富氮多孔纳米炭纤维的制备及其用作超级电容器电极材料
2015-06-05史景利李亚娟
马 昌, 史景利, 李亚娟, 宋 燕, 刘 朗
富氮多孔纳米炭纤维的制备及其用作超级电容器电极材料
马 昌1, 史景利1, 李亚娟1, 宋 燕2, 刘 朗2
(1.天津工业大学材料科学与工程学院,天津300387; 2.中国科学院山西煤炭化学研究所中国科学院炭材料重点实验室,山西太原030001)
以商业聚酰亚胺树脂为前驱体,经过静电纺丝和一步炭化制备出富含氮原子的纳米炭纤维,采用扫描电镜、低温氮吸附和XPS等手段对纳米炭纤维的结构进行表征,考察不同炭化温度下纳米炭纤维的孔结构与表面含氮官能团的演变。结果显示,所得聚酰亚胺纤维经过一步高温处理便可得到微孔发达且富含氮原子的纳米炭纤维。随着炭化温度的升高,纳米炭纤维的比表面积与氮含量均逐渐降低。700℃炭化得到的纳米炭纤维的比表面积达到447 m2/g、纤维平均直径为234 nm、表面氮含量达到4.1%。将所得纳米炭纤维直接用作超级电容器电极,采用循环伏安法、恒流充放电和交流阻抗对其电化学性能进行考察。所得富氮纳米炭纤维表现出优异的电容量和表面电化学活性,其比电容达到214 F/g,单位比表面的电容量达到0.57 F/m2。
富氮;电容器;纳米炭纤维;多孔炭
1 前言
多孔纳米炭纤维作为新型炭材料家族中重要的一员,不仅具有传统多孔炭的固有特性,例如优异的电子导电性、高的比表面积、良好的化学稳定性和温度稳定性,还具有大比表面效应和量子尺寸效应等纳米材料的特性,同时它还具有一维材料的高机械强度和较好的成型性,因此,它在开发新的纳米复合材料、吸附材料、催化材料和电极材料等研究领域中扮演着重要的角色[1]。
近年来,人们发现将氮原子引入纳米多孔炭会带来一些新的特性,譬如,表面含氮官能团能够增加材料表面润湿性,体内氮原子则能提高材料的电导率,某些具有给电子能力的表面氮则能增加炭表面的电化学活性[2]。因此,含氮多孔炭在电容器、燃料电池和锂离子电池等应用领域显现出出色的电化学性能[3]。将掺氮原子带来的电化学特性和多孔纳米炭纤维特有的结构特性进行结合,制备富氮的纳米炭纤维具有重要的研究意义与应用价值。
目前,制备多孔纳米炭纤维的方法主要有: CVD法、模板法和静电纺丝法[2,4]。其中,CVD法得到纳米炭纤维比表面积低,而模板法则必须经繁复的模板制备与洗涤工序。相比之下,静电纺丝法更易得到高比表面积的纳米炭纤维。目前,采用静电纺丝法制备多孔纳米炭纤维的报道不少,但关于富氮纳米炭纤维的制备以及氮原子与电化学性能的关联研究却并不多。Ra等[5]以聚丙烯腈为前驱体,以静电纺丝法制备了聚丙烯腈纳米纤维,然后经炭化和活化制备富氮的多孔纳米炭纤维,考察了其作为超级电容器的电化学性能。Ma等[6]以三聚氰胺树脂为前驱体,采用静电纺丝工艺制备了富氮多孔的纳米炭纤维。Kim等[7]以聚酰胺酸为前驱体制备多孔炭纤维,却并没有考察氮原子对电化学性能的影响。因此,富氮纳米炭纤维的制备以及氮原子与电化学性能的关联研究还有待进一步开展。
聚酰亚胺树脂是一类含氮芳杂环高分子,其通过简单加热便能实现固化,且残炭率可观。笔者直接以商业化聚酰亚胺树脂作为碳/氮前驱体,采用静电纺丝和一步炭化便制备富含氮原子的多孔纳米炭纤维,主要考察纳米炭纤维的结构,例如表面氮氧官能团和孔结构,对其作为超级电容器电极材料的电化学性能的影响。
2 实验与表征
2.1纳米炭纤维的制备
将商业聚酰亚胺树脂溶液PI-G2525(美国杜邦。溶剂为N,N-二甲基乙酰胺,固含量为20%)以相同的溶剂稀释到15%制得纺丝原液。静电纺丝时,正负极电压为25 kV,距离为18 cm,喷头为直径0.62 mm的不锈钢针头。将初纺纤维在空气中于200℃固化1 h后升至300℃固化1 h,然后在氮气气氛中以3℃/min的升温速率加热到不同的温度下炭化1 h。将炭化纤维记为PICF-T(T代表炭化温度700,800,900℃)。
2.2表征
炭纤维的表面形貌采用扫描电子显微镜(SEM,Hitachi S-4800)进行观察,纤维的直径采用ImageJ软件进行测量,直径分布是基于50根随机纤维的直径统计。纤维的表面化学状态采用X射线光电子能谱仪(XPS,Thermo ESCALAB 250, USA)进行表征;表面积与孔结构是采用ASAP 2020型物理吸附仪(Micromeritics,USA)进行表征,吸附介质为N2,测试温度为77 K。测试前样品均在300℃下脱气10 h。
电化学性能:采用三电极体系进行电化学性能表征,将所得炭纤维薄膜直接压在两片泡沫镍中间作为工作电极,以铂片电极和Hg/HgO电极分别作为对电极和参比电极,以6 mol/L KOH作为电解液。测试前,先将工作电极在电解液中浸渍24 h以保证电解液在电极材料中的充分浸润。以电化学工作站(CHI660C,上海辰华仪器有限公司)进行电极的恒流充放电、循环伏安和交流阻抗测试。其中,交流阻抗测试频率范围为10 kHz至10 mHz,施加的交流信号振幅为5 mV;恒流充放电的电流范围为100 mA/g到10 A/g;循环伏安的扫描速率为10 mV/s。
3 结果与讨论
3.1结构特征
图1(a),(b),(c)是PICFs在不同倍率下的电镜照片。可以看到纤维的形态良好,但纤维薄膜上有少量颗粒,这是未牵伸的液滴形成的。图1(d)是纤维的直径分布图,纤维直径为50-400 nm,平均直径为234 nm。纤维之间并没有明显的粘并现象,说明纺丝过程中溶剂能够从纤维中顺利脱除。
图2(a)为PICFs的脱吸附曲线。所有样品在低压区吸附就达到饱和,显示出典型的微孔特征。吸附等温线在高压区吸附量呈现轻微的下降,这一现象也出现在其他的纯微孔材料中[8-10],这可能是吸附过程微孔结构发生形变或坍塌所致。图2(b)为不同炭化温度下PICFs的孔径分布图,所有样品的孔均集中在0.9 nm和1.1 nm附近,显示典型的微孔分布,没有中孔或大孔。随着温度的变化,孔的尺寸和分布基本没有变化,表明到700℃时碳骨架基本定型。

图1 (a,b,c)PICFs的扫描电镜照片与(d)直径分布直方图Fig.1 (a,b,c)SEM images of PICFs;(d)histogram displays the corresponding fiber diameter distributions.
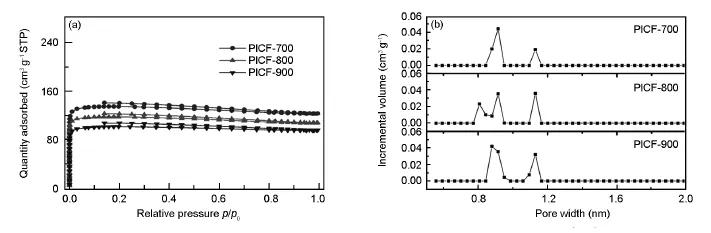
图2 (a)PICFs的脱吸附和(b)孔径分布曲线Fig.2 (a)N2adsorption/desorption isotherms and(b)pore size distributions for PICFs.
表1列出了PICFs的孔结构参数。PICF-700的表面积最大,为447 m2/g,大于目前研究最多的PAN基纳米炭纤维。随着温度的升高,比表面积略下降,碳骨架在更高的温度下出现一定的塌陷会导致一些微孔闭合或孔道融合都可能是表面积下降的原因。表面积的下降幅度并不显著,900℃处理的炭纤维仍具有376 m2/g的比表面积。具有纳米直径的聚酰亚胺炭纤维显示出明显大于树脂基颗粒炭的比表面积[11],这可能与材料的尺寸有关,小尺寸更利于分解气的逸出而形成孔道,大颗粒则易形成闭孔。
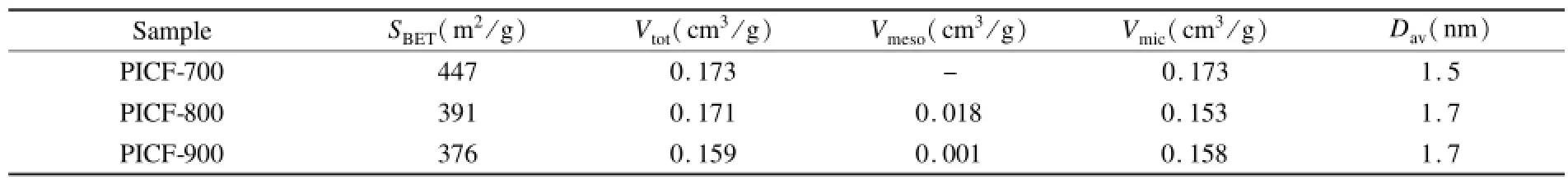
表1 PICFs的孔结构参数Table 1 Texture properties of PICFs.

表2 XPS表面元素组成Table 2 Surface elemental composition derived from XPS.
表2是XPS测试的样品表面元素含量。由表可见所得炭纤维含有丰富的氮原子和氧原子,其中PICF-700含有4.1%的氮和10.57%的氧,可见聚酰亚胺树脂中的氮原子经高温处理得到较好的保留。随炭化温度上升,氮含量下降,氧含量变化不大,表明在高温下氧原子比氮原子具有更高的稳定性。
为了考察表面氮和氧的化学状态,进一步对N1s峰和O1s峰进行拟合。图3为样品N1s峰和O1s峰的拟合谱图,各种官能团含量见表3。

图3 PICFs的XPS谱图:(a)N1s和(b)O1sFig.3 XPS spectra for PICFs:(a)N1s and(b)O1s.
结果显示,表面氮原子有3种存在状态,分别是吡啶型氮(N6,取代边缘六元芳香环中裸露炭的氮原子,(398.7±0.3)eV)、吡咯型氮(N5,取代边缘五元环中裸露炭的氮原子,(400.3±0.3)eV)和四价氮(NQ,取代内部芳香环炭的氮原子,(401.4± 0.3)eV)[12-14]。其中N6和N5占主导。随着炭化温度的升高,N6和N5均不断下降,这是因为它们具有较低的结合能,高温稳定性差。由于具有相对较高的结合能和热稳定性,NQ的原子百分数在高温下得到了提高。表面氧原子则有2种存在状态,即((531.9±0.2)eV和(533.3±0.2)eV),分别对应=-C O(O I)和C-OH/C-O-C(OⅡ)[14,15]。随着炭化温度的升高,两类含氧官能团的含量变化均不大,显示出较高的温度稳定性。研究显示,暴露于表面的官能团,包括N6、N5、O I和OⅡ,因为与水分子具有更高的亲和力而能够润湿表面,加速电解质离子的迁移速度。而具有一定给电子能力的官能团,包括N6、N5和O I,具有一定的电化学反应活性,对于多孔炭电极而言,它们能够提供非常可观的赝电容。研究表明,氮原子具有比氧原子更高的电化学活性[16],尤其在碱性电解质体系中,氮原子能够提供更多的赝电容。从官能团组成看,所得聚酰亚胺基纳米炭纤维所含有的表面官能团大部分是具有电化学活性的,可以期待它们在碱性电解质体系中会有较高的单位表面电容。
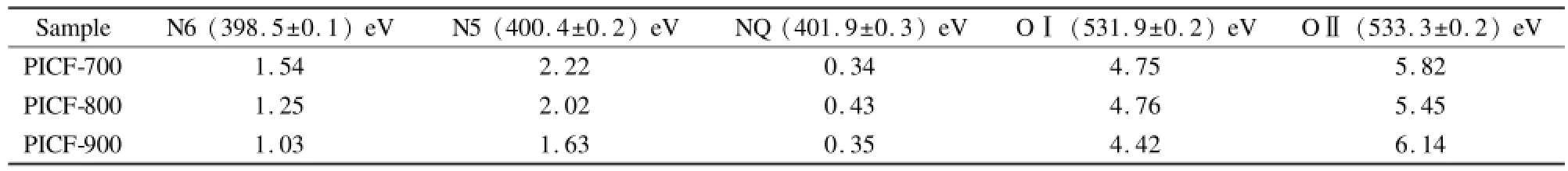
表3 PICFs的XPS表面官能团组成Table 3 Surface functional compositions of PICFs derived from XPS.
3.2电化学性能
图4为样品在100 mA/g下的质量比电容和比表面电容。3个样品虽然比表面积并不高,但具有较高的比电容,分别达到:195 F/g(PICF-700), 196 F/g(PICF-800)和214 F/g(PICF-900)。计算得到3个样品的比表面电容分别为:0.43 F/m2(PICF-700)、0.50 F/m2(PICF-800)和0.57 F/m2(PICF-900),均高于一般的多孔炭[17,18],这主要归因于所得材料高含量的表面活性官能团和特定尺寸的微孔。从XPS结果可知,3个样品均含有丰富的活性官能团,其含量分别达到:3.76%N和4.75% O(PICF-700)、3.27%N和4.76%O(PICF-800)和2.66%N,和4.22%O(PICF-900)。这些活性官能团显著提高了材料的表面电容。另外,最近的研究显示[19],在特殊尺寸的微孔中,离子以去溶剂化的状态进出,不仅使得单位表面的电荷密度更高,还能缩短两极间距,因而能够存储更多的电能。
从孔径分布上看,3个样品的孔径分布基本一致,另外,由于3个样品均来自于相同前驱体,处理温度也相差不大,可以认为它们的孔形状、结构以及深度上相差无异。也就是说,孔径与孔结构对表面电容的贡献基本相同。另外,电化学活性基团对表面电容起着积极作用,即相同条件下,活性官能团越多,单位表面的电容越大。然而,对于所制得的聚酰亚胺树脂基炭纤维,反而是活性基团低的样品比表面电容更高,这显然不合常理。因此,笔者推测表面活性官能团,尤其是纳米孔道表面的官能团,提供的赝电容需要较高的电导率来保证,处理温度越高,基体材料电子传导能力越强,孔道表面的官能团对电容的贡献才能得到最大程度的发挥。图5为3个样品的放电曲线。可以看出,PICF-900的电压降最小,其次是PICF-800,然后是PICF-700。可以看出,更高的处理温度对于电极内阻的降低以及容量的最大化有着显著的影响。对于只提供双电层电容的多孔炭而言,放电曲线为直线。3个样品的放电曲线呈现出不同的弯曲程度。这是表面活性官能团发生氧化还原反应所致。就弯曲程度而言,PICF-700最大,其次是PICF-800,PICF-900最小,体现了表面活性官能团在含量上的差异。表面官能团含量越高,发生的氧化还原反应越多,放电曲线的弯曲程度越明显,这与文献报道结果一致[20]。
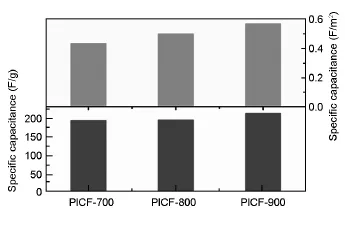
图4 PICFs的比电容和比表面电容Fig.4 Specific capacitance and specific surface capacitance for PICFs.
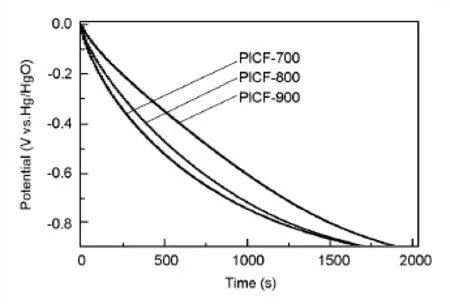
图5 100 mA/g下PICFs的放电曲线Fig.5 Discharging curves of PICFs at100 mA/g.
图6 为比电容保持率与电流密度的关系曲线。由图可知当电流密度增大100倍,即从100 mA/g升为10 A/g时,3个样品的电容保持率分别为: 37%(PICF-700)、43%(PICF-800)和57%(PICF-900)。随着炭化温度升高,样品电容保持率增加。一般来讲,大电流下的电容保持率主要由离子扩散和电子传导决定,3个样品的孔径基本一致,离子的传输环境相差不大,因此可以认为孔道离子扩散速率基本一致。高温处理的样品具有高的电导率,因而,电导率在倍率性能起到了决定性的作用。这一点从循环伏安曲线上也能明显地体现(图7),在电压拐点,PICF-900的电流响应十分迅速,PICF-800和PICF-700则较为缓慢。

图6 PICFs随电流密度增加时的电容保持率Fig.6 Capacitance retention of PICFs at current densities.

图7 PICFs在10 mV/s下的循环伏安曲线Fig.7 CV curves of PICFs at10 mV/s.
为了更清晰地考察电极的阻抗行为,测试了各个电极的交流阻抗图(图8)。高频区的半圆弧反映电极的内阻,包括电极的界面电阻、接触电阻和纤维内部电阻。半圆越大,说明电极内阻越大。由图可见:各个电极的内阻存在较大的差异。相同的制备工艺和测试条件下,电极的界面电阻和接触电阻是相同的,因此半圆的大小主要反映了炭纤维电阻的差异。PICF-900具有最小的电阻,PICF-700和PICF-800次之。图8(b)为电极的Bode曲线,45°相角对应的频率(f0)反映了电极的离子响应频率,在此特征频率,电容器的电阻和电容达到平衡。由图可得,3个电极的响应频率分别为:0.08 Hz(PICF-700),0.12 Hz(PICF-800)和0.89 Hz(PICF-900),根据特征频率计算了电极的时间常数(τ0=1/f0),分别为:12.5 s(PICF-700),8.3 s(PICF-800)和1.1 s(PICF-900)。可见,PICF-900具有最小的时间常数,表明其具有最快的离子响应速度,因此在大电流下,其表面的利用率最高,电容保持率也最高。

图8 PICFs在6 mol/L KOH中的交流阻抗谱图:(a)Nyquist谱图和(b)Bode谱图Fig.8 AC impedance spectra of PICFs in 6 mol/L KOH:(a)Nyquist plot and(b)Bode plot.
4 结论
以聚酰亚胺树脂为前驱体通过电纺法制备出富含氮原子的多孔纳米炭纤维,其氮含量达3.01%~4.1%,氧含量达9.56%~10.56%。该富氮纳米炭纤维为典型的微孔炭,显示出较高的比表面积376~447 m2/g和优异的比电容(195~214 F/g),并表现出优于一般多孔炭的比表面电容(0.43~0.57 F/m2),这主要归因于其丰富的表面活性官能团和特殊的微孔结构。表面官能团能够提供可观的赝电容,特殊尺寸的微孔提供更高的双电层电容,而高的电导率为表面电容的提升创造了条件。所得富含氮多孔纳米炭纤维展示出良好的电化学活性,是一类具有潜力的电容器电极材料。
[1] Dong Z,Kennedy S J,Wu Y.Electrospinning materials for energy-related applications and devices[J].Journal of Power Sources,2011,196(11):4886-4904.
[2] Qie L,Chen W M,Wang Z H,et al.Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability[J].Advanced Materials, 2012,24(15):2047-2050.
[3] Xiaojiao W,Chuanxiang Z,Baolin X.Research progress in the nitrogen‘containing porous carbon electrode materials for supercapacitor[J].Materials Review,2011,25(4):24-32.
[4] Shuang L,Yan S,Chang M,et al.The electrochemical performance of porous carbon nanofibers produced by electrospinning[J].New Carbon Materials,2012,27(2):129-134.
[5] Ra EJ,Raymundo-Piñero E,Lee YH,et al.High power supercapacitors using polyacrylonitrile-based carbon nanofiber paper [J].Carbon,2009,47(13):2984-2992.
[6] Ma C,Song Y,Shi J,et al.Preparation and electrochemical performance of heteroatom-enriched electrospun carbon nanofibers from melamine formaldehyde resin[J].Journal of Colloid and Interface Science,2013,395(0):217-223.
[7] Kim C,Choi YO,Lee WJ,et al.Supercapacitor performances of activated carbon fiber webs prepared by electrospinning of PMDA-ODA poly(amic acid)solutions[J].Electrochimica Acta, 2004,50(2-3):883-887.
[8] Guo P,Gu Y,Lei Z,etal.Preparation of sucrose-based microporous carbons and their application as electrode materials for supercapacitors[J].Microporous and Mesoporous Materials, 2012,156(0):176-180.
[9] Wang H,Gao Q,Hu J,et al.High performance of nanoporous carbon in cryogenic hydrogen storage and electrochemical capacitance[J].Carbon,2009,47(9):2259-2268.
[10] Xie J,Wang X,Deng J.Modifying the pore structure of Pit-ACF with the chemical vapor deposition of methane and propylene[J].Microporous and Mesoporous Materials,2004,76(1-3):167-175.
[11] Shuo Z,Cheng-Yang W,Ming-Ming C,et al.Preparation and electrochemical performance of polyimide derived activated carbon[J].Jounal of Inorganicl Materials,2008,23(5):923-927.
[12] Kim YJ,Abe Y,Yanagiura T,et al.Easy preparation of nitrogen-enriched carbon materials from peptides of silk fibroins and their use to produce a high volumetric energy density in supercapacitors[J].Carbon,2007,45(10):2116-2125.
[13] Raymundo-Piñero E,Cazorla-Amorós D,Linares-Solano A,et al.Structural characterization of N-containing activated carbon fibers prepared from a low softening pointpetroleum pitch and a melamine resin[J].Carbon,2002,40(4):597-608.
[14] Seredych M,Hulicova-Jurcakova D,Lu G Q,et al.Surface func-tional groups of carbons and the effects of their chemicalcharacter, density and accessibility to ions on electrochemical performance [J].Carbon,2008,46(11):1475-1488.
[15] Zhou J H,Sui Z J,Zhu J,et al.Characterization of surface oxygen complexes on carbon nanofibers by TPD,XPS and FT-IR[J].Carbon,2007,45(4):785-796.
[16] Kwon T,Nishihara H,Itoi H,et al.Enhancement mechanism of electrochemical capacitance in nitrogen-/Boron-doped carbons with uniform straight nanochannels[J].Langmuir,2009, 25(19):11961-11968.
[17] Zhu Y,Hu H,Li W,et al.Resorcinol-formaldehyde based porous carbon as an electrode material for supercapacitors[J].Carbon,2007,45(1):160-165.
[18] Qu D.Studies of the activated carbons used in double-layer supercapacitors[J].Journal of Power Sources,2002,109(2): 403-411.
[19] Chmiola J,Yushin G,Gogotsi Y,et al.Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer[J].Science,2006,313(5794):1760-1763.
[20] Zhao L,Fan LZ,Zhou MQ,et al.Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors [J].Advanced Materials,2010,22(45):5202-5206 .
Instructions to Authors
NewCarbonMaterialsis a bimonthly journal published with the permission of the Ministry of Science and Technology and of the State News and Publication Agency.The journal is sponsored by the Institute of Coal Chemistry,Chinese Academy of Sciences,and is published by Science Press. Aims and Scope
NewCarbonMaterialspublishes research devoted to the physics,chemistry and technology of those organic substances that are precursors for producing aromatically or tetrahedrally bonded carbonaceous solids,and of the materials that may be produced from those organic precursors.These materials range from diamond and graphite through chars,semicokes,mesophase substances,carbons,carbon fibers,carbynes,fullerenes and carbon nanotubes,etc.Papers on the secondary production of new carbon and composites materials(for instance,carbon-carbon composites)from the above mentioned various carbons are also within the scope of the journal.Papers on organic substances willbe considered if research has some relation to the resulting carbon materials.
Manuscript Requirements
1.NewCarbonMaterialsaccepts Research Paper,Short Communication and Review.The number of words in each Research Paper should be less than 6000 words.Short Communication<3500 words.There is no maxium of words for Review.
2.Manuscript including an abstract,graphical abstract,highlight,keywords,reference list,original figures and captions,tables.Manuscripts can be written both in Chinese and English.
3.Manuscript should be accompanied with key words placed after Abstract and a short resume of first author(name,academic degree,professional position)placed in the end of 1st page of text as foot-note.Corresponding author and his(her)E-mail address should also be mentioned.
4.All illustrations,photographs,figures and tables should be on separate sheets,figure captions should be typed separately,notincluded on the diagram.Authors are requested to submit original photographs,which should have good contrast and intensity.
5.References should be individually numbered in the order in which they are cited in the text,and listed in numerical sequence on separate sheets at the end of the paper,typed in double spacing.Remember that"unpublished works"are not references!In the reference list,periodicals[1],books [2],multi-author books with editors[3],proceedings[4],patents[5],and thesis[6]should be cited in accordance with the following examples:
[1] Mordkovich V Z,Baxendale M,Yoshimura S,et al.Intercalation into nanotubes.Carbon,1996,34(10):1301-1303.
[2] Lovell D R.Carbon and High-Performance Fibers Directory.5th ed.,London:Chapman&Hall,1991:66.
[3] Mochida I,Korai Y.Chemical characterization and preparation of the carbonaceous mesophase.In:Bacha J D,Newman J W,White J L, eds.Petroleum-Derived Carbons.Washington DC:ACS,1986,29-31.
[4] Su J,Li G,Hao Z.The research and application of copper impregnated coarse-grain graphite throat.23rd Int'l Biennial Conference on Carbon,Extended Abstract and Program,July 18-23,Pennsylvania 1997,256-258.
[5] Shigeki T,Jinichi M,Hiroshi H.Manufacture of mesocarbon microbeads.JP 61-222913,1986.
[6] Jones L E.The Effect of Boron on Carbon Fiber Microstructure and Reactivity.Ph.D.Thesis.Penn State University,University Partk, PA 1987.
Note:For the references with more than three authors,please give the first three and mark"et al".
6.Publication of papers in the journal is free of charge.Authors whose paper is published in the journal will receive 10 free offprints and 2 copy of this journal soon after its coming out.
7.Manuscript Submission:Online submission:http://xxtcl.sxicc.ac.cn/EN/volumn/home.shtml
Preparation of nitrogen-enriched porous carbon nanofibers and their electrochemical performance as electrode materials of supercapacitors
MA Chang1, SHI Jing-li1, LI Ya-juan1, SONG Yan2, LIU Lang2
(1.SchoolofMaterialsScienceandEngineering,TianjinPolytechnicUniversity,Tianjin300387,China;2.KeyLaboratoryofCarbonMaterials,InstituteofCoalChemistry,ChineseAcademyofSciences,Taiyuan030001,China)
Nitrogen-enriched porous carbon nanofibers were prepared from a commercial polyimide resin by electrospinning,followed by carbonization.The products were characterized by scanning electron microscopy,nitrogen sorption and X-ray photoelectron spectroscopy.As-prepared carbon nanofibers were directly used as a supercapacitor electrode,and their electrochemical performance was investigated by cyclic voltammetry,charge-discharge tests and electrochemical impedance spectroscopy.The evolution of the porous structure and the surface nitrogen-containing functionality of the carbon nanofibers with carbonization temperature was also investigated.Results showed the carbon nanofibers with developed micropores and enriched with nitrogen were obtained by carbonization of polyimide nanofibers.Both the specific surface area and surface nitrogen content decreased gradually with the carbonization temperature.The carbon nanofibers obtained at700℃had the highest specific surface area of 447 m2/g,a fiber diameter of 234 nm and a nitrogen content of 4.1%.They also exhibited a specific capacity of 214 F/g or 0.57 F/m2.
Nitrogen-enriched;Supercapacitor;Carbon nanofibers;Porous carbon
SHI Jing-li.E-mail:shijingli1963@163.com;SONG Yan.E-mail:songyan1026@126.com
TQ127.1+1
A
2014-12-30;
:2015-07-05
山西省自然科学基金(2012011219-3).
史景利.E-mail:shijingli1963@163.com;宋 燕.E-mail:songyan1026@126.com
马 昌,博士,讲师.E-mail:fdoy_lt54@163.com
1007-8827(2015)04-0295-07
Foundation item:National Natural Science Foundation of Shanxi Province,China(2012011219-3).
Author introduction:MA Chang,Ph.D.,Lecturer.E-mail:fdoy_lt54@163.com
