Design of the Metal Precursors Molecular Structures in Impregnating Solutions for Preparation of Ef ficient NiMo/Al2O3Hydrodesulfurization Catalysts
2015-06-22LiHuifengLiMingfengChuYangLiuFengNieHong
Li Huifeng; Li Mingfeng; Chu Yang; Liu Feng; Nie Hong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Design of the Metal Precursors Molecular Structures in Impregnating Solutions for Preparation of Ef ficient NiMo/Al2O3Hydrodesulfurization Catalysts
Li Huifeng; Li Mingfeng; Chu Yang; Liu Feng; Nie Hong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
The molecular structures of metal precursors in the impregnating solution were designed so as to prepare ef ficient NiMo/Al2O3hydrodesulfurization (HDS) catalysts. At first, five typical impregnating solutions were designed; the existing metal precursors, such as [Mo4(citrate)2O11]4--like, [P2Mo18O62]6--like and [P2Mo5O23]6--like species in the solutions were confirmed by laser Raman spectroscopy (LRS). The UV-Vis spectra results indicated that the solutions containing both phosphoric acid and citric acid could change the existing form of nickel species. Five corresponding NiMo/Al2O3catalysts were prepared by the incipient wetness impregnation method. The LRS analysis results of dried catalysts showed that the above metal precursors could be partly retained on alumina support after impregnation and drying, although the interface reaction between different metal precursors and alumina support unavoidably took place. Then the catalysts were sulfided and characterized by N2physisorption, TEM and XPS analyses. The results showed that different metal precursors in impregnating solution could mainly result in the difference in both the morphology of (Ni)MoS2slabs and the promoting effect of Ni species. The catalyst prepared mainly with [P2Mo5O23]6--like species used as precursors exhibited worse dispersion of (Ni)MoS2slabs and lower ratio of Ni–Mo–S active phases than the one with [Mo4(citrate)2O11]4--like species. Promisingly, the catalyst prepared with co-existing [Mo4(citrate)2O11]4--like, [P2Mo18O62]6--like and [P2Mo5O23]6--like species showed better hydrodesulfurization activity for 4,6-DMDBT thanks to its more well-dispersed Ni–Mo–S active phases.
molecular structures of metal precursors; impregnating solution; citric acid; phosphorous; hydrodesulfurization
1 Introduction
The development of highly active hydrotreating catalysts is considered to be the most cost-effective means for implementing ultra-deep hydrodesulfurization (HDS)[1-10]. So far, various methods have been tried to prepare efficient catalysts. All these methods can be mainly summarized into two technical routes.
The first technical route is the design of surface characteristics by using novel supports[11-12], or modified ones through introducing some additives[6,8,13], such as fluorine, phosphorus, boron, carbon, etc., so as to avoid strong metal-support interaction occurring on conventional alumina support.
The second technical route is to design the molecular structures of metal precursors in the impregnating solution for preparing highly active HDS catalysts. For example, the addition of chelating agents can improve the activity of the sulfided catalysts by optimizing the existing state of oxidic metal species and their sulfidation behavior[6,8,10,14-17]. Klimov, et al.[15-16]synthesized the bimetallic Co-Mo compounds from ammonium heptamolybdate, citric acid and cobalt acetate for the preparation of catalysts. Moreover, in recent years the alumina supported HDS catalysts prepared with new heteropolycompounds (such as Co3/2PMo12O40, etc.), which are characteristic of definite molecular structures, have received more and more attention on theoretical research[18-20].
For commercial application, conventional Co(Ni)-Mo-P impregnating solutions are generally prepared with industrial materials. For example, by adding phosphoric acid in an aqueous solution of ammonium heptamolybdate followed by adding cobalt (nickel) nitrate, or dissolvinga mixture containing MoO3, H3PO4and Co (Ni) CO3in water under reflux, the P/Mo molar ratio is usually highly capable of forming a clear solution without precipitation. And the chemistry of conventional Co(Ni)-Mo-P impregnating solutions is extensively studied in the last century. However, the addition of chelating agents (especially citric acid) into conventional Co(Ni)-Mo-P impregnating solutions can bring about new changes to the chemistry of the impregnating solution. During our work, it has been found out that the P/Mo molar ratio of conventional Ni(Co)-Mo-P solutions can be adjusted flexibly due to the existence of citric acid, and various metal precursors can be obtained according to the pre-designed preparation formula[21]. And it can indeed shed light on the design of new industrial HDS catalysts from a molecular approach. Therefore, the aim of our present work is to design different types of impregnating solutions and investigate the essential role of different metal precursors on the active phase morphology and HDS activity of NiMo/Al2O3catalysts. So far, according to our best knowledge, such work has been rarely reported comprehensively in the literature.
2 Experimental
2.1 Design of impregnating solutions
To clearly verify the changes in existing state of the metal precursors in the impregnating solutions, we presented a novel and convenient synthesis route[21]. For a typical example, a mixture of deionized water, nickel carbonate basic tetrahydrate, molybdenum trioxide and citric acid was stirred under 373 K for 4 h to obtain a clear aqueous solution as the mother solution (labeled as P-0). Then different amounts of phosphoric acid (with a molar ratio of P/Mo=0.1, 0.3, and 0.5, respectively) were added into the above P-0 solution to prepare another three impregnating solutions (labeled as P-1, P-2 and P-3, respectively). For comparison, a mixture of deionized water, nickel carbonate basic tetrahydrate, molybdenum trioxide and phosphoric acid (with a molar ratio of P/Mo=0.5) was dissolved at the same conditions to get a clear solution and labeled as P-4. All the chemical reagents were provided by the SINOPEC Changling Catalyst Company.
2.2 Catalyst preparation
Commercial alumina support (provided by SINOPEC Changling Catalyst Company) was obtained without further treatment. Five NiMo/Al2O3catalysts were prepared by the incipient wetness impregnation method with the corresponding impregnating solutions (P-0, P-1, P-2, P-3 and P-4) to ensure the same loading of 5% of NiO and 21% of MoO3(calculated by reference to alumina support). After drying, five catalysts were obtained and labeled as CP-0, CP-1, CP2, CP-3 and CP-4, respectively.
2.3 Characterization
The laser Raman spectra (LRS) of the samples were recorded on a Raman spectrometer (LabRAM HR UVNIR, Jobin Yvon) with a microscope operating at room temperature. The ultraviolet-visible (UV-Vis) spectra of impregnating solutions were recorded by a Varian Cary 300 spectrophotometer. The textural parameters of the sulfided samples were analyzed by N2physisorption using a Micromeritics ASAP 2405N automated system. The transmission electron micrographs (TEM) of the sulfided samples were recorded by a Tecnai G2 F20 S-Twin microscope. The X-ray photoelectron spectra (XPS) of the sulfided samples were acquired by a Thermo Fischer-VG Escalab 250 spectrometer. For more details please see the literature[8,12-14].
2.4 Catalytic activity evaluation
The HDS tests were carried out in a continuous flow fixedbed micro-reactor using 0.4% of 4, 6-DMDBT and 0.4% of decalin (as internal standard) in decane as the model feed at a feed flow rate of 0.20 cm3/min under the following conditions: the catalyst loading was 0.15 g (40—60 mesh), the temperature was 553 K, the reaction pressure was 4.0 MPa, and the H2flow rate was 180 cm3/min. The catalysts were first presulfided with a sulfiding feed composed of 5% CS2in cyclohexane, at a temperature of 633 K and a pressure of 4.0 MPa for 2.5 h. The liquid effluents were collected and determined by an Agilent 6890N gas chromatograph when the steady-state conditions were reached. For more details please refer to the literature[8,12-14].
3 Results and Discussion
3.1 LRS analysis
To determine the existing state of the metal precursors in the impregnating solutions, five impregnating solutions (P-0, P-1, P-2, P-3 and P-4) were first characterized by LRS. It can be seen from the LRS analysis results presented in Figure 1 that the metal species in these solutions varied in their molecular structures. The P-0 solution was mainly characterized by its [Mo4(citrate)2O11]4--like species with the bands centered at approximately 942 cm-1, 897 cm-1and 860 cm-1[22]. After the addition of phosphoric acid, two new small bands (975 cm-1and 712 cm-1) characteristics of the [P2Mo18O62]6--like species were found in the LRS spectra of P-1 and P-2 solutions, but such bands considerably weakened in the spectra of P-3 solution. In contrast, the P-4 solution was mainly characterized by its [P2Mo5O23]6--like species with the strong bands centered at approximately 945 cm-1and 897 cm-1[23], similar to those of P-0 solution. Anyway, it can be expected that the relative amounts of [Mo4(citrate)2O11]4--like, [P2Mo18O62]6--like and [P2Mo5O23]6--like species would vary depending on different formulations of P-1, P-2 and P-3 solutions with an increasing addition of phosphoric acid. But it is difficult to determine the exact amounts of different metal precursors in each impregnating solution.
In addition, the dried catalysts (CP-0, CP-1, CP-2, CP-3 and CP-4) were also characterized by LRS. It can be seen from the LRS analysis results shown in Figure 2 that the peaks of the dried catalysts became broader compared with those of the corresponding impregnating solutions due to the interaction between metal precursors and alumina support. For the catalyst CP-0, the bands were centered around 942 cm-1, 897 cm-1and 860 cm-1which were assigned to [Mo4(citrate)2O11]4--like species. It indicated that the [Mo4(citrate)2O11]4--like species were still retained on the dried catalyst CP-0.
For the catalysts CP-1, CP-2 and CP-3, generally, there was a broad peak between 700 cm-1and 1 000 cm-1; however, upon taking the catalyst CP-3 as an example, the bands centered about 942 cm-1, 897 cm-1and 860 cm-1were found; although the bands (at 975 cm-1and 712 cm-1) characteristics of [P2Mo18O62]6--like species were not clearly seen, it is probable that such small bands were covered under the broad peak. The reasons can be attributed to the fact that the addition of citric acid can effectively prevent the heteropolyacids from being decomposed in the pores of alumina[24], and thus the [P2Mo18O62]6--like species can be partly retained on the dried catalysts.
In regard to the catalyst CP-4, the peaks at 947 cm-1, 899 cm-1, 572 cm-1, 355 cm-1, and 218 cm-1were identified, indicating the existence of the Anderson-type alumino-heteropolymolybdate [Al(OH)6Mo6O18]3-anions. The formation of such species could be explained as follows. Firstly, the [P2Mo5O23]6--like species could react with the alumina surface and partly decompose into phosphate and molybdate (MoO42-) anions[25]; secondly, under acidic conditions the formed MoO42-also could react with Al3+dissolved from alumina surface to form [Al(OH)6Mo6O18]3-anions. In addition, it should be noted that the decomposition of [P2Mo5O23]6--like species by alumina could be partly inhibited by the presence of free phosphate[25]. Upon considering that the molar ratio of P/Mo in the P-4 solution was 0.5, which was higher than the theoretical P/ Mo ratio of molecular formula (0.4), it would be expected that [P2Mo5O23]6--like species could be partly retained onthe dried catalyst CP-4.
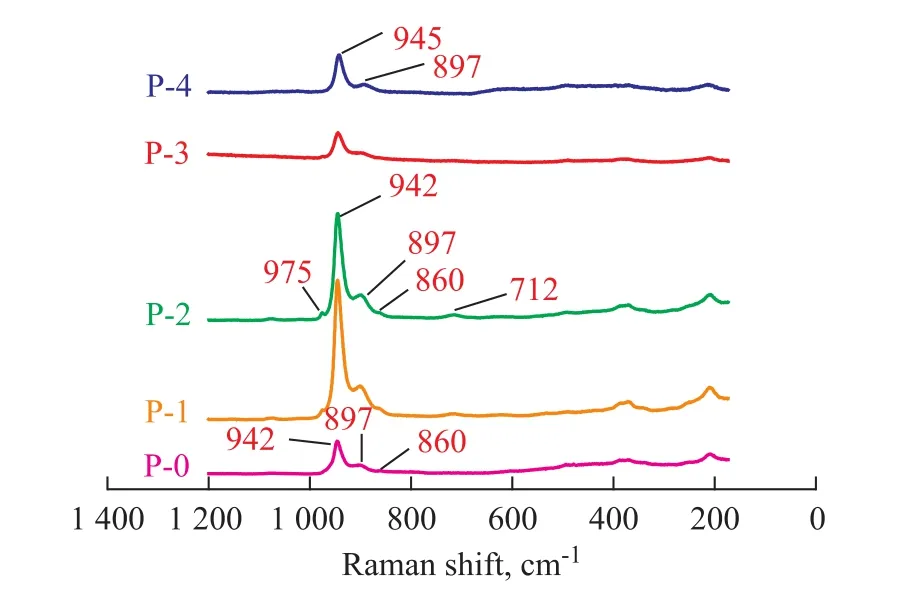
Figure 1 LRS spectra of different solutions
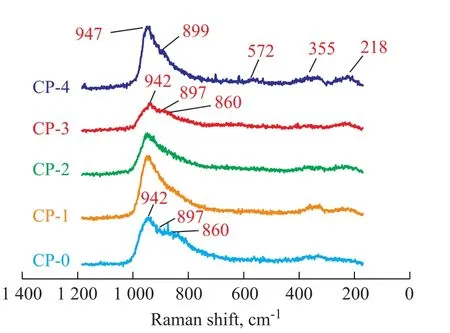
Figure 2 LRS spectra of different dried catalysts
Based on the above characterization and analysis, it can be inferred that all the metal precursors detected in the solutions could be partly retained on alumina support after impregnation and drying, although the interface reaction between metal precursors and alumina support could not be avoided.
3.2 UV-Vis spectra
To compare the chemical coordination of nickel ions in different impregnating solutions, their UV-Vis spectra recorded thereby are shown in Figure 3. In the UV-Vis spectra of P-0 solution (with merely added citric acid) and P-4 solution (with merely added phosphoric acid), only two bands at 652 nm and 718 nm, namely the characteristic bands of [Ni(H2O)6]2+species were observed; but in comparison with the P-0 and P-4 solutions, the P-1, P-2 and P-3 solutions with an increasing addition of phosphoric acid would firstly cause the gradual weakening of bands at around 652 nm, and secondly the remarkable broadening of bands at around 750 nm and shift to higher wavelength. It indicated that adding phosphoric acid and citric acid simultaneously might change the existing form of nickel species.

Figure 3 UV-Vis spectra of different solutions(a)—nickel nitrate; (b)—P-0; (c)—P-4; (d)—P-1; (e)—P-2; (f) P-3
3.3 N2physisorption
The pore size distribution of the freshly sulfided samples (labeled as S-CP-0, S-CP-1, S-CP-2, S-CP-3 and S-CP-4) is illustrated in Figure 4. Generally speaking, all the samples had similar pore size distribution. But compared with S-CP-4, the samples after adding citric acid (S-CP-0, S-CP-1, S-CP-2 and S-CP-3) exhibited a more noticeable pore diameter (3-4 nm in diameter). Such phenomena were also observed in the freshly sulfided NiW supported catalysts[8].
In regard to the textural properties of the freshly sulfided samples, it is clear that, firstly, in comparison with S-CP-4 (111 m2/g; 0.35 cm3/g), the samples S-CP-0 (149 m2/g; 0.37 cm3/g), S-CP-1 (143 m2/g; 0.37 cm3/g), S-CP-2 (146 m2/g; 0.35 cm3/g) and S-CP-3 (140 m2/g; 0.32 cm3/g) all had larger surface area and equivalent pore volume, and the larger surface area of the latter could be attributed to the increase in the amount of narrow pores (3—4 nm). Secondly, in comparison with S-CP-0, with an increasing phosphoric acid dosage in the impregnating solutions, there was a gradual decrease in the surface area and pore volume of the samples S-CP-1, S-CP-2 and S-CP-3, because excessive phosphoric acid could cause corrosive action on the alumina support.

Figure 4 Pore size distribution of the sulfided samples■—S-CP-0; ●—S-CP-1;▲—S-CP-2; ▼—S-CP-3; ◆—S-CP-4
3.4 XPS analysis
To further study the influence of different metal precursors on the sulfidation extent and the distribution of active metals on the surface of alumina, the freshly sulfided samples were characterized by XPS, with the results shown in Figure 5. Considering that no NiSxphase species are clearly observed in Figure 4, according to the deconvolution method reported by Qiu, et al.[26], the binding energies at about 226.2 eV, 227.5 eV and 233.2 eV were assigned to the S2s peak from sulfide and terminal S22-, the S2s peak from oxysulfide and bridging S22-, and the S2s peak from sulfate, respectively; the binding energies ataround 229.0 eV, 230.5 eV and 232.8 eV were ascribed to Mo3d5/2of Mo(IV), Mo(V) and Mo(VI) species, respectively; the binding energies at around 232.2 eV, 233.8 eV and 236.0 eV were ascribed to Mo3d3/2of Mo(IV), Mo(V) and Mo(VI) species, respectively; the binding energies at around 854.0 eV, 856.7 eV and 861.5 eV were ascribed to Ni–Mo–S phase, Ni2+in an oxidic environment and its shake-up peak, respectively. It is interesting to find from the XPS analysis results listed in Table 1 that with an increasing phosphoric acid in the impregnating solutions, there was a notable rise in the P/Mo molar ratio of the freshly sulfided samples. Especially with respect to S-CP-4, its P/Mo molar ratio determined by XPS even rose up to 1.0, which was two times those used in the impregnating solutions. It could be attributed to the fact that phosphomolybdates of different molecular structureswere gradually decomposed during sulfidation and finally were transformed into MoS2phases; but the free phosphorus species were liable to be anchored on the surface of alumina.

Figure 5 XPS results: (a) Mo3d-S2s spectra and (b) Ni2p3/2spectra of the sulfided samples
Compared with S-CP-4, the sample S-CP-0 showed higher degree of Mo sulfidation (Mosulfide/Mototal=Mo(IV)/Mototal) and NiNi–Mo–S/Nitotal. It indicates that the P-0 solution characteristic of [Mo4(citrate)2O11]4--like species was better than the P-4 solution characteristic of [P2Mo5O23]6--like species, in regard to promoting the sulfidation of supported nickel and Mo species and the formation of Ni-Mo-S active phases. It can be explained that the formation of [Al(OH)6Mo6O18]3-anions on the dried CP-4, as evidenced by the LRS analysis results in Figure 2, could partly hinder the sulfidation of Mo species[27]and the formation of Ni-Mo-S active phases. Moreover, compared with S-CP-0, the samples S-CP-1, S-CP-2 and S-CP-3 exhibited an equivalent degree of Mo sulfidation, while undergoing a slight decrease in NiNi–Mo–S/Nitotalwith an increasing addition of phosphoric acid. It could be attributed to the notable change of the relative amounts of [Mo4(citrate)2O11]4--like, [P2Mo18O62]6--like and [P2Mo5O23]6--like species in the impregnating solutions of P-1, P-2 and P-3, as evidenced by LRS test results in Figure 1. In addition, it is interesting to find that S-CP-3 had higher ratio of NiNi–Mo–S/Nitotalthan S-CP-4, although they had the same high P/Mo molar ratio; the reasons can be explained by the fact that the existence of citric acid might weaken the negative effect on the formation of Ni-Mo-S active phases brought about by large amounts of phosphorus species. It can be seen from the above results that the introduction of a proper amount of phosphorus together with citric acid in impregnating solution could bring forth synergetic effect to promote the formation of Ni-Mo-S active phases.
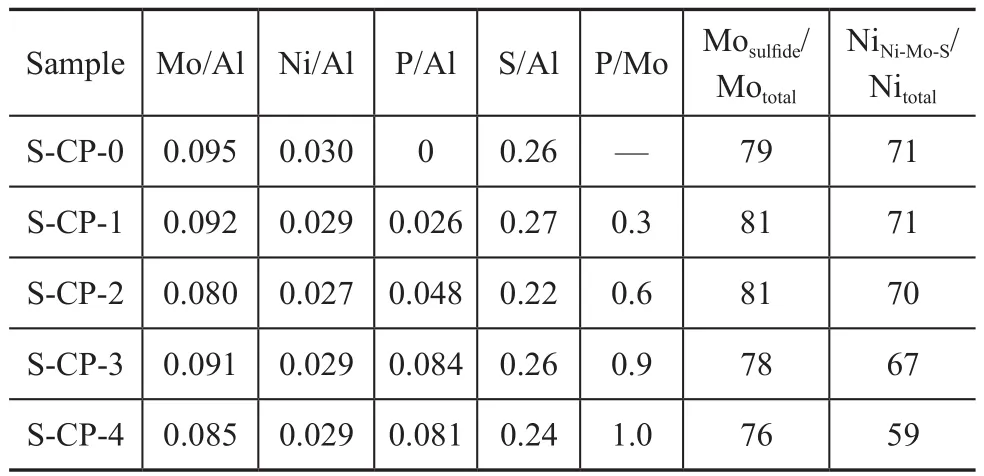
Table 1 XPS analysis results of the sulfided samples
3.5 TEM analysis
To find the morphology difference of sulfided catalysts prepared by different metal precursors, the freshly sulfided samples were characterized by TEM. Representative TEM micrographs are shown in Figure 6. Compared with S-CP-0, the S-CP-4 sample showed a notable increase in both average slab length (from 4.3 nm to 5.6 nm) and stacking number (from 3.1 to 3.3) of (Ni)MoS2slabs. It can be explained as follows. Firstly, the S-CP-4 sample was prepared by P-4 solution characteristic of [P2Mo5O23]6--like species; during impregnation the [P2Mo5O23]6--like species were in contact with alumina support, and the phosphorus atoms located at the external surface of its molecular structure were ready to react with alumina surface; moreover, the free phosphorus species would prefer to be anchored on alumina surface strongly than molybdenum species, which would result in the formation of phosphorus-rich shell (AlPO4-like species), as reported by Cheng, et al.[25]In fact, this explanation can also be veri fied by the highest P/Mo molar ratio (1.0) of S-CP-4, as evidenced by our XPS analysis results in Table 1. Therefore, after sulfidation the larger MoS2slabs were prone to form on the alumina surface pre-occupied by phosphorus excessively because of too much AlPO4-like species formation on the support surface. In contrast, S-CP-0 was prepared with the P-0 solution characteristic of [Mo4(citrate)2O11]4--like species, and it could bene fit from two major roles of citric acid to improve the dispersion of active phases[8]. The first one is “tailoring the alumina surface”. When strong adsorption sites of alumina surface[28-29]were occupied by citric acid, it could weaken the metal–support interaction and facilitate the sulfidation of metal species. The second one is “isolating the metal species”. After sulfidation the citric acid could partly be retained in the form of in situ coke or carbonaceous materials to further impede excessive agglomeration of MoS2slabs.
In addition, it is interesting to find that compared with S-CP-0, with an increasing addition of phosphoric acid, a noticeable decrease in both average slab length and stacking number of (Ni)MoS2slabs in S-CP-1 (3.8 nm; 2.5), S-CP-2 (3.3 nm; 2.8) and S-CP-3 (4.0 nm; 2.7) was identified. This phenomenon can be attributed to the following reasons. Firstly, unlike the [P2Mo5O23]6--like species, the [P2Mo18O62]6--like species had their phosphorus atomslocated in the internal part of their molecular structure; the [P2Mo18O62]6--like species were liable to be well-dispersed on alumina support in the presence of citric acid. Secondly, even if the [P2Mo18O62]6--like species were decomposed and transformed into MoS2during sulfidation, proper amounts of phosphorus species anchored on alumina surface could also play a role in isolating the newly formed small (Ni)MoS2active phases and inhibiting their excessive aggregation, especially at high MoO3loading.
3.6 Catalytic activity evaluation
4,6-DMDBT was used to compare HDS activity of the catalysts prepared with different metal precursors. According to the method reported by relevant researchers[13-14], the total HDS activity, HYD activity and DDS activity were calculated. It is clear that the total HDS activity decreased in the following order: CP-2 (0.98 mol/kg·h) > CP-3 (0.91 mol/kg·h) > CP-1 (0.88 mol/kg·h) > CP-0 (0.84 mol/kg·h) > CP-4 (0.79 mol/kg·h). Moreover, it was found out that the DDS activity of five catalysts was equivalent, and thus their catalytic difference especially widened in terms of the HYD activity. Indeed, the HYD activity decreased in the same order: CP-2 (0.94 mol/kg·h)> CP-3 (0.87 mol/kg·h)> CP-1 (0.84 mol/kg·h)> CP-0 (0.79 mol/kg·h)> CP-4 (0.74 mol/kg·h).
Based on the above characterization results, the lowest HDS activity of CP-4 could be attributed to its poorest dispersion of (Ni) MoS2slabs and lowest ratio of NiNi–Mo–S/Nitotal. However, compared with CP-0 and CP-4, the catalysts CP-1, CP-2 and CP-3 had better HDS activity; especially CP-2 catalyst exhibited a highest HDS activity on 4,6-DMDBT. It can be attributed to the fact that the co-existing multiple metal precursors prepared by an optimal P/Mo molar ratio together with citric acid could be retained on the support after impregnation and drying, and then achieve a better dispersion of (Ni) MoS2slabs and a higher NiNi–Mo–S/Nitotalratio after sulfidation. Moreover, it should be noted that proper amounts of phosphorus and carbonaceous materials derived from citric acid could closely interact with both the alumina support and the (Ni)MoS2active phases, and could play important roles in changing the morphology and electronic structure of the Ni-Mo-S active phases. This means that its first role is the “isolating effect” to avoid excessive agglomeration of (Ni)MoS2slabs, and its second role is the “tailoring effect” to decrease the polarization of the Mo-S bonding of Ni-Mo-S active phases caused by Mo-O-Al groups. Both roles could mainly contribute to the highest HDS activity of CP-2 catalyst.
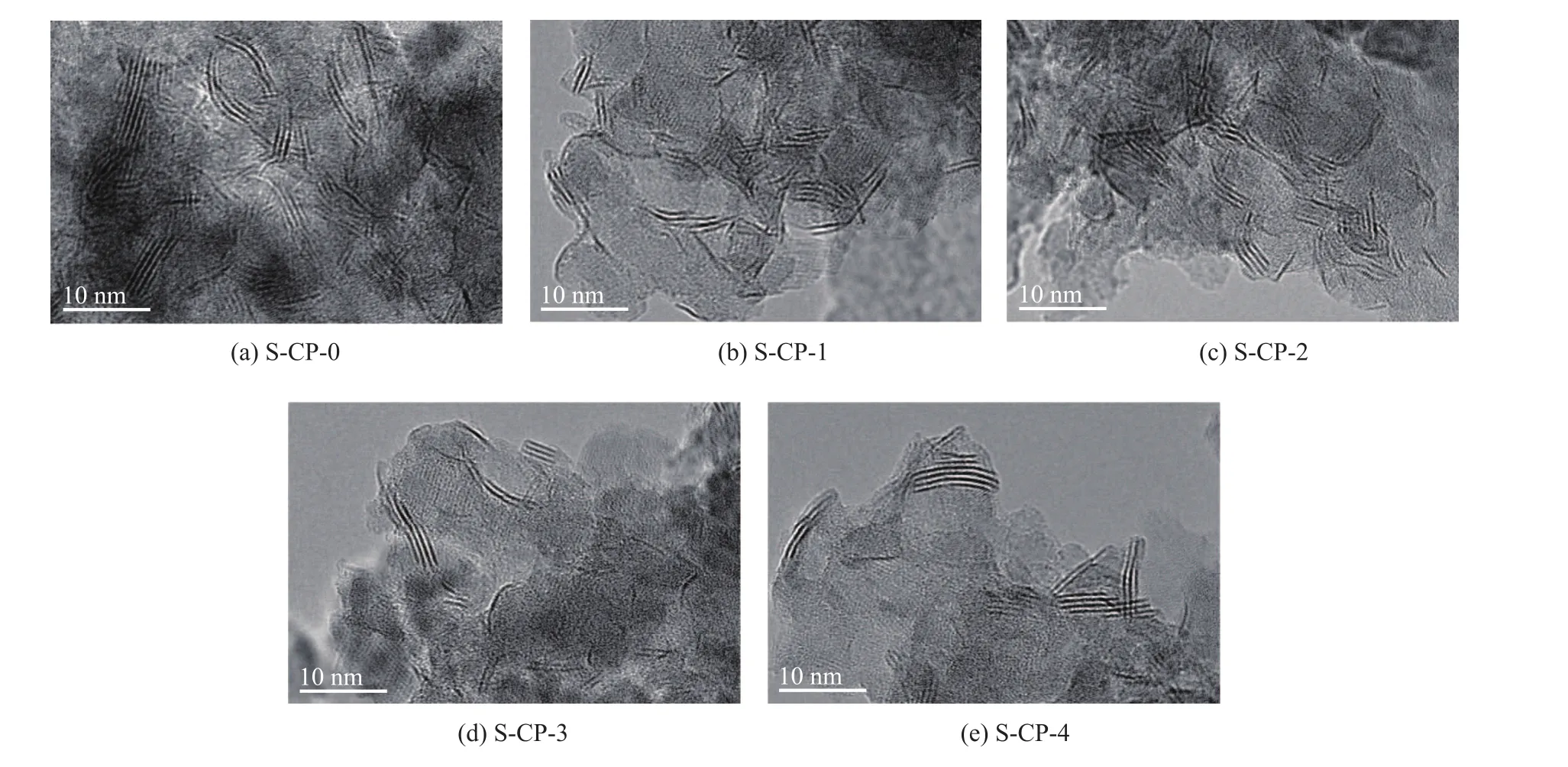
Figure 6 TEM images of the sulfided samples
4 Conclusions
The interface reaction between different molecular structures of metal precursors and alumina support plays an important role in the dispersion of (Ni)MoS2slabs andthe ratio of NiNi–Mo–S/Nitotal. Promisingly, the CP-2 catalyst, characteristic of co-existing [Mo4(citrate)2O11]4--like, [P2Mo18O62]6--like and [P2Mo5O23]6--like species in its impregnating solution, exhibited the highest HDS activity on 4,6-DMDBT thanks to its well-dispersed (Ni)MoS2slabs and optimal ratio of Ni–Mo–S in all the MoS2slabs.
Acknowledgements:This work was supported by the National Key Basic Research Program of China (973 Program, 2012CB224802) and the SINOPEC project (No. 114013).
[1] Li D. Crucial technologies supporting future development of petroleum refining industry[J]. Chin J Catal, 2013, 34(1): 48-60 (in Chinese)
[2] Nie H, Li M, Gao X, et al. Hydrogenation catalyst and technology in petroleum processing[J]. Acta Petrolei Sinica ( Petroleum Processing Section), 2010, 26(S): 77-81 (in Chinese)
[3] Nie H, Yang Q, Dai L, et al. Development and commercial application of key technology for efficient conversion of heavy oil[J]. Petroleum Processing and Petrochemicals, 2012, 43(1): 1-6 (in Chinese)
[4] Zhang Le, Li Mingfeng, Nie Hong. Study on sulfidation degree and morphology of MoS2catalyst derived from various molybdate precursors[J]. China Petroleum Processing and Petrochemical Technology,2014 16(2): 1-7
[5] Song C, Ma X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization[J]. Appl Catal B, 2003, 41(1/2): 207-238
[6] Sun M, Nicosia D, Prins R. The effects of fluorine, phosphate and chelating agents on hydrotreating catalysts and catalysis[J]. Catal Today, 2003, 86: 173-189
[7] Nie H, Li H, Long X, et al. Application of chelating agents in preparation of hydrotreating catalysts[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31(2): 250-258 (in Chinese)
[8] Li H, Li M, Chu Y, et al. Essential role of citric acid in preparation of efficient NiW/Al2O3HDS catalysts[J]. Appl Catal A, 2011, 403: 75-82
[9] Li M, Li H, Jiang F, et al. Effect of surface characteristics of different alumina on metal–support interaction and hydrodesulfurization activity[J]. Fuel, 2009, 88(7): 1281-1285
[10] Li M, Li H, Jiang F, et al. The relation between morphology of (Co)MoS2phases and selective hydrodesulfurization for CoMo catalysts[J]. Catal Today, 2010, 149: 35-39
[11] Liu X, Li X, Yan Z. Facile route to prepare bimodal mesoporous γ-Al2O3as support for highly active CoMo-based hydrodesulfurization catalyst[J]. Appl Catal B, 2012, 121-22: 50-56
[12] Li H, Li M, Nie H. Tailoring the surface characteristic of alumina for preparation of highly active NiMo/Al2O3hydrodesulfurization catalyst[J]. Micropor Mesopor Mat, 2014, 188: 30-36
[13] Chen W, Maugé F, van Gestel J, et al. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts[J]. J Catal, 2013, 304: 47-62
[14] Nie H, Long X Y, Liu Q H, et al. Effect of citric acid on sulfidation behavior of NiW/Al2O3hydrodesulfurization catalyst[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26(S): 329-335 (in Chinese)
[15] Klimov O V, Pashigreva A V, Bukhtiyarova G A, et al. Bimetallic Co–Mo complexes: A starting material for highly active hydrodesulfurization catalysts[J]. Catal Today, 2010, 150: 196-206
[16] Klimov O V, Pashigreva A V, Fedotov M A, et al. Co–Mo catalysts for ultra-deep HDS of diesel fuels prepared via synthesis of bimetallic surface compounds[J]. J Mol Catal A, 2010, 322: 80-89
[17] Lin L, Yi X D, Qiu B, et al. Preparation and characterization of Mo-Ni-P solution[J]. Acta Petrolei Sinica (Petrol Proc Sect), 2009, 25(2): 173-177 (in Chinese)
[18] Griboval A, Blanchard P, Gengembre L, et al. Hydrotreatment catalysts prepared with heteropolycompound: Characterisation of the oxidic precursors[J]. J Catal, 1999, 188: 102-110
[19] Guo Rong, Shen Benxian, Fang Xiangchen, et al. Study on relationship between microstructure of active phase and HDS performance of sulfided Ni-Mo catalysts: Effect of metal loading[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(2): 12-19
[20] Pettiti I, Botto I L, Cabello C I, et al. Anderson-type heteropolyoxomolybdates in catalysis: 2. EXAFS study on γ-Al2O3-supported Mo, Co and Ni sulfided phases as HDS catalysts[J]. Appl Catal A, 2001, 220: 113-121
[21] Li H, Li M, Nie H. Design the molecular structures of metal precursors in the impregnating solution for preparing efficient HDS catalysts[Z]. MACS VI, France, 2013
[22] Bergwerff J A, Jansen M, Leliveld B R G, et al. Influenceof the preparation method on the hydrotreating activity of MoS2/Al2O3extrudates: A Raman microspectroscopy study on the genesis of the active phase[J]. J Catal, 2006, 243: 292-302
[23] Wang Z, Lu W. Raman spectroscopic study on the impregnation solution of hydrotreating catalyst Mo-Ni-P/H2O[J]. Chin J Catal, 1983, 4(1): 66-74 (in Chinese)
[24] Yin H, Zhou T, Liu Y, et al. Study on the structure of active phase in NiMoP impregnation solution using Laser Raman spectroscopy II. Effect of organic additives[J]. Journal of Fuel Chemistry and Technology, 2011, 39: 109-114
[25] Cheng W-C, Luthra N P. NMR study of the adsorption of phosphomolybdates on alumina[J]. J Catal, 1988, 109: 163-169
[26] Qiu L, Xu G. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts[J]. Appl Surf Sci, 2010, 256: 3413-3417
[27] Li H, Li M, Chu Y, et al. Effect of different preparation methods of MoO3/Al2O3catalysts on the existing states of Mo species and hydrodesulfurization activity[J]. Fuel, 2014, 116: 168-174
[28] Li H, Li M, Chu Y,et al. The dispersion characteristics of molybdenum and tungsten on alumina surface[J]. Chin J Catal, 2009, 30(2): 165-170 (in Chinese)
[29] Li Sukui, Wang Haiyan, Yan Jingsen. Effect of phosphorus pretreatment on γ-Al2O3and activity of its nickel phosphide hds catalyst[J]. Petroleum Processing and Petrochemicals, 2014, 45(5): 60-65(in Chinese)
CFHL Technology for Hydro-upgrading of Syncrude from Low-Temperature Fischer-Tropsch Synthesis Successfully Applied on the First in China One-Million-Ton-Class Indirect Coal Liquefaction Unit
On August 25, 2015 the CFHL technology for hydroupgrading of syncrude obtained from low-temperature Fischer-Tropsch synthesis had been successfully applied on the first in China 1.0 Mt/a indirect coal liquefaction unit. This achievement is realized by the SINOPEC Research Institute of Petroleum Processing (RIPP) in the area of coal-to-oil production following its development of RCHU technology for hydro-upgrading of syncrude obtained from direct coal liquefaction process. Hence RIPP has been capable of realizing hydro-upgrading of syncrude oil obtained from either direct or indirect coal liquefaction.
The indirect coal liquefaction process (Fischer-Tropsch synthesis for producing syncrude) can convert coal through gasification to syngas followed by transforming syngas to syncrude oil through the Fischer-Tropsch synthesis, resulting in qualified petrochemical products. The products from hydro-upgrading of F-T syncrude oil are characteristic of sulfur-free, nitrogen-free and aromaticsfree liquid, among which the naphtha fraction can be used as the steam cracker feedstock that can produce a total yield of ethylene, propylene and butadiene reaching up to 65% of liquid products, while the diesel fraction features low density and a cetane number equating to over 75. In addition, the hydrotreated products after precise rectification can be separated into a series of high quality solvent oils.
The Shaanxi Future Energy Chemical Company Limited as the owner of the first in China 1.0 Mt/a indirect coalto-oil unit has selected the process design package of CFHL technology developed by RIPP as the basis for design of the syncrude oil hydro-upgrading unit. In order to guarantee the successful startup of the hydro-upgrading unit at the first attempt, RIPP has organized a group of technical personnel to engage in the operation of facilities, while working hard in compiling the startup and operating manual and discussing emergency cases and response measures. After many days of strenuous work the startup team has grappled with success all issues arising in the course of equipment operation.
date: 2015-08-12; Accepted date: 2015-10-19.
Prof. Li Mingfeng, Telephone: +86-10-82368907; Fax: +86-10-62311290; E-mail: limf.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol
