载气对炭/炭复合材料沉积速率、体密度和微观结构的影响
2015-06-05侯振华郝名扬罗瑞盈商海东许怀哲
侯振华, 郝名扬, 罗瑞盈, 向 巧, 杨 威, 商海东, 许怀哲
载气对炭/炭复合材料沉积速率、体密度和微观结构的影响
侯振华, 郝名扬, 罗瑞盈, 向 巧, 杨 威, 商海东, 许怀哲
(北京航空航天大学物理科学与核能工程学院,北京100191)
分别采用H2和CO2作为载气,CH4为前躯体,通过等温化学气相渗积制备炭/炭复合材料,通过偏光显微镜、拉曼光谱、X射线衍射和透射电镜对材料微观结构表征以及渗积过程密度变化,研究载气对沉积速率、体密度和微观结构的影响规律。结果表明:在渗积前50 h,CH4-H2体系的沉积速率明显大于CH4-CO2体系,但在其余渗积时间里,CH4-H2体系的沉积速率小于CH4-CO2体系。当载气从H2变成CO2时,复合材料的体密度从1.626 g/cm3增加到1.723 g/cm3,最大径向密度梯度从0.074 g/cm3减小到0.056 g/cm3。同时,基体炭从纯的粗糙体炭转变为杂化粗糙体炭含有过度生长锥,且平均石墨化度从62.7%下降到50.8%。这些显著的变化是由于CO2的氧化作用降低了表面沉积速率,却没有降低孔内沉积速率,同时大量的缺陷形成于层状石墨烯结构中导致形成过度生长锥,降低了热解炭织构。
炭/炭复合材料;微观结构;化学气相渗积;载气
1 Introduction
It is of prime importance to achieve high bulk densities of carbon/carbon(C/C)composites with rough laminar(RL)pyrolytic carbon(PyC),which is the key point for fabricating C/C braking materials with high mechanical,outstanding thermal and braking properties[1-5].But the high bulk density is frequently limited by blocking of the pore entrances through chemical vapor infiltration(CVI)route.To overcome this problem,the reduction of the infiltration rates on the surface and inhibition an overgrowthof the pore entrances from the outside are recommended[6].Therefore,the new processes,such as temperature and pressure gradients,forced and pulsed flow processes,have been investigated[7,8], but they do not replace the conventional isothermal CVI techniques for mass production of carbon brake disks due to their homogeneous deposition of PyC. Moreover,Zhang[6]have proved that the inside-out infiltration can be obtained by adjusting the processing parameter(e.g.temperature,pressure,residence time and the ratio of C/H).Further,the new carrier gas,H2,has been proposed by Becker et al.[9]because it can inhibit carbon deposition by blocking the free active sites,and they obtained the high bulk density C/C composites in the laboratory[10].Tang et al[11]has employed hydrogen as carrier gas to prepare large size carbon brake disks fabricated by thermal gradient CVI.The average bulk density,the radial density gradient and the texture of PyC with H2as carrier gas were improved compared with N2.
Although numerous works have been devoted to improve traditional CVIefficiency and reduce the processing time,the high bulk density usually comes off second-best.To gain the high bulk density,especially above 1.70 g/cm3,brake disk often needs to remove the surface crusts in the last stage of infiltration.Unfortunately,this is very inefficient and even futile.In this work,a new carrier,CO2,was proposed because it can obviously reduce the surface deposition and do not obviously inhibit the in-pore infiltration,and the effect of the type of carrier gas(H2and CO2)on the densification rate,bulk density and microstructure of the carbon disks fabricated by isothermal CVI was investigated.
2 Experimental
2.1Preparation of C/C composites
In the present work,a quasi three dimensional needled carbon fiber preform was used as a substrate for CVI.The density of the preform was about 0.55 g/cm3.The size of preforms isΦ450-Φ230× 20 mm.Carbon fiber preforms were firstly heat-treated at 2 300℃for 2 h,and infiltrated by isothermal CVI at 1 080-1 130℃with a total pressure of 1-3 kPa.Methane(CH4)was used as the precursor. H2and CO2were used as carrier gases.The ratio of precursor to carrier gas was about 7∶1.All the infiltration experiments were performed stepwise.The density of preforms was tested after each infiltration run of 50 h.The preforms were machined with 300# corundum abrasive papers after an initial infiltration for 50 h in order to measure the bulk volume.The preforms were notmachined to remove the crustin the rest infiltration time in order to obtain the real surface topography and density gradient.Finally,the carbon disks were graphitized at2 300℃for 2 h.
2.2Characterization of C/C composites
Specimens of 20×10×6 mm3were sliced from each sample at different positions along the radialand thickness directions as shown in Fig.1,to evaluate the homogeneity of density.The surface topography of the composites was characterized by scanning electron microscopy(SEM,S-4800).The microstructure of the composites perpendicular to needle punched surface was observed under a polarized light microscope(PLM,Neophot21).Then,the polished surfaces of C/C composites were analyzed by Raman spectroscopyu(LabRAM,HR800),with two laser excitation wavelengths of 514.5 nm and 325 nm. Meanwhile,the powder samples were examined by X-ray diffraction(XRD,D/M-2200)between 15° and 80°(2θ)with monochromatic(40 kV,40 mA) Cu Kαradiation to determined d002-spacing and crystallite size(Lc).Powdered samples of the composites were characterized by transmission electron microscopy(TEM,JEOL2100).
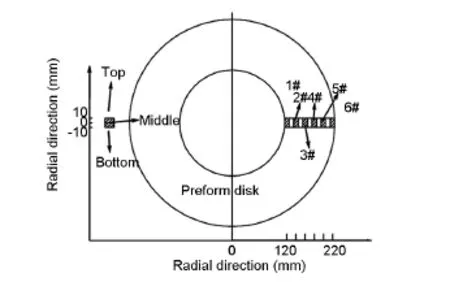
Fig.1 Configuration of specimens sliced from the carbon disk for density measurements.
3 Results and discussion
3.1Effect of carrier gases on densification rate and bulk density
The effect of carrier gases on the densification rate of the carbon disks is shown in Fig.2.In the initial 50 h,the densification rate obtained from CH4-H2is obviously higher than that from CH4-CO2,while the densification rate from CH4-H2is lower than that from CH4-CO2in the rest of infiltration time,especially in the last 200 h.The average bulk density of the carbon disk obtained from CH4-H2(1.626 g/cm3)is obviously lower than that obtained from CH4-CO2(1.723 g/cm3).In comparison to H2, CO2acting as an oxidizing carrier gas,plays a quitedifferent role in CVI.H2can inhibitboth the homogeneous pyrolysis reactions and the heterogeneous deposition reactions.For the gas-solid heterogeneous reaction,on the one hand,CO2favors the CO2+C(PyC) =2CO reaction leading to a reduction of the carbon deposition rate effectively.For the homogeneous pyrolysis reactions,on the other hand,CO2plays an active role in the methane pyrolysis[12].Only a small fraction of reactive species formed by pyrolysis of CH4in the gas phase can be chemisorbed on out surface of the preform under a low ratio of the surface area to the deposition volume([A/V]).A large fraction of the reactive species is removed by the flowing gases.Because of a much high ratio of [A/V]inside the preform,the reaction species formed should immediately be chemisorbed and pyrolyzed into PyC by several complex gas-solid heterogeneous reactions inside pores.Therefore,the gas-gas homogeneous reaction only plays a minor role,and the adsorption and gas-solid surface reactions play a critical role in CVI.
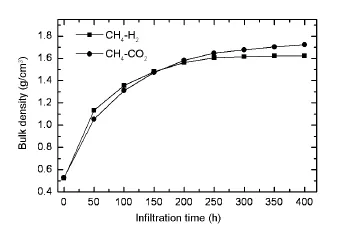
Fig.2 Average bulk densities of the carbon disks obtained from CH4-H2and CH4-CO2as a function of infiltration time.
In the initial stage,the low densification rate from CH4-CO2may resultfrom the oxidizing effect of CO2because the CVI are controlled by chemical reaction.With an increase of the degree of pore filling, diffusion gradually becomes dominant,and the diffusion rate of the reaction species largely determines the densification rate.The diffusion rate of H2is so much high that the inhibition effectof H2willnotobviously decrease.While the oxidizing effect of CO2gradually reduces with increasing depth of pores or decreasing diameter of pores due to the low diffusion rate of CO2and the volume expansion feature of CH4pyrolysis. Thus,the in-pore deposition rate of CH4-CO2will be obviously higher than that of CH4-H2.Moreover,the small amount of CO2even promote pyrolysis of CH4in the pore because of the special pyrolysis feature of CH4,in which the first step is a“third-body enhanced”reaction:CH4+M=CH3+H+M[13].Thus, the high densification rate is obtained from CH4-CO2in the restof infiltration time.
It should be pointed out that the bulk density difference of the carbon disks is mainly resulted from the unfilled pores,not from the intrinsic density of PyC.Moreover,the CVI parameters are optimized for the desired RL PyC in this work.Therefore,it is reliable to conclude that the different densification levels are caused by different carrier gases.The higher average bulk density of the carbon disks from CH4-CO2than that from CH4-H2may result from the higher ratio of the in-pore to the surface deposition rate and the architecture of the preforms.Obviously,the surface deposition rate from CH4-H2should be higher than that from CH4-CO2because the oxidizing effect of CO2is stronger than the inhibition effect of H2. Moreover,the in-pore deposition rate from CH4-CO2is higher than that from CH4-H2,which is mentioned above.Thus,the homogeneous infiltration from outside to inside is obtained easier from CH4-CO2than from CH4-H2.In this work,the needled carbon fiber felts were used as preforms,which is a typical architecture that the macro-pores in the non-woven long carbon fiber cloth and the micro-pores in the short-cut fiber web are alternately superposed in the thickness direction.The diffusion in the thickness directions mainly depends on the macro-pores formed by the needle punching.The blocking of these macro-pore entrances often means the end of the pore infiltration.
The surface topography in the center of carbon disks after 250 h infiltration is given in Fig.3.The crusts obtained from CH4-H2have been formed(Fig. 3(a))and the macro-pores are almost sealed off (Fig.3(b)).However,a large proportion of the surfaces obtained from CH4-CO2are not covered by crusts(Fig.3(c)).Moreover,the macro-pores can maintain permeability,and the inside-out infiltration is observed in the macro-pores(Fig.3(d)).These results further indicate that a high bulk density of the carbon disks is expected from CH4-CO2.The crusts both from CH4-H2and CH4-CO2are formed because the precursor concentration on the surface is apparently higher than that in pores in CVI,and the concentration gradient between surface and pores increases gradually with a reduction of pore diameter.Therefore the blocking ofsmallpore entrances is inevitable.
3.2Effect of carrier gases on bulk density distribution
Fig.4 presents the density distribution of the carbon disks obtained from CH4-H2and CH4-CO2.For the density distribution obtained from CH4-H2(Fig.4(a)),the average bulk density of the carbon disk is 1.626 g/cm3,and the radial density gradient of the bottom,middle and top regions are 0.067, 0.074 and 0.068 g/cm3,respectively.

Fig.3 Surface topography images of the center of carbon discs after 250 h infiltration,(a)and(b)CH4-H2,(c)and(d)CH4-CO2.
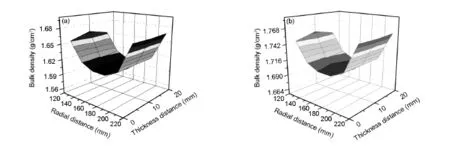
Fig.4 Bulk density distributions of(a)CH4-H2and(b)CH4-CO2.
The thickness direction density gradientof specimens 1#,3#and 5#are 0.002,0.005 and 0.002 g/cm3,respectively.In the radial direction, the small-region density exhibits a minimum with increasing the distance from 120 to 220 mm(from the inner to middle,then to exterior).In the thickness direction,the small-region density exhibits a maximum from the bottom to the top atthe inner and exterior direction of the disk,while the small-region density exhibits a minimum from the bottom to the top near the middle direction of disk.
When CO2is used as a carrier gas,the average bulk density of the carbon disk is 1.723 g/cm3, which is obvious higher than that obtained from CH4-H2.The maximum radial density gradient found at the bottom area is 0.056 g/cm3,which is improved as compared with H2.But the maximum thickness direction density gradient in the inner areas is 0.010 g/cm3,which is higher than that obtained from CH4-H2.The most important of all is that the highest small-region density is always at the middle for both the radialand thickness direction(Fig.4(b)).
In order to better understand the density distribution,the preforms can be approximately divided into 9 zones as shown in Fig.5,in the radial and thickness directions.For infiltration from CH4-H2,2Mregion is the most difficult deposition zone because of the strongly diffusion limited process.The carbon deposition rates of1T,1B,3Tand 3Bzones are expected to be higher than thatof1M,3M,2Tand 2Bdue to the higher concentration gradient of the reaction species and the more surface porosity.Taking the temperature gradientarising from the gas flow during CVI into account,the deposition rate of 3Tand 3Bshould be higher than that of 1Tand 1B.Thus,the density distribution of the carbon disk has the following characteristics:
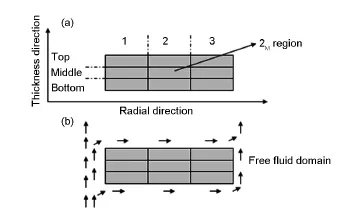
Fig.5 Schematic illustration of(a)regional division and (b)gas flow direction in the free fluid domain during CVI (arrows stand for gas flow direction).
ρ3T≈ρ3B>ρ1T≈ρ1B>ρ3M≈ρ1M>ρ2B>ρ2M(ρrepresents the bulk density of the small-region).When CO2is used as carrier gas,the deposition rate of the PyC on the surface(1B,1M,1T,2T,3T,2B,3B,3Mand 3Tregions)is reduced effectively due to theoxidizing effectof CO2,especially in the 1T,1B,3Tand 3Bzones.This means that the blocking of the surface pores is delayed.As a result,2Mregion is most adequately deposited in a sharp contrastwith CH4-H2, where 2Mis the lowest density zone.Correspondingly,the density of the rest regions is increased due to the higher ratio of the in-pore deposition rate than the surface deposition rate.However,the thickness direction density gradient(1 and 3 zones)is also increased because the most serious oxidation is found in the 1T, 1B,3Tand 3Bzones.
3.3Effect of carrier gases on the microstructureThe radial optical microstructure of the specimens obtained from CH4-H2and CH4-CO2are shown in Fig.6 and 7,respectively.In the case of H2,a pure single RL PyC is observed both in specimen 1# (Fig.6(a))and 3#(Fig.6(b)).When CO2is used as a carrier gas,a complex hybrid RL PyC with overgrowth cones can be observed in the specimens (Fig.7).

Fig.6 Radial distribution of the PyC microstructure obtained from CH4-H2,(a)specimen 1#and(b)specimen 3#.

Fig.7 Radial distribution of the PyC microstructure obtained from CH4-CO2,(a)specimen 1#and(b)specimen 3#.
These indicate that no matter which of these two carrier gases used,the texture distribution of PyC is uniform,but the anisotropy of PyC is apparently different.This can be further confirmed by the results of Raman spectra in Fig.8 and 9.

Fig.8 Raman spectra of the PyC obtained from CH4-H2,(a)specimen 1#and(b)specimen 3#.
Allthe firstorder Raman spectra were fitted with Lorentzian functions for the D and G peaks.It can be observed that the intensity ratios(R=ID/IG)of specimens from CH4-H2is obviously lower that of specimens from CH4-CO2,which suggests that the defects density in the graphene of specimens from CH4-H2is lower than that of specimens from CH4-CO2.

Fig.9 Raman spectra of the PyC obtained from CH4-CO2,(a)specimen 1#and(b)specimen 3#.
As for the specimens from CH4-CO2,two new peaks at 1 140 and 1 450 cm-1are observed,which are either taken as a simple criterion for a nanocrystalline diamond phase in deposited diamond films[14],or considered as the modes originating from trans-polyacetylene in the diamond films[15].To further investigate the state of these two peaks,the UV Raman spectra were used to characterize the hybrid matrix,as shown in Fig.10.

Fig.10 UV Raman spectra of the PyC obtained from CH4-CO2,(a)specimen 1#and(b)specimen 3#.
The UV Raman spectra only show the two features,at approximately 1 395 and 1 580 cm-1,which are labeled as the D and G peaks,respectively.The T peaks at 1 060 cm-1that is due to C—C sp3vibrations does not appear,which further confirms that the distinct Raman peak around 1 140 and 1 450 cm-1is not resulted from a nanocrystalline diamond phase,and indirectly supports the view of the modes originating from trans-polyacetylene.But it is very unthinkable for the existence of trans-polyacetylene in the PyC matrix after the high temperature treatment.Altogether,these two peaks are not clearly known,and they can be attributed to some faulty structures that deteriorate the integrity of the graphitic stacking and enhance the defect density in the graphene layers.These can be further confirmed by the results of the TEM images of the different matrices,as shown in Fig.11.
For the H2-prepared composites,the straight graphene stripes are observed(Fig.11(a)),on the other hand,some ring-like graphene stripes are formed for the CO2-prepared composites(Fig.11(b)),and the nuclei responsible for the formation of the overgrowth cones is observed.Those results indicate that the texture of Pyc is deteriorated by the introduction of CO2in gas phase.This view can be also confirmed by the results of XRD given in Table 1.The d002-spacing is higher and Lcis lower for the specimens obtained from CH4-CO2than those from CH4-H2.
It has been generally recognized that the microstructure of PyC is determined by the composition of the gas phase within the porous preforms.RL PyC arises from a gas with an optimum ratio of small light hydrocarbon(especially acetylene C2)to the aromatic hydrocarbons(especially benzene C6).It has beenreported that H2,as a reaction product,has a direct influence on the reaction kinetics,especially on the formation of benzene from acetylene[9].Thus,a pure single RL PyC is obtained when H2is used as a carrier gas.While CO2,as an oxidizing gas,has a significant effect on the PyC deposition,but a limited effect on the composition of the gas phase within the porous preforms due to the low diffusion rate of CO2.Therefore,the RL PyC is also produced,but defects are formed in the laminar deposit in presence of CO2, starting from nodules around which overgrowth cones are formed according to the model of Coffin[16], where defects are amplified layer after layer with progress of deposition.

Fig.11 TEM images of the specimens,(a)from CH4-H2and(b)from CH4-CO2.
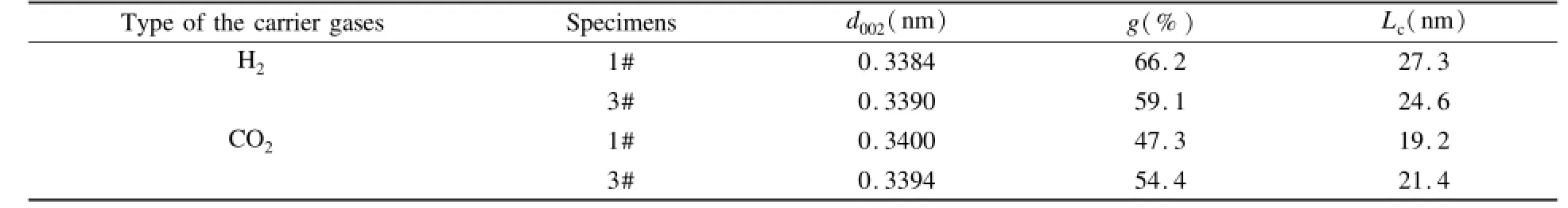
Table 1 Microcrystalline parameters of the specimens sliced from the sample disks from CH4-H2and CH4-CO2.
4 Conclusions
The effects of the type of carrier gases on the densification rate,bulk density and microstructure of C/C composites fabricated by isothermal CVI,have been comprehensively evaluated,and the following conclusions are drawn from the present work:
In the initial 50 h,the densification rate obtained from CH4-H2is obviously higher than that from CH4-CO2,while the densification rate from CH4-H2is lower than that from CH4-CO2with a further increase of infiltration time.The average bulk density of the disk from CH4-CO2(1.723 g/cm3)is higher than that (1.626 g/cm3)from CH4-H2and the maximum radial density gradient is lower for the former (0.056 g/cm3)than that for the latter (0.074 g/cm3).However,the maximum thickness direction density gradient is higher(0.010 g/cm3) for the former than the latter(0.005 g/cm3).These significant changes are caused by the fact that CO2can effectively reduce the surface deposition rate due to the oxidizing effect,but does not inhibit the in-pore infiltration due to its lowe diffusion rate.When the carrier gas is switched from H2to CO2,the matrix is changed from the pure RL to hybrid RL PyC with overgrowth cones,and the average degree of graphitization reduces from 62.7%to 50.8%.These are resulted from the fact that defects are formed in the deposits by introducing CO2in the gas phase and the overgrowth cones deteriorate the texture degree of Pyc.
[1] Delhaes P.Chemical vapor deposition and infiltration processes of carbon materials[J].Carbon,2002,40(5):641-657.
[2] Luo R Y,Liu T,Li J S,et al.Thermophysical properties of carbon/carbon composites and physical mechanism of thermal expansion and thermal conductivity[J].Carbon,2004,42 (14):2887-2895.
[3] SUN Chao,ZHANG Bo,YANG Xiao-guang,et al.Effect of cycle time of in-situ polymerization of naphthalene on the densification and performance of C/C composites[J].New Carbon Materials,2012,27(1):49-54.
(孙 超,张 博,杨晓光,等.原位聚合增密次数对C/C复合材料性能的影响[J].新型炭材料,2012,27(1):49-54.)
[4] Reuge N,Vignoles G L.Modeling of isobaric-isothermalchemical vapor infiltration:effects of reactor control parameters on a densification[J].Journal of Materials Processing Technology, 2005,166(1):15-29.
[5] Ozcan S D,Tezcan J,Filip P.Microstructure and elastic properties of individual components of C/C composites[J].Carbon, 2009,47(15):3403-3414.
[6] Zhang W G,Hüttinger K J.Simulation studies on chemical va-por infiltration of carbon[J].Composites Science and Technology,2002,62(15):1947-1955.
[7] Rovillain D,Trinquecoste M,Bruneton E,et al.Film boiling chemical vapor infiltration:An experimental study on carbon/ carbon composite materials[J].Carbon,2001,39(9):1355-1365.
[8] Dupel P,Bourrat X,Pailler R.Structure of pyrocarbon infiltraed by pulse-CVI[J].Carbon,1995,33(9):1193-1204.
[9] Becker A,Hu Z,Hüttinger K J.A hydrogen inhibition modelof carbon deposition from light hydrocarbons[J].Fuel,2000,79 (13):1573-1580.
[10] Zhang WG,Hu Z J,Hüttinger K J.Chemical vapor infiltration of carbon fiber felt:optimization of densification and carbon microstructure[J].Carbon,2002,40(14):2529-2545.
[11] Tang Z H,Xiong X,Zhang H B.Effect of carrier gas on bulk densiy and microstructure distribution of carbon/carbon composties fabricated by thermal gradient chemical vapor infiltration [J].Carbon,2012,50(3):1243-1252.
[12] Kermiotis Ch,Vourliotakis G,Skevis G,et al.Experimental and computationalstudy of methane mixtures pyrolysis in a flow reactor under atmospheric pressure[J].Energy,2002,43(1): 103-110.
[13] Vignoles G L,Langlais F,Descamps C,et al.CVD and CVI of pyrocarbon from various precursors[J].Surface and Coatings Technology,2004,188-189:241-249.
[14] Ferrari A C.Determination of bonding in diamond-like carbon by Raman spectroscopy[J].Diamond and Related Materials, 2002,11(3-6):1053-1061.
[15] Kuzmany H,Pfeiffer R,Salk N,et al.The mystery of the 1 140 cm-1Raman line in nanocrystalline diamond films[J]. Carbon,2004,42(5-6):911-917.
[16] Coffin L F.Structure-property relations for pyrolytic graphite [J].Journal of the American Ceramic Society,1964,47 (10):473-478.
Effect of carrier gases on densification rate,bulk density and microstructure of carbon/carbon composites
HOU Zhen-hua, HAO Ming-yang, LUO Rui-ying, XIANG Qiao, YANG Wei, SHANG Hai-dong, XU Huai-zhe
(SchoolofPhysicsandNuclearEnergyEngineering,BeijingUniversityofAeronauticsandAstronautics,Beijing100191,China)
Effect of carrier gases(H2and CO2)on the densification rate,bulk density and microstructure of carbon/carbon composites fabricated by isothermalchemical vapor infiltration from methane(CH4)was investigated.In the initial 50 h,the densification rate obtained from CH4-H2is obviously higher than that from CH4-CO2,while the densification rate from CH4-H2is lower than that from CH4-CO2with a further increase of infiltration time.When the carrier gas is switched from H2to CO2,the average bulk density of the compositeincreases from 1.626 to 1.723 g/cm3,the maximum radial density gradient decreases from 0.074 to 0.056 g/cm3,the matrix changes from the pure rough laminar to hybrid rough laminar pyrocarbon with overgrowth cones,and the average degree of graphitization reduces from 62.7%to 50.8%.These significant changes are caused by the fact that CO2can effectively reduce the surface deposition rate but does not inhibit the in-pore infiltration,and thatdefects are formed in the deposits by a CO2introduction in gas phase and the resulting overgrowth cones deteriorate the texture degree of pyrocarbon.
Carbon/carbon composites;Microstructure;Chemical vapor deposition;Carrier gas
LUO Rui-ying,Professor.E-mail:ryluo@buaa.edu.cn
TQ342+.76
A
1007-8827(2015)04-0364-08
国家自然科学基金(21071011).
罗瑞盈,教授.E-mail:ryluo@buaa.edu.cn
侯振华,博士研究生.E-mail:houzhenhualove@126.com
Received date:2015-02-25;Revised date:2015-07-20
Foundation item:National Natural Science Foundation of China(21071011).
Author introduction:HOU Zhen-hua,Ph.D.E-mail:houzhenhualove@126.com
English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).
10.1016/S1872-5805(15)60196-2
