Developments of mucus penetrating nanoparticles
2015-05-16MinLiu,JianZhang,WeiShan等
Developments of mucus penetrating nanoparticles
ARTICLEINFO
Article history∶
Received 6 December 2014
Accepted 31 December 2014
Available online 16 February 2015
∶
Mucus can effectively protect the exposed mucosal surfaces due to its adhesive and viscoelastic properties.Most foreign particulates are ef fi ciently trapped in mucus layers via steric obstruction and adhesion.Trapped particles are typically removed from the mucosal tissue within seconds to a few hours depending on their location sites.This article focuses on describing the tenacious mucus barrier properties,the strategies to investigate the interaction of nanoparticles with the mucus as well as the novel developments of mucus penetrating nanoparticles.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1. Introduction
Nanocarriers have emerged as an effective strategy for mucosa delivery of drugs,which possess a series of desirable properties,including small steric obstruction due to their nanometer size,and protection of cargo therapeutics at both the extracellular and intracellular levels[1].However,one of the greatest challenges that limit the success of nanoparticles (NPs)is their ability to penetrate quickly through mucus to reach the underlying cells.
Mucus is a viscoelastic and adhesive hydrogel that covers in the surface of lung airways,gastrointestinal(GI)tract,female reproductive tracts,eye and other mucosa[2].Mucus protects underlying epithelium by ef fi ciently trapping pathogens and foreign particulates,then rapidly removing them.Therefore, mucus is not only vital for human health,but also represents a substantial barrier to mucosal drug delivery.Mucus forms adhesive interactions with particulates via electrostaticinteractions,van derWaalsforces,hydrophobicforces, hydrogen bonding,and chain entanglement[3,4].Mucoadhesive NPs is to prolong the retention timeof particles in mucosal surface by maximize these interactions[5],which would undergo either direct transit or elimination.Different mucoadhesive systems have been well reviewed previously[6,7].
Another strategy to overcome the mucus barrier and achieve longer retention time in cell surface is to develop a nanocarrier which can effectively penetrate the mucus layer and accumulate in epithelial surface.Justin Hanes and coworkers fi rst proposed mucus penetrating particles(MPP)by mimicking the essential surface properties of viruses that allow them to avoid mucoadhesion[5],showing great promise in mucosal drug delivery.Thereby,the aim of present study is to summarize the properties of mucus,approaches for designing NPs to conquer the mucus barrier as well as the strategies used to investigate the interactions between mucus and NPs.
2. Barrier of mucus layer
Mucus is a viscoelastic,adhesive gel that coats and protects most epithelial surfaces,which ef fi ciently trap most foreign particles and pathogens through adhesive and steric interactions,followed by rapid clearance.Fig.1 illustrated the fate of foreign particulates,including penetrating through mucus(A),trapping in mucus(B)and excluding by mucus(C) [8].The following will elaborate the reasons resulting in various fates of foreign particulates.
2.1. Composition of mucus
Mucus is a hydrogel complex composed of carbohydrates, protein,lipids,antibody,cellular debris bacteria and inorganic salts[9].The barrier properties of mucus are rooted in its dense network of mucin fi bers,which contain highly glycosylated(negatively charged)segments[5],thus show high affi nity with positively charged particles.For example,Laf fl eur F et al.reported that the diffusion rate of neutral polyacrylic acid(PAA)-polypropylene amide(PAM)nanoparticles(NPs)is 2.5-fold higher than positively charged PAM NPs[8].Similar phenomenon also has been reported by other groups that NPs with positively charged surface can be trapped in mucus effectively own to strong electrostatic interactions[10].
Additionally,there exist periodic hydrophobic domains along the mucin strains[11],which can bind hydrophobic particles with high avidity.Although hydrophobic interactions effectively limit the transport of some harmful agents such as bacteria[12,13],italso represents a challenge for the delivery of drug carriers,since the commonly used biomaterials are hydrophobic,like poly(lactic-co-glycolic acid)(PLGA)[14,15]and polystyrene(PS)[16].However,after coating PLGA NPs with hydrophilic DNA,the average transport rates can be improved 10-fold in reconstituted pig gastric mucus[17].
2.2. Viscoelasticity
The viscoelasticity of mucus are essential for its protective properties.As reported,a rather moderate decrease of viscoelasticity can signi fi cantly promote bacterialand sperm motility[11].Mucin,as the main component of mucus,directly affects the viscoelastic properties of mucus. A series studies have shown that the mucins can be changed in amount,type and size in disease[18-20].For instance,compared with healthy secretions,the mucin concentration increased approximately 7-fold for patient with asthma,further increasing the dif fi culty of mucosal drug delivery[21].Apart from mucin,other factors also play a key role in regulating mucus viscoelasticity,including lipids,inorganic salts,pH and cellular debris.The cell debris DNA can further increase the viscoelasticity of mucus due to its fi bers are even longer than mucin fi bers[18].Besides, highly acidic environments(pH<4)would cause the aggregation of mucin fi bers and greatly increase the mucus viscoelasticity[22].Therefore,to ensure free diffusion of NPs in mucus,large amounts of mucus mucolytic agents can be adopted to reduce the viscoelasticity of mucus (Fig.2),including papain[23,24],recombinant human DNase (rhDNase)[10,25],N-acetyl-L-cysteine(NAC)[26]and guluronate oligomers[27].
2.3. Steric obstruction
Mucus gel is composed of highly cross-linked mucin fi bers by hydrophobic interactions and disul fi des link,creating a dense porous structure.However,mucus displays different pore size depending on its location on the body,such as,the average pore size of human cervicovaginal mucus(CVM)is 340±70 nm[28]and 550±50 nm for fresh bovine vitreous [29],while smaller mesh spacing inherent to cystic fi brosis (CF)sputum(140±50 nm)own to the higher concentrations of mucins,DNA,and actin[30].Thereby,to penetrate mucus,nanocarriersmustbesmallenoughtoavoid steric obstruction in spite of NPs with larger size are preferred to improve drug loading and release kinetics[5]. As reported,a 2-fold increase in particle size,from 510 nm to 1190 nm for PS-PEG NP,would led to a 30-fold decrease in the ensemble-averaged mean squared displacement[29]. Italsohasbeen reported thatthenanospheressize approaching 560 nm were almost completely blocked by the sputum[25].Similarly,Norris and Sinko studied the diffusion of variously sized PS particles in reconstituted porcine gastric mucin gel,and observed a sharp decrease in translocation permeability when particle sizes reach 300 nm[31].
2.4. Dynamic properties
Mucus is constantly secreted,subsequently shed and discarded or digested and recycled.Its turnover time is short, especiallyforthelooselyadherentmucuslayer,often measured in minutes to hours.For oral drug delivery,the intestinal mucus turnover time is 50-270 min[32],resulting in ef fi cient clearance of administered particulates.
In conclusion,the understanding of mucus compositions and properties is important to design nanocarriers which can avoid the blockagein mucus,meanwhile penetratemucusat a rate signi fi cantly higher than mucus turnover cycle.
3. MPP by modi fi cation their surface physicochemical properties
NPs properties including charge and hydrophobicity have a great in fl uence on their behavior of penetrating through mucus.As a consequence,to prepare NPs with a suf fi ciently hydrophilic and uncharged surface to effectively minimize the adhesive interactions betweenmucin and NPs by reducing hydrophobicorelectrostatic interactions,show promise prospect on mucus penetrating.
3.1. PEG-modi fi ed NPs
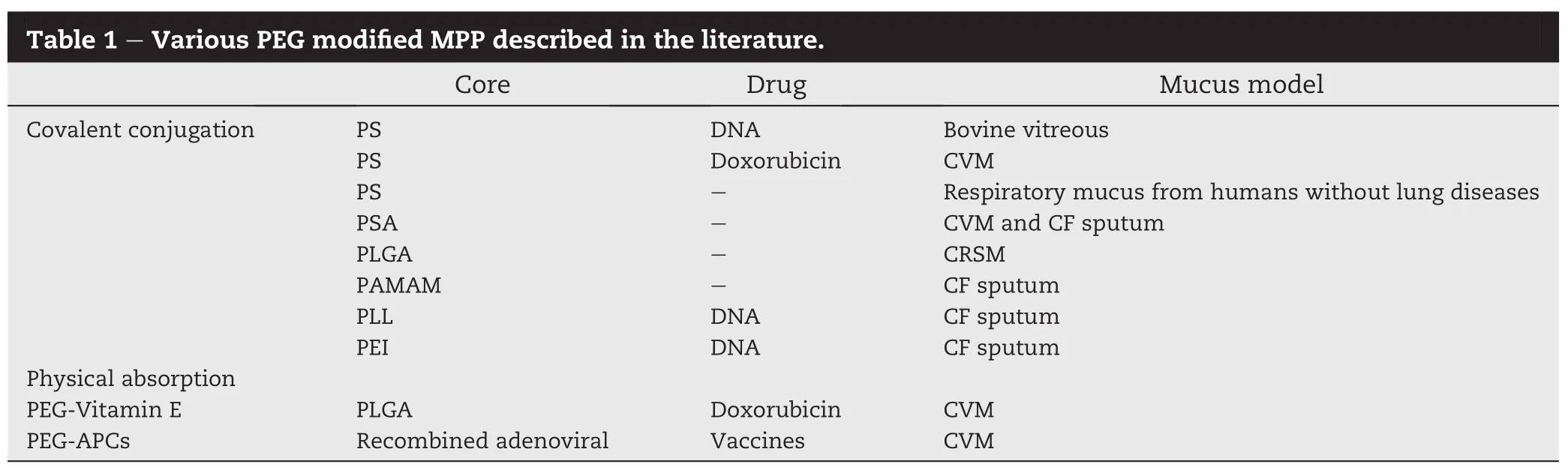
Coating NPs with low molecular weight(MW)PEG is the most widely studied mucus penetrating strategy(Fig.3A)[33].There are various PEG modi fi ed MPP described in the literature (Table 1).PEG is an uncharged hydrophilic polymer which was used to increase the mucus adhesion in earlier studies[34,35]. While later researches have shown that coating NPs with a high density of low MW PEG can reduce the interactions between particle and mucus.The possible reasons are as followings:the MW of PEG was too low to support adhesion via polymer chains interpenetration and the PEG density is suffi cient to shield the hydrophobic core effectively[36].To determine the effect of PEG MW on the interactions of coated particles with mucus,Hanes et al.studied the diffusion rate of PS NPs modi fi ed by different MW(2,5 and 10 kDa)and densities(42±3%,65±1%and 69±1%)of PEG in CVM.The experimental results showed that low MW(e.g.2 kDa)and high-density(e.g.65-70%)PEG coating can facilitate the NPs to pass through mucus[36].Apart from modi fi ed PS NPs,PEG also can be used to conjugate with other polymeric materials to prepare MPP,like PLGA[37],poly sebacic acid(PSA)[38], polyethylenimine (PEI)[39]and poly-L-lysine(PLL)[40]. Furthermore,densely PEGylated particles are able to readily penetrate chronic rhinosinusitis mucus(CRSM)samples and sputum expectorated from the cystic fi brosis(CF)patients with higher viscosity[37].
In addition to covalent conjugation of PEG to the particles core,PEG also can be physically absorbing on the particle surface by hydrophobic or electrostatic interactions[41,42]. This kind of NPs has similar mucus penetrating properties as long as their surface is densely coated by low MW PEG.
3.2. Pluronic F-127 modi fi ed NPs
Triblock copolymer of poly(ethylene glycol)-poly(propylene oxide)-poly(ethylene glycol)(PEG-PPO-PEG;known as Pluronics)has a long application history in oral,intravenous and ophthalmic administration,which can coat hydrophobic particle surfaces by adsorption of the hydrophobic PPO segments,leaving a dense brush of uncharged,hydrophilic PEG segments protruding from the particle surface[43](Fig.3B). Studies have shown that Pluronics containing PPO segments with MW>3 kDa,such as F-127,can produce MPP.Coating PLGA nanoparticles with Pluronic F127 can effectively block adhesive interactions between the PLGA core and mucus constituents,which are similar to covalent conjugation of PEG toPLGA,simultaneously without changingthestructureofthe PLGA carrier materials[44].Other studies also indicated that the average speed for PLGA/F127 particles was only 20-fold reduced compared to their theoretical speed in water(uncoated PLGA particles were slowed by>1000-fold)[37].Similarly,X.Li et al.designed a core shell corona nanolipoparticles (CSC)which contains chitosan NPs as a core component and pluronic F127-lipid vesicles as a shell with hydrophilic chain and polyethylene oxide PEO as a corona for oral protein delivery.CSC can further improve the absorption of drug through enhanced intestinal mucus penetration[45].Additionally,otherstudycomparedthemucuspenetrating behavior of Pluronic F127-modi fi ed liposomes(PF127-Lip)and chitosan-modi fi ed liposomes(CS-Lip).Pharmacokinetic analysis in rats shows that the Cmaxand AUC0-tof F127-Lip were 1.73-fold and 1.84-fold higher than those of CS-Lip,respectively.This indicated F127-Lip more suitable for drug nanocarriers[46].Besides,it is interesting to found that PS NPs can rapidly penetrate CVM if the CVM is pretreated with suf fi cient concentrations of F127[47].
However,it needs to be noticing that not all hydrophilic and neutral modi fi cation can facilitate mucus penetration. Like hydrophilic and uncharged polyvinyl alcohol coated PS NPs were immobilized,with speeds at least 4000-fold lower in mucus than in water,regardless of the MW or incubation concentration of PVA[48].
4. MPP by disrupting the mucus barrier
Due to the tenacious and sticky network of mucin fi bers,the diffusion of foreign particles is restricted by trapping and steric hindrance.According to the nature of mucus,a technique to reach the underlying cell layer is presented by disrupting the mucoglycoprotein substructures using mucolytic agents[49] (Fig.3C).For example,Mu¨ller C and coworkers prepared NPs composed of polyacrylic acid(PAA)and papain.The presence of papain on the surface and inside of the particles could strongly decrease viscosity of the mucus thus leading to particle transition across the mucus layer quickly[23,24].
In addition,recombinant human DNase(rhDNase)is the most commonly used mucolytic in CF,which can hydrolyzes the DNA that forms dense entanglements with mucin glycoproteins and other mucus constituents,thus reducing the number of viscoelasticity and crosslinks of mucus[50]. Sanders et al.observed rhDNase moderately facilitated the transport of nanospheres through CF sputum[25].However, Dawson et al.found that treatment with rhDNase dramatically narrowed the distribution of individual particle diffusion rates and reduced macroviscoelastic properties of CF sputum by up to 50%,but did not signi fi cantly alter the ensembleaverage particle diffusion rate.This might attribute to the hydrolytic cleavage of DNA into smaller fragments which diffuse freely into micropores and then increased the microviscosity within the pores[10].
N-acetyl-L-cysteine(NAC)is another common mucolytic for its ability to facilitate the penetration of NPs across CF sputum.NAC disrupts the structure of the mucus polymer by substituting free thiol(sulfhydryl)groups for the disul fi de bonds connecting with mucin proteins[26].As a result,both the elasticity and viscosity of the mucus are lowered.Alton et al.found that the mucus barrier to non-viral gene vectors can be overcome partially by treatment with NAC in an ex vivo model of sheep tracheal epithelium[51].However,it is still need further investigation to clarify if the combination of polymeric NPs with mucolytic agents will promote more endotoxins and other toxic substances absorption by decreasing the local viscosity of mucus layer.
5. Research strategies of mucus penetration
5.1. Diffusion experiments
Methods used to study particle diffusion in mucus include multiple particle tracking(MPT),Ussing chamber or Transwell-Snapwelldiffusion chamberand so on.Transwell-Snapwelldiffusion chamberwas fi rstused for quantitatively measuring the diffusivity of particles in mucus, which consists of two chambers with the placement of the mucus layer in the middle.Although this method is conceptually straightforward,it is also sensitive to parameters that are dif fi cult to control,including the thickness of the mucussample,alterations in mucus properties and blockage of fi lter pores by mucus[52].
In order to avoid the defects in diffusion chamber experiments,many studies recorded the NPs dynamic transit in the mucus using fl uorescence microscopy,such as fl uorescence recovery after photobleaching(FRAP)and multiple particle tracking(MPT).FRAP is the fl uorescently labeled NPs exposure to a laser beam to form a fl oating white spot.The diffusion coef fi cient is obtained by the recovery of the fl uorescence intensity,which results following diffusion of the fl uorescently labeled molecules into this area with the fl ow of NPs[53].Shen etal.applied FRAPtoexamine the diffusion ofplasmid DNAsin mucus[54].Additionally,FRAP also has been used to investigate the effect of guluronate oligomers on mobility of NPs in mucous matrices,and results showed that guluronate oligomers can improve NPs mobility in native pig gastric mucus (Fig.4A and B)[27].FRAPcan beused to investigate the mobility of labeled molecules in mucus and biogels,but it provides only ensemble-averaged diffusion rates and cannot be used to quantify the transport rates of individual particles.A lack of information at the individual particle level may limit its application in complex mucus[55].For this purpose,Hanes et al.have pioneered MPT to measure the transport of NPs in mucus.As shown in Fig.4C and D[56],it can record each particletrajectoriesbyinverted fl uorescencemicroscope meanwhile MPT can be used for analysis of NPs in some complex biological fl uids,such as sputum[57].Apart from this, the diffusion behavior of NPs in the mucus also can be studied by rotating diffusion tubes[58]and mucus slices[59]etc.
5.2. Cell models
5.2.1. HT29-MTX cell model
HT29 cells belong to human colonic adenocarcinoma cell line. Under the in fl uence of methotrexate(MTX)[60],HT29 differentiated into mature goblet cells,such as E12 cells,which can secrete mucus.Therefore,HT29-MTX cells can be used to study thein fl uenceofmucuslayeronNPstransport[61].However,this modelhassomedrawbackscomparedtotheinvivosituationdue to it is based on only one cell type of the intestinal epithelium.
5.2.2. Caco-2/HT29-MTX co-cultures cell model
Human colon carcinoma Caco-2 and HT29 cells were established to represent the two most abundant cell populations in the intestinal epithelium,absorptive cells and goblet cells. Therefore,co-cultures of Caco-2 cells and mucus-producing goblet cells HT29-MTX would provide a drug absorption model incorporating the mucus barrier[62].Wilkman-Larhead fi rst characterized co-cultures of Caco-2 and goblet-like HT29-MTX cells as in vitro drug and peptide absorption models[63]. In general,a higher amount of Caco-2 cells leads to higher transepithelial electrical resistance(TEER)values probably due to more intensely formed tight junctions.When HT29 cell ratio is 25%,the change of TEER value is 0-790 Ω·cm2in 0-23 days. whentheratiois75%,theTEERvaluedowngradeto 0-310 Ω·cm2.Goblet cells comprise a quantitatively signi fi cant component of the GI tract,comprising approximately 10%of the duodenal epithelium and increasing to 24%in the distal colon.Thus,to maintain better in vivo/in vitro correlationrelevance,the co-cultures proportion of Caco-2/HT29-MTX is 90%/10%or 75%/25%mostly[64,65].
5.2.3. Caco-2/HT29-MTX/Raji B triple culture model
Apart from enterocytes and goblet cells,M cells located in the epithelium that overlay the Peyer's patches also play a dominant role.Several reportssuggest that NPs are capable to enter intestinal epithelia via M cells while uptake by absorptive enterocytes only plays a minor role[66,67].In order to design a model which can mimic the small intestinal epithelial more accurately,some studies establish an in vitro cellular model based on Caco-2,mucus-producing HT29-MTX,and human Burkitt's lymphoma Raji B cells which represent M cells.The model was set up by seeding co-cultures of Caco-2 and HT29 cells into Transwell fi lters and maintained under identical conditions following the addition of Raji B to the basolateral chamber[68,69].
In addition,there were also studies designed and characterized biosimilar mucus compatible with Caco-2 cell monolayers cultured in vitro to establish a more representative in vitro model for the intestinal mucosa to predict the in fl uence of mucus on intestinal drug absorption[70].
5.3. Animal models
To better understand the fate of the particles and how the results might translate in humans,many studies adopt animal models to investigate the therapeutic effects or pharmacokinetics of NPs,which mainly include isolated intestinal experiments,in situ experiments and in vivo experiments.
5.3.1. Isolated intestinal model
To avoid the shortcomings of cell monolayer model such as lack of three-dimensional macrostructure and cells of varying degrees of differentiation,some studies adopted isolated intestinal experiments(including everted intestinal sac and permeability study by Ussing chamber[71])to measure the mucoadhesive properties of NPs.However,in such model,the intestinalneed toberemoved,opened,washed and segmented,which may change the intestinal property and failed to predict what occurred in vivo[72].
5.3.2. In situ model
Intestinal loop models have been used for decades to investigate systemic absorption of drugs.In this model,a portion of the small intestine is excised from the abdominal cavity, subsequently ligated at both ends to make an isolated“loop”, and the test NPs is directly injected into the loop.After a chosen time period,the animal is sacri fi ced and the intestinal loop is removed from the body cavity for further morphology or quantitative analysis[73].Several studies have used this modelto investigate thein fl uenceofmucusonNPsabsorption and the amounts of NPs trapped in mucus[45,46].
5.3.3. In vivo model
No matter how sophisticated an in vitro model,eventually in vivo evaluation is necessary to validate the true performance of a drug delivery system.For example,the signi fi cant difference existed in mucus composition and thickness with the position of the GI tract,it is dif fi cult to simulate these in vitro experiments.However,a shortcoming existed in all models so far is their non-human nature,which shows great difference in human studies[72].
6. Conclusions
Mucus layer covering in exposed epithelial surfaces of the body has vital protective and lubrication effect.However,the adhesive and rapidly update properties of mucus is one of the main barriers for mucosal drug delivery.A promising strategy to tacklethisproblemisuse ofMPPwhichcanreadilyin fi ltrate into the mucus layer before turnover occurring.While some problems for MPP including the epithelial barrier,the security of MPP and suitable mucus models still need to be further investigated.
Acknowledgement
We gratefully acknowledge fi nancial support from the National Natural Science Foundation of China(81173010).
REFERENCES
[1]Panyam J,Labhasetwar V.Biodegradable nanoparticles for drug and gene delivery to cells and tissue.Adv Drug Deliv Rev 2003;55:329-347.
[2]Ensign LM,Schneider C,Suk JS,et al.Mucus penetrating nanoparticles:biophysical tool and method of drug and gene delivery.Adv Mater 2012;24:3887-3894.
[3]Ponchel G,Irache J.Speci fi c and non-speci fi c bioadhesive particulate systems for oral delivery to the gastrointestinal tract.Adv Drug Deliv Rev 1998;34:191-219.
[4]Woodley J.Bioadhesion:new possibilities for drug administration?Clin Pharmacokinet 2001;40:77-84.
[5]Lai SK,Wang YY,Hanes J.Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues.Adv Drug Deliv Rev 2009;61:158-171.
[6]Sosnik A,das Neves J,Sarmento B.Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes:a review.Prog Polym Sci 2014;39:2030-2075.
[7]Takeuchi H,Yamamoto H,Kawashima Y.Mucoadhesive nanoparticulate systems for peptide drug delivery.Adv Drug Deliv Rev 2001;47:39-54.
[8]Laf fl eur F,Hintzen F,Shahnaz G,et al.Development and in vitro evaluation of slippery nanoparticles for enhanced diffusion through native mucus.Nanomedicine(Lond) 2014;9:387-396.
[9]Ensign LM,Cone R,Hanes J.Oral drug delivery with polymeric nanoparticles:the gastrointestinal mucus barriers.Adv Drug Deliv Rev 2012;64:557-570.
[10]Dawson M,Wirtz D,Hanes J.Enhanced viscoelasticity of human cystic fi brotic sputum correlates with increasing microheterogeneity in particle transport.J Biol Chem 2003;278:50393-50401.
[11]Cone RA.Barrier properties of mucus.Adv Drug Deliv Rev 2009;61:75-85.
[12]Sajjan U,Reisman J,Doig P,et al.Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucinand respiratory mucin from patients with cystic fi brosis.J Clin Invest 1992;89:657-665.
[13]Drumm B,Neumann AW,Policova Z,et al.Bacterial cell surface hydrophobicity properties in the mediation of in vitro adhesion by the rabbit enteric pathogen Escherichia coli strain RDEC-1.J Clin Invest 1989;84:1588-1594.
[14]Sellers DL,Kim TH,Mount CW,et al.Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion.Biomaterials 2014;35:8895-8902.
[15]Zheng F,Wang S,Wen S,et al.Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly(lactic-co-glycolic acid)composite nano fi bers.Biomaterials 2013;34:1402-1412.
[16]Lunov O,Syrovets T,Loos C,et al.Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line.ACS Nano 2011;5:1657-1669.
[17]Dawson M,Krauland E,Wirtz D,et al.Transport of polymeric nanoparticle gene carriers in gastric mucus.Biotechnol Prog 2004;20:851-857.
[18]Thornton DJ,Sheehan JK.From mucins to mucus:toward a more coherent understanding of this essential barrier.Proc Am Thorac Soc 2004;1:54-61.
[19]Thornton DJ,Davies JR,Kraayenbrink M,et al.Mucus glycoproteins from'normal'human tracheobronchial secretion.Biochem J 1990;265:179-186.
[20]Thornton DJ,Sheehan JK,Lindgren H,et al.Mucus glycoproteins from cystic fi brotic sputum.Macromolecular properties and structural‘architecture’.Biochem J 1991;276(Pt 3):667-675.
[21]Sheehan JK,Richardson PS,Fung DC,et al.Analysis of respiratory mucus glycoproteins in asthma:a detailed study from a patient who died in status asthmaticus.Am J Respir Cell Mol Biol 1995;13:748-756.
[22]Hong Z,Chasan B,Bansil R,et al.Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules 2005;6:3458-3466.
[23]Muller C,Perera G,Konig V,et al.Development and in vivo evaluation of papain-functionalized nanoparticles.Eur J Pharm Biopharm 2014;87:125-131.
[24]Mu¨ller C,Leithner K,Hauptstein S,et al.Preparation and characterization of mucus-penetrating papain/poly(acrylic acid)nanoparticles for oral drug delivery applications.J Nanopart Res 2012;15:1-13.
[25]Sanders NN,De Smedt SC,Van Rompaey E,et al.Cystic fi brosis sputum:a barrier to the transport of nanospheres. Am J Respir Crit Care Med 2000;162:1905-1911.
[26]Henke MO,Ratjen F.Mucolytics in cystic fi brosis.Paediatr Respir Rev 2007;8:24-29.
[27]Nordgard CT,Nonstad U,Olderoy MO,et al.Alterations in mucus barrier function and matrix structure induced by guluronate oligomers.Biomacromolecules 2014;15:2294-2300.
[28]Lai SK,Wang YY,Hida K,et al.Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses.Proc Natl Acad Sci U S A 2010;107:598-603.
[29]Xu Q,Boylan NJ,Suk JS,et al.Nanoparticle diffusion in,and microrheology of,the bovine vitreous ex vivo.J Control Release 2013;167:76-84.
[30]Suk JS,Lai SK,Wang YY,et al.The penetration of fresh undiluted sputum expectorated by cystic fi brosis patients by non-adhesive polymer nanoparticles.Biomaterials 2009;30:2591-2597.
[31]Norris DA,Sinko PJ.Effect of size,surface charge,and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin.J Appl Polym Sci 1997;63:1481-1492.
[32]Lehr C-M,Poelma FGJ,Junginger HE,et al.An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop.Int J Pharm 1991;70:235-240.
[33]Nance E,Zhang C,Shih TY,et al.Brain-penetrating nanoparticles improve Paclitaxel ef fi cacy in malignant glioma following local administration.ACS Nano 2014;8:10655-10664.
[34]Huang Y,Leobandung W,Foss A,et al.Molecular aspects of muco-and bioadhesion:tethered structures and site-speci fi c surfaces.J Control Release 2000;65:63-71.
[35]Bures P,Huang Y,Oral E,et al.Surface modi fi cations and molecular imprinting of polymers in medical and pharmaceutical applications.J Control Release 2001;72:25-33.
[36]Wang YY,Lai SK,Suk JS,et al.Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that“slip”through the human mucus barrier.Angew Chem Int Ed Engl 2008;47:9726-9729.
[37]Lai SK,Suk JS,Pace A,et al.Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials 2011;32:6285-6290.
[38]Tang BC,Dawson M,Lai SK,et al.Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier.Proc Natl Acad Sci U S A 2009;106:19268-19273.
[39]Suk JS,Kim AJ,Trehan K,et al.Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier.J Control Release 2014;178:8-17.
[40]Boylan NJ,Suk JS,Lai SK,et al.Highly compacted DNA nanoparticles with low MW PEG coatings:in vitro,ex vivo and in vivo evaluation.J Control Release 2012;157:72-79.
[41]Mert O,Lai SK,Ensign L,et al.A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles.J Control Release 2012;157:455-460.
[42]Xie Z,Ji Z,Zhang Z,et al.Adenoviral vectors coated with cationic PEG derivatives for intravaginal vaccination against HIV-1.Biomaterials 2014;35:7896-7908.
[43]Illum L,Davis SS.The organ uptake of intravenously administered colloidal particles can be altered using a nonionic surfactant(Poloxamer 338).FEBS Lett 1984;167:79-82.
[44]Yang M,Lai SK,Wang YY,et al.Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus.Angew Chem Int Ed Engl 2011;50:2597-2600.
[45]Li X,Guo S,Zhu C,et al.Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles.Biomaterials 2013;34:9678-9687.
[46]Chen D,Xia D,Li X,et al.Comparative study of Pluronic((R)) F127-modi fi ed liposomes and chitosan-modi fi ed liposomes for mucus penetration and oral absorption of cyclosporine A in rats.Int J Pharm 2013;449:1-9.
[47]Ensign LM,Lai SK,Wang YY,et al.Pretreatment of human cervicovaginal mucus with pluronic F127 Enhances nanoparticle penetration without compromising mucus barrier properties to Herpes Simplex Virus. Biomacromolecules 2014;15:4403-4409.
[48]Yang M,Lai SK,Yu T,et al.Nanoparticle penetration of human cervicovaginal mucus:the effect of polyvinyl alcohol. J Control Release 2014;192:202-208.
[49]Bell AE,Sellers LA,Allen A,et al.Properties of gastric and duodenal mucus:effect of proteolysis,disul fi de reduction, bile,acid,ethanol,and hypertonicity on mucus gel structure. Gastroenterology 1985;88:269-280.
[50]Shah PL,Scott SF,Knight RA,et al.In vivo effects of recombinant human DNase I on sputum in patients with cystic fi brosis.Thorax 1996;51:119-125.
[51]Ferrari S,Kitson C,Farley R,et al.Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene Ther 2001;8:1380-1386.
[52]Saltzman WM,Radomsky ML,Whaley KJ,et al.Antibody diffusion in human cervical mucus.Biophys J 1994;66:508-515.
[53]Groo AC,Lagarce F.Mucus models to evaluate nanomedicines for diffusion.Drug Discov Today 2014;19:1097-1108.
[54]Shen H,Hu Y,Saltzman WM.DNA diffusion in mucus:effect of size,topology of DNAs,and transfection reagents.Biophys J 2006;91:639-644.
[55]Suh J,Dawson M,Hanes J.Real-time multiple-particle tracking:applications to drug and gene delivery.Adv Drug Deliv Rev 2005;57:63-78.
[56]Zagato E,Forier K,Martens T,et al.Single-particle tracking for studying nanomaterial dynamics:applications and fundamentals in drug delivery.Nanomedicine(Lond) 2014;9:913-927.
[57]Suk JS,Suh J,Lai SK,et al.Quantifying the intracellular transport of viral and nonviral gene vectors in primary neurons.Exp Biol Med(Maywood)2007;232:461-469.
[58]Dunnhaupt S,Barthelmes J,Hombach J,et al.Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int J Pharm 2011;408:191-199.
[59]Gradauer K,Barthelmes J,Vonach C,et al.Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J Control Release 2013;172:872-878.
[60]Lesuf fl eur T,Barbat A,Dussaulx E,et al.Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells.Cancer Res 1990;50:6334-6343.
[61]Behrens I,Stenberg P,Artursson P,et al.Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells.Pharm Res 2001;18:1138-1145.
[62]Mahler GJ,Shuler ML,Glahn RP.Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability.J Nutr Biochem 2009;20:494-502.
[63]Wikman-Larhed A,Artursson P.Co-cultures of human intestinal goblet(HT29-H)and absorptive(Caco-2)cells for studies of drug and peptide absorption.Eur J Pharm Sci 1995;3:171-183.
[64]Walter E,Janich S,Roessler BJ,et al.HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: in vitro-in vivo correlation with permeability data from rats and humans.J Pharm Sci 1996;85:1070-1076.
[65]Hilgendorf C,Spahn-Langguth H,Regardh CG,et al.Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion,inside-and outside-directed carrier-mediated transport.J Pharm Sci 2000;89:63-75.
[66]Jepson MA,Clark MA,Hirst BH.M cell targeting by lectins:a strategy for mucosal vaccination and drug delivery.Adv Drug Deliv Rev 2004;56:511-525.
[67]des Rieux A,Ragnarsson EG,Gullberg E,et al.Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium.Eur J Pharm Sci 2005;25:455-465.
[68]Schimpel C,Teubl B,Absenger M,et al.Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles.Mol Pharm 2014;11:808-818.
[69]Antunes F,Andrade F,Araujo F,et al.Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs.Eur J Pharm Biopharm 2013;83:427-435.
[70]Boegh M,Baldursdottir SG,Mullertz A,et al.Property pro fi ling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur J Pharm Biopharm 2014;87:227-235.
[71]Fan T,Chen C,Guo H,et al.Design and evaluation of solid lipid nanoparticles modi fi ed with peptide ligand for oral delivery of protein drugs.Eur J Pharm Biopharm 2014;88:518-528.
[72]Gamboa JM,Leong KW.In vitro and in vivo models for the study of oral delivery of nanoparticles.Adv Drug Deliv Rev 2013;65:800-810.
[73]Jin Y,Song Y,Zhu X,et al.Goblet cell-targeting nanoparticles for oral insulin delivery and the in fl uence of mucus on insulin transport.Biomaterials 2012;33:1573-1582.
Min Liu,Jian Zhang,Wei Shan,Yuan Huang*
Key Laboratory of Drug Targeting and Drug Delivery System,Ministry of Education,West China School of Pharmacy, Sichuan University,No.17,Block 3,Southern Renmin Road,Chengdu 610041,China
*Corresponding author.West China School of Pharmacy,Sichuan University,Chengdu,Sichuan 610041,China.Tel./fax:+86 028 85501617. E-mail address:huangyuan0@163.com(Y.Huang).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.12.007
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Mucus barrier
Mucus penetrating nanoparticles Mucus properties
Experimental strategies
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Overview of milling techniques for improving the solubility of poorly water-soluble drugs
- Technical crystallization for application in pharmaceutical material engineering:Review article
- Hot melt extrusion:An industrially feasible approach for casting orodispersible fi lm
- Dendritic macromolecules as nano-scale drug carriers:Phase solubility,in vitro drug release, hemolysis and cytotoxicity study
- Effect of formulation variables on in vitro release of a water-soluble drug from chitosan-sodium alginate matrix tablets
- Design and development of novel bioadhesive niosomal formulation for the transcorneal delivery of anti-infective agent:In-vitro and ex-vivo investigations
