炎症反应,那么局部注射NSAID是否也可以因为其抗炎作用获得良好疗效的同时还可以避免激素的副作用?本研究的目的就是了解局部注射派瑞昔布钠对减轻肩关节撞击综合征与激素的优劣。
资 料 与 方 法
一、纳入和排除标准
选取我院门诊中确诊为肩关节撞击综合征的患者。纳入标准:(1)肩外展60°~120°时存在痛弧;(2)Neer′s 症阳性;(3)Hawkin′s 症阳性;(4)B超或MRI诊断肩峰下滑囊炎。排除标准:(1)年龄<18岁;(2)病程<1个月;(3)过去3周有肩关节注射史;(4)既往有肩袖撕裂病史;(5)既往有肩关节骨性关节炎病史;(6)有全身炎症反应性疾病;(7)既往有患肩手术病史;(8)既往有患肩感染病史;(9)既往有肩关节黏连或不稳定病史;(10)既往有消化道溃疡及血液病史。
向每位纳入研究的患者说明本研究的目的、方法,需要采集的个人信息,可能发生的问题,并签署知情同意书。本研究经天津市第五中心医院伦理委员会批准实施。
二、研究设计
本研究为随机对照试验,为确保双盲,纳入患者由门诊医师开具局部注射医嘱后,由一位固定药师进行注射药物开具及配制,随机采用信封法(由患者抽取信封)。配置好的两种药物:(1)激素组:40 mg曲安奈德(昆明积大制药有限公司)加入2%的利多卡因(中国大冢制药公司)配置到5 ml;(2)NSAID组:派瑞昔布钠(辉瑞制药有限公司)加入2%利多卡因配制到5 ml。吸入5 ml注射器并由药师编号记录(外加封套无法看到内容物)后由开具医嘱的医师完成局部注射。每位患者均采用标准的注射方法,后外侧“软点”作为进针点,肩峰后角向下2 cm,向外1 cm进针,指向喙突的方向。注射完成后,肩关节外展活动时疼痛减轻作为注射位置正确的标志。
三、评价指标
由本文第一作者和第二作者分别对每一位患者注射前、注射后30 min,注射后3周肩关节活动度(外展)、HSS评分、疼痛(VAS评分)进行评估及记录,完成后每一位患者取两位医师评估和记录的平均值。
四、统计学分析
两组间结果的比较采用双侧t检验,P<0.05为差异有统计学意义。根据样本量公式计算所需的每组患者数,两组各需纳入14例患者。
结 果
2012年10月至2014年9月,59例确诊为肩关节撞击综合征患者进入研究,经纳入和排除标准评价后48例患者入组。随机分为激素注射组和NASID注射组,每组24例,4例失访(激素组2例,NSAID组2例),2例没有按时随访(激素组1例,NSAID组1例),7例注射后2周MRI检查发现为肩袖撕裂(激素组2例,NSAID组3例),最终获得完全随访37例(激素组19例,NSAID组18例)均行MRI或超声检查,明确没有肩袖撕裂。两组患者一般资料见表1。

表1 纳入患者一般资料汇总
两种药物注射组均在注射后3周随访时两种药物均显示出明显的疼痛减轻(NSAID组1.12±2.11,P=0.04;激素组1.48±2.97,P=0.05),但两种药物相比疼痛减轻差异无统计学意义(P=0.170)。与注射前相比,3周随访时NSAID注射组肩关节HSS评分与注射前相比差异有统计学意义(P=0.02);激素组HSS评分与注射前相比差异无统计学意义(P=0.143)(图1)。NSAID组与激素组外展功能均较术前有明显改善,差异均有统计学意义(NSAID组P=0.03;激素组P=0.04),其中3周随访时,NSAID组肩关节外展度大于激素组,P=0.03(图2)。
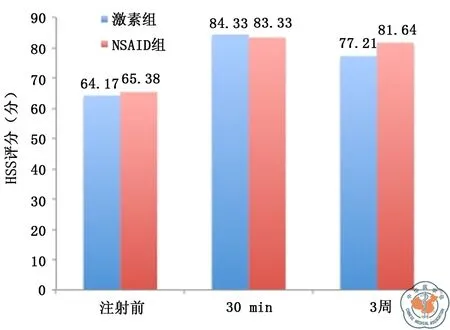
注:NSAID为非甾体消炎药图1 激素组与NSAID组HSS评分比较
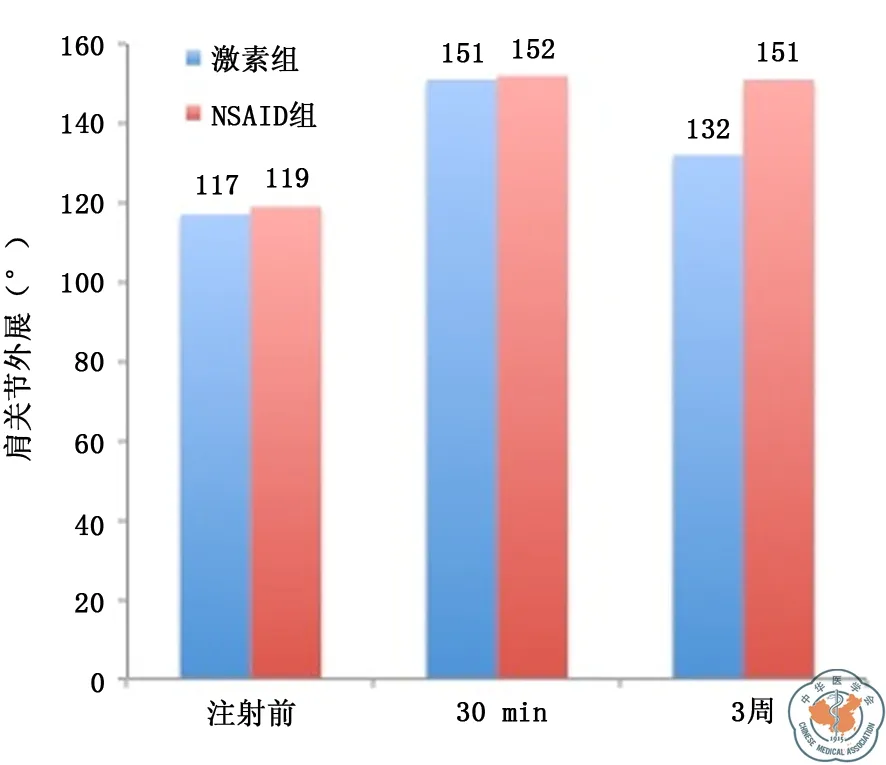
注:NSAID为非甾体消炎药图2 激素组与NSAID组肩外展度比较
讨 论
我们通过在肩峰下间隙注射NSAID来获得局部的无菌性炎症的抑制区,从而达到治疗肩关节撞击综合征的目的。国外研究已有局部注射NSAID治疗肩关节撞击综合征的报道,但均是和安慰剂组相比,缺乏与其他有效治疗手段之间的比较。派瑞昔布钠是近年来推出的NSAID镇痛效果较好的药物,其具有选择性抑制COX-2来抑制前列腺素合成的作用,同时对心血管及胃肠道安全性以及副作用方面优于其他NSAID。人工膝关节置换后,局部注射的“鸡尾酒”镇痛剂就包含此药物。本研究通过随机对照实验的方法,证明了局部注射派瑞昔布钠和激素具有同样的镇痛效果,而3周随访时,肩关节活动度的改善优于注射激素组。
肩关节撞击综合征的病因很多,常见的为肩峰下肌腱、滑囊炎,肩峰形态异常,肩峰下骨赘形成,肩锁关节炎等[5]。近年来随着肩关节外科的发展,肩袖损伤导致的肱骨头上移,肩胛骨位置异常等原因也逐渐被认为是肩关节撞击的病因[6]。上述原因引起的局部组织炎性改变,充血水肿,导致肩关节活动受限。部分肩峰撞击征患者通过有效地保守治疗可以获得很好地疼痛减轻和功能恢复,避免了因病情加重而带来的手术治疗。保守治疗的方法包括局部理疗、手法康复、注射激素、口服NSAID(2~4周),但疗效孰优孰劣尚无定论。Cochrane协作组发表的系统综述中也提到,局部注射激素是治疗肩关节撞击综合征的有效方法,但其疗效不一定优于口服NSAID[4]。
尽管局部注射激素已经被证明在治疗肩关节撞击综合征中是有效的,但激素带来的副作用远超过我们的想象。以往认为局部应用激素是安全的观点早已被否定,局部注射激素带来的肱骨头软骨退变、冈上肌腱萎缩和自发断裂等逐渐引起肩关节外科医师的重视[7-8]。临床中局部注射NSAID尚没有见到影响关节软骨和肌腱组织的报道,很多动物实验证实,即使将NSAID注入软骨内,也不会引起软骨性状的改变[9]。一些基础实验发现,NSAID对成纤维细胞的增殖没有任何作用,而对于其成熟和塑形具有一定的影响,但影响的程度目前尚不明了[10]。因此局部注射NSAID的安全性明显优于激素。
局部注射激素和NSAID均具有减轻注射区域炎症反应的效果,虽然其作用机制不同,但肩关节撞击综合征导致活动受限和疼痛的原因都是局部炎症反应,有效抑制局部炎症就能获得较好的治疗效果。通过本研究,证明了局部注射派瑞昔布钠具有与激素相同甚至更好的止痛效果,3周后患者肩关节活动度的改善优于激素组。当然肩峰下注射NSAID并非能够改变肩关节撞击综合征的病程,但减轻疼痛和水肿后,患者可以配合更好的功能康复,增加肩袖肌的力量,恢复正常的肩峰下间隙,从而减少甚至消除撞击的发生。
本研究存在随访时间短,失访患者数量多,仅在肩峰下注射1次,未对肩峰形态进行分类等不足。
[1] Alvarez CM, Litchfield R, Jackowski D, et al. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis[J]. Am J Sports Med, 2005,33(1): 255-262.
[2] Hawkins RJ, Kennedy JC. Impingement syndrome in athletes[J]. Am J Sports Med, 1980,8(3):151-158.
[3] Adebajo AO, Nash P, Hazleman BL. A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis[J]. J Rheumatol, 1990,17(2): 1207-1210.
[4] Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain[J]. Cochrane Database Syst Rev, 2003, (1): CD004016.
[5] Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report[J]. J Bone Joint Surg Am, 1995,7(7): 1682-1691.
[6] Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment[J]. J Shoulder Elbow Surg, 2009,18(1): 172-177.
[7] Blair B, Rokito AS, Cuomo F, et al. Efficacy of injections of corticosteroids for subacromial impingement syndrome[J]. J Bone Joint Surg Am, 1996,78(22): 1685-1689.
[8] Jean YH,Wen ZH, Chang YC, et al. Intraarticular injection of the cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transected knee in rats: role of excitatory amino acids[J]. Osteoarthritis Cartilage, 2007,15(1): 638-645.
[9] Ozyuvaci H, Bilgic B, Ozyuvaci E, et al. Intraarticular injection of tenoxicam in rats: assessment of the local effects on the articular cartilage and synovium[J]. J Int Med Res, 2004,32(2): 312-316.
[10] Riley GP, Cox M, Harrall RL, et al. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro[J]. J Hand Surg Br, 2001,26(1): 224-228.
(本文编辑:李静)
郁凯,杨剑,刘敏,等.肩峰下间隙药物注射治疗肩关节撞击综合征疗效[J/CD]. 中华肩肘外科电子杂志,2015,3(3):146-150.
Analysis on the curative effects of subacromial space drug injection therapy on shoulder impingement syndrome
YuKai1,YangJian1,LiuMin2,YangJie1,YangRenjun1,ZhangDianying1,3.
1DepartmentofOrthopedics,TianjinFifthCentralHospital,Tianjin300450,China;2Departmentofpharmacy,TianjinFifthCentralHospital,Tianjin300450,China;3DepartmentofOrthopedicsandTrauma,People′sHospital,PekingUniversity,Beijing100044,China
ZhangDianying,Email:zdy8016@163.com
Background The common symptoms of shoulder impingement syndrome include shoulder pain and shoulder activity limitation, and shoulder pain is the chief complaint of majority of patients. There are many causes for shoulder pain, and the common causes include bursitis and tendon degeneration which are resulted from the impingement between subacromial bursa/rotator cuff and humerus/ acromion in the abduction activity of shoulder. The abnormal activity of humeral head and scapula which is resulted from rotator cuff injury may also cause shoulder impingement and generate symptoms. To alleviate pain and increase joint range of motion, such methods as no-load rest, cold compress, physical therapy, oral administration of nonsteroidalanti-inflammatory drugs (NSAID) and injection of cortical hormone are adopted for non-operational treatment of shoulder impingement. An international multicenter study shows that, injection of cortical hormone is an effective means for the patients for whom the other methods such as rest are ineffective without exception. However, the mechanism of its significant effect has not been made clear, and an widely accepted interpretation to its mechanism is that hormone can relieve sterile inflammatory reaction. However, the adverse effects of application of hormone for many times are obvious, and the common adverse effects include spontaneous rupture of supraspinatus tendon, skin atrophy on local injection site and cartilage degeneration at humeral head etc. The reason why local injection of hormone is effective in treatment of shoulder impingement syndrome is that hormone can relieve local inflammatory reaction. Then, whether local injection of NSAID can also avoid the adverse effects resulted from hormone in the mean time of achieving good curative effects thanks to its anti-inflammatory action? It is the objective of this study to know the effects of local injection of parecoxib sodium in reducing shoulder impingement syndrome as well as the advantages and disadvantages of hormone.Methods Ⅰ. Inclusion and exclusion criteria:The patients with shoulder impingement syndrome confirmed in Outpatient department of our hospital were selected. The inclusion criteria:(1) there exists painful arc when shoulder is abduced by 60°-120°; (2) Neer′s disease positive; (3) Hawkin′s disease positive; (4) subacromial bursitis confirmed through B ultrasound or MRI. Exclusion criteria:(1) Age <18 years; (2) course of disease <1 month; (3) has history of shoulder joint injection in the past 3 weeks; (4) has past history of rotator cuff tear; (5) Has past history of shoulder osteoarthritis; (6) Has systemic inflammatory reaction disease; (7) Has past history of shoulder operation; (8) has past history of shoulder infection; (9) has past history of shoulder joint adhesion or instability; (10) has past history of alimentary tract ulcer and hematopathy. For each patient included in this study, our hospital described the objective and method of this study, the personal information to be acquired, the probable problems and signed informed consent. This study was performed upon approval by the Ethics Committee of Tianjin No.5 Central Hospital. Ⅱ. Study design:This study is a randomized control test. To ensure double blind trial, after the clinic doctor has issued medical advice for local injection, the patients included in study should take medicine from pharmaceutist; one fixed pharmaceutist should issue and prepare the drug for injection, and randomized evelop method is adopted (envelops randomly drawn by patients). Two kinds of drugs are prepared: (1) Hormone group: To 40mg Triamcinolone acetonide(provided by Kunming Jida Pharmacy Co.,Ltd), add 2% lidocaine(China Otsuka Pharmaceutical Co., Ltd), prepare the solution to 5 ml; (2) NSAID group: Add 2% lidocaine to Parecoxib sodium (Pfizer Pharmaceuticals Limited), and prepare the solution to 5ml. The drug is sucked into a 5 ml syringe. After the pharmacist has made numbering and recording (the content cannot be seen since envelope is added), the doctor who issued the medical device should complete local injection. Each patient is injected according to standard injection method. The "soft spot" on posterior-lateral side serves as the needle insertion point, and needle is inserted from posterior angle of acromion and inserted downwards by 2 cm and outwards by 1cm, pointing to the direction of coracoid. After completion of injection, pain relief in the abduction of shoulder joint is used as the mark of correct injection position. Ⅲ. Evaluation indicators:For each patient, the first author and the second author of this article respectively make evaluation and recording for the shoulder joint range of motion(abduction), HSS score and ache (VAS score) at 30 min prior to injection, 30 min after injection and 3 weeks after injection; After completion of evaluation and recording, for each patient, take the mean value evaluated and recorded by two physicians.Ⅳ. Statistical analysis:For the comparison on the results between two groups, two sided t test is performed. IfP<0.05, it is meant that the different has statistical significance. According to the Equation for sample size, calculate the required number of patients in each group. Two groups need to respectively include 14 patients.Results During the period from October 2012 to September 2014, 59 patients with confirmed shoulder impingement syndrome entered the study; After evaluation based on inclusion/exclusion criteria, 48 patients were incorporated into the study groups. Every 24 cases were randomly divided into hormone injection group and NASID injection group, 4 cases lost to follow up (2 cases in hormone group and 2 cases in NSAID group); 2 cases failed to receive follow-up visit on schedule (1 case in hormone group and 1 case in NSAID group). Through MRI at 2 weeks after injection, 7 case were confirmed as rotator cuff tear (2 cases in hormone group and 3 cases in NSAID group). Finally, 37 cases obtained complete follow-up visit (19 cases in hormone group and 18 cases in NSAID) and
MRI or ultrasonic examination, and it was made clear that they did not suffer from rotator cuff tear. Both drug injection groups received follow-up visit at 3 week after injection, when both drugs shown obvious ache relief (NSAID group:1.12±2.11,P=0.04; hormone group:1.48±2.97,P=0.05). However, there was no difference in ache relief when two kinds of drugs were compared (P=0.170). In the 3-week follow-up visit, the difference of shoulder joint HSS score of NSAID injection group in comparison with the value prior to injection has statistical significance (P=0.143). Both NSAID group and hormone group shown obvious improvement in abduction function in comparison with the abduction function prior to operation, with both differences having statistical significance (NSAID group:P=0.03;hormone groupP=0.04). In the 3-week follow-up visit, the shoulder joint abduction range of NSAID group is greater than that of hormone group, withP=0.03.Conclusion Injection of hormone and NSAID drug under acromion can help relieve the pain of patient with shoulder impingement syndrome. The shoulder joint range of motion and HSS score of NSAID injection group are superior to those of hormone group.
Shoulder impingement syndrome;Nonoperative treatment;Nonsteroidal anti-inflammatory drugs;Corticosteroids
10.3877/cma.j.issn.2095-5790.2015.03.004
天津市滨海新区卫生局科技项目(2011BHKY008、2012BWKZ002、2013BWKY009);卫生公益性行业
300450天津市第五中心医院骨科1,药剂科2;100044北京大学人民医院创伤骨科3
张殿英,Email:zdy8016@163.com
2014-12-16)
科研专项(201002014、201302007);教育部创新团队(IRT1201);北京市科委重大专项基金(Z101107052210001)
