Two new Energetic Ionic Salts with Environmental Protection: Preparation and Thermal Properties of IMI·TNR and 4-AT·TNR
2015-05-10LIYingBIYangangZHAOWenyuanGUOWeimingZHANGTonglai
LI Ying, BI Yan-gang, ZHAO Wen-yuan, GUO Wei-ming, ZHANG Tong-lai
(State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China)
1 Introduction
The high energy density materials (HEDMs), especially those materials with excellent performance and environmental compatibility, have been concerned[1-8]. In which, five-azole heterocycles and their derivatives are desired due to their high nitrogen content, enthalpy of formation, density, easily achieved oxygen balance[9-16], among which salts and complexes based on IMI and 4-AT (IMI=imidazolium, 4-AT=4-amino-1,2,4-triazolium) are well researched.
On the other hand, styphnate (2,4,6-trinitro resorcinol, TNR), is the main ingredient of a famous traditional primary explosives lead styphnate, which is utilized as primary explosive, and contribute to an environment in both military and civilian fields. Although energetic styphnate salts may exhibit comparative excellent performance in their designed complexes or salts with PA[17-21], the studies on them are rarely mentioned, and the reports are focused on energetic nitrate, perchlorate or azide salts.
In this contribution, two energetic materials IMI·TNR and 4-AT·TNR based on styphnate (TNR=2,4,6-trinitro resorcinol) (Scheme 1) were obtained and characterized by X-ray diffraction analysis. Both materials were fully characterized by elemental analysis, FT-IR spectroscopy, and their thermal effects, sensitivities and performances were gained.
2 Experimental
2.1 Materials and Physical Techniques
All the reagents and solvents were of analytical grade and used without further purification as commercially obtained.
Elemental analyses were performed on a Flash EA 1112 full-automatic trace element analyzer. The FT-IR spectra were recorded on a Bruker Equinox 55 infrared spectrometer (KBr pellets) in the range of 4000-400 cm-1with a resolution of 4 cm-1. DSC and TG measurements were carried out by using a Pyris-1 differential scanning calorimeter and a Pyris-1 thermogravimetric analyzer (Perkin Elmer, USA) under dry nitrogen as atmosphere with flowing rate of 20 mL·min-1. The energy of combustion was measured by an oxygen bomb calorimeter (Parr 6200, USA).
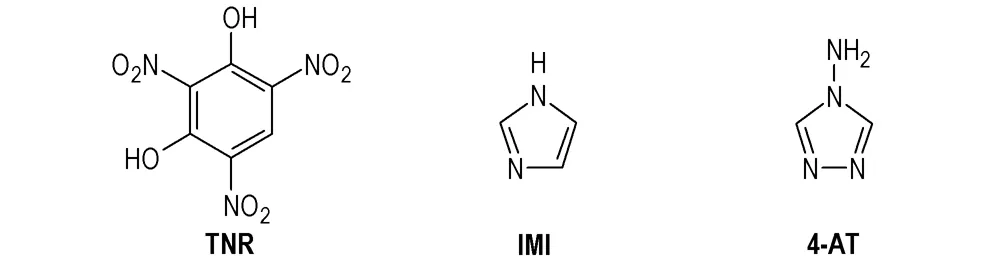
Scheme 1 Structural formulas of TNR, IMI, 4-AT
Impact sensitivity was determinedwith a Fall Hammer Apparatus. Salt (30 mg) was placed between two steel poles and was hit by a 5.0 kg drop hammer.
Friction sensitivity was determined on a MGY-1 pendularfriction sensitivity apparatus by a standard procedure using 20 mg of the sample. When salt was compressed between two steel poles with mirror surfaces at the pressure of 3.92 MPa, and then was hit horizontally with a 1.5 kg hammer fell from 90° angle.
Flame sensitivity was determined by following a standard method, in which the sample was ignited by standard black powder pellet. Salt (20 mg) was compacted to a copper cap under the press of 58.8 MPa and was ignited by standard black powder pellet.
2.2 Synthesis of the compounds
As shown in Scheme 2, the IMI·TNR(1), 4-AT·TNR(2) were synthesized by the reactions between the appropriate free bases and styphnate acid in water with 1∶1 molar quantities.

Scheme 2 Synthesis of the salts of IMI·TNR (1), 4-AT·TNR (2)
IMI(0.14 g, 2 mmol) and TNR (0.49 g, 2 mmol) were dissolved in 30 mL H2O and stirred for 30 min at 70 ℃. The suspension was stirred for 1 h and filtrated immediately into a cup. The synthesis conditions of 4-AT·TNR are basically the same, but only change IMI to 4-AT in the same mole ratio, two kinds of yellow crystals would be obtained after 1d with yield of 75% and 70%, respectively. IR for IMI·TNR (KBr,ν/cm-1): 3421, 2601, 1632, 1533, 1473, 1415, 1266, 1184, 1102, 904, 840, 790, 705, 630. Anal. calcd for IMI·TNR: C 34.50, N 22.36, H 2.24; found: C 34.42, N 22.29, H 2.31. IR for 4-AT·TNR (KBr,ν/cm-1): 3363, 3139, 2684, 1633, 1574, 1529, 1455, 1380, 1340, 1288, 1187, 1086, 930, 833, 717, 617. Anal. calcd for 4-AT·TNR: C 29.18, N 29.79, H 2.13; found: C 29.11, N 29.69, H 2.19.
2.3 X-ray Crystallography
The crystal data of IMI·TNR(1), 4-AT·TNR(2) were collected with a Bruker Smart CCD diffractometer with graphite monochromatic Mo Kαradiation (λ=0.71073Å) at 294(2) K usingφandωscan modes. Their structures were determined and refined by direct methods using SHELXS-97[22]and SHELXL-97[23]programs. All hydrogen atoms were located from difference Fourier electron-density maps and refined isotropically, while all non-hydrogen atoms were obtained from the difference Fourier map and refined anisotropically. The results concerning crystallographic data collection and structure refinements are given in Table 1.
CCDC-951714 and CCDC-951715 contain the supplementary crystallographic data for the title compound (1) and (2), and these data can be acquired free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data- request/cif (or through the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-Mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
3 Results and Discussion
3.1 Molecular Structures
Fig.1 shows themolecular structure and packing diagram of IMI·TNR and 4-AT·TNR, respectively. The selected bond lengths and angles are listed in Table 2, and the hydrogen bond lengths and bond angles of IMI·TNR and 4-AT·TNR in Tables 3 and 4, respectively.
IMI·TNR crystallizes in a monoclinic cell,which belongs to space groupP21/cwith cell parameters ofa=6.006(1)Å,b=13.170(3)Å andc=14.816(4)Å. For 4-AT·TNR, it is triclinic, space groupP-1 with a density of 1.772 g·cm-3and cell parameters ofa=8.157(2)Å,b=8.2047(19) Å andc=10.159(3) Å.
Table 1 Crystal data and structure refinements for IMI·TNR and 4-AT·TNR

compoundIMI·TNR4-AT·TNRCCDCNo.951714951715formulaC9H7N5O8C8H7N7O8formulamass/g·mol-1313.20329.21crystalsystemmonoclinictriclinicspacegroupP21/cP-1crystalsize/mm0.33×0.32×0.290.53×0.53×0.19Z42a/Å6.006(1)8.157(2)b/Å13.170(3)8.2047(19)c/Å14.816(4)10.159(3)α/(°)-78.844(9)β/(°)93.818(4)89.602(11)γ/(°)-68.005(7)volume/Å31169.4(5)616.9(3)ρc/g·cm-31.7791.772μ(MoKα)/mm-10.1590.160F(000)640.0336.0θ/(°)6.32-58.265.4-58.22reflectioncollected/unique10192/30917561/3230R1,wR2[I>2σ(I)]0.0412/0.10690.0451/0.1237R1,wR2(alldata)0.0522/0.11560.0564/0.1334GOFonF21.0010.999largestdiff.peakandhole/e·Å-30.32/-0.230.76/-0.27
In IMI anions, the C—N bond lengths range from 1.321(2) Å[N(1)—C(3)] to 1.375(2)Å[N(1)—C(1)] with an average value of 1.351 Å, which is longer than the normal CN bond length (1.270Å) and shorter than the normal C—N bond length (1.450 Å). In 4-AT anions, the C—N bond lengths range from 1.307(2) Å[N(1)—C(1)] to 1.362(2) Å[N(3)—C(1)] with an average value of 1.328Å, which is longer than the normal CN bond length (1.270 Å) and shorter than the normal C—N bond length (1.450 Å)[24]. There are two N—N bond[N(1)—N(2), 1.369(2) Å and N(3)—C(4), 1.412(2)Å], longer than the normal NN bond length of 1.252 Å and shorter than the normal N—N bond length of 1.470 Å.[24]
In IMI·TNR molecule, there is only one ionic bond between every IMI anion and TNR cation. Plane of the imidazole ring and the phenyl ring are not in one plane but parallel substantially to each other (Angle between the two planes is 1.696(59)°). Conversely, in 4-AT·TNR the benzene and triazole ring lie in different planes, which are angulated by 75.212(56)° towards each other.
As shown in Fig.1c, each TNR anion within the crystal structure is surrounded by five TNR anions linked by hydrogen bonds to oxygen atoms on the phenolic hydroxyl and nitro and some van der Waals forces. The hydrogen bonds′ length of the crystal structure are from 2.5684 Å to 3.4641 Å, only one strong hydrogen bonds connected O4, which results in a smaller crystal density,Dc=1.779 g·cm-3. In Fig.1d, each 4-AT anion connected with three TNR cations through four hydrogen bonds[N(2)—H(2)N…O(4), N(2)—H(2)N…O(5), N(4)—H(4)B…O(3), N(4)—H(4B)…O(7)]. The hydrogen bonds′ lengths of the crystal structure are from 2.5843 Å to 3.3896 Å.
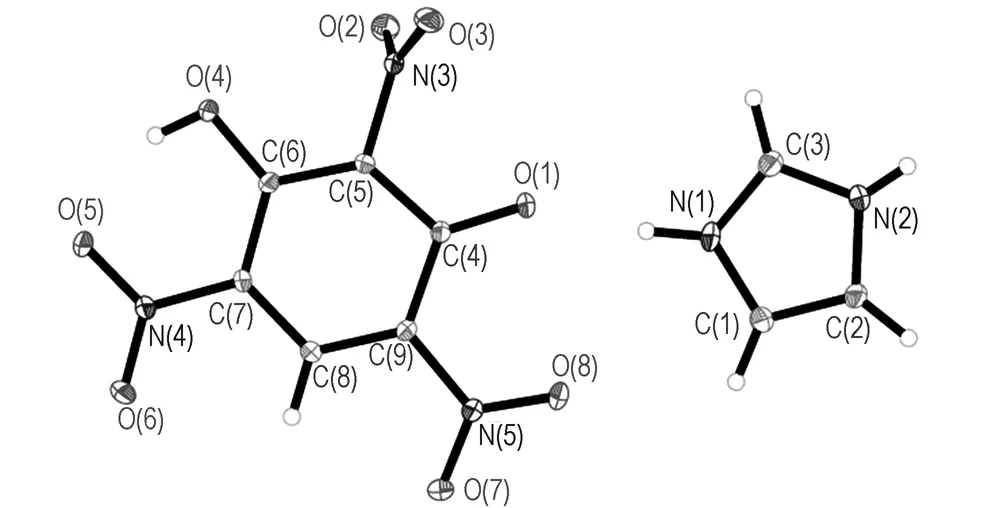
a. molecular structure of IMI·TNR

b. molecular structure of 4-AT·TNR

c. packing diagram of IMI·TNR

d. packing diagram of 4-AT·TNR′s
Fig.1 Molecular structure and packing diagram of IMI·TNR and 4-AT·TNR
Table 2 Selected bond lengths and bond angles
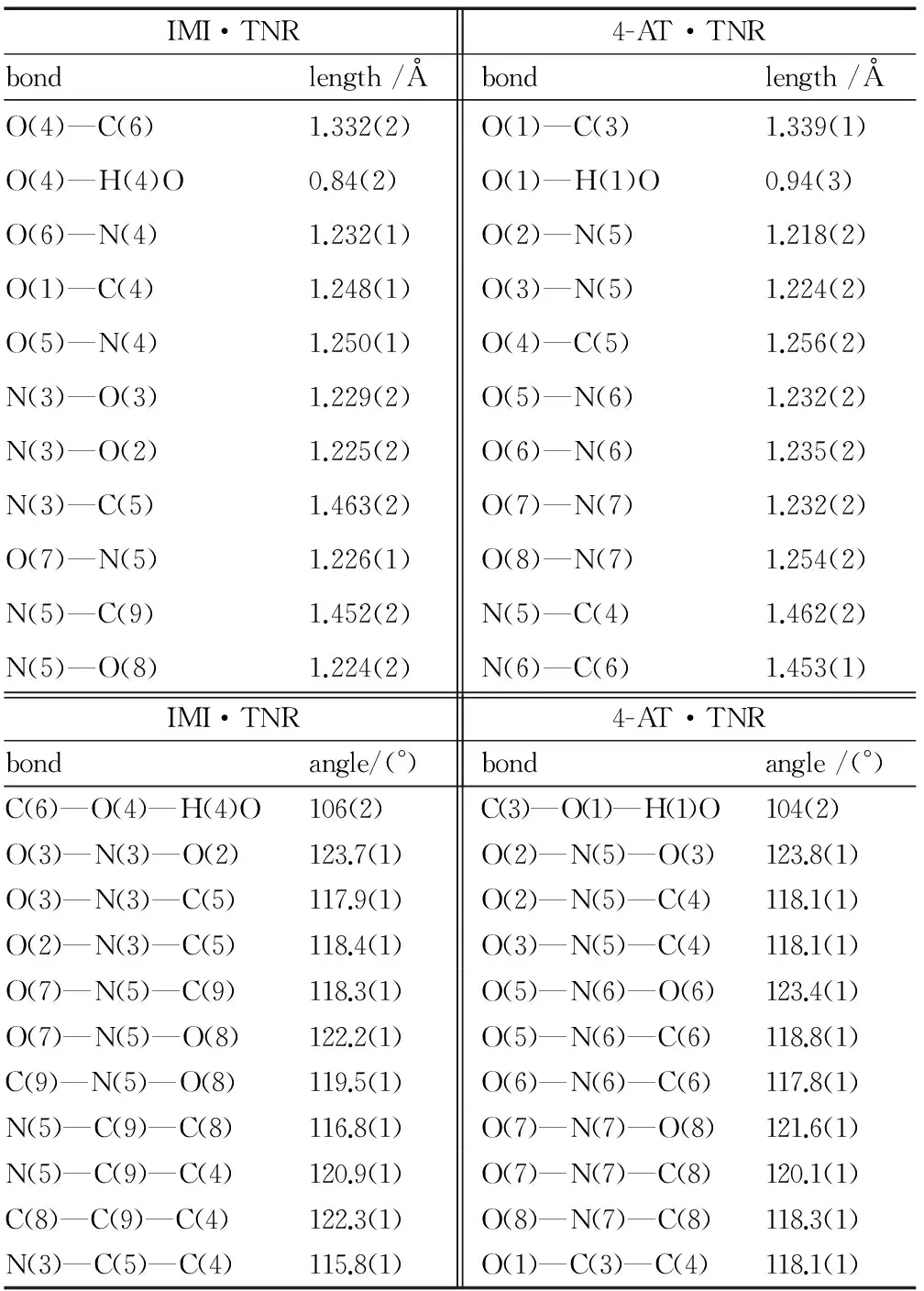
IMI·TNRbondlength/Å4-AT·TNRbondlength/ÅO(4)—C(6)1.332(2)O(1)—C(3)1.339(1)O(4)—H(4)O0.84(2)O(1)—H(1)O0.94(3)O(6)—N(4)1.232(1)O(2)—N(5)1.218(2)O(1)—C(4)1.248(1)O(3)—N(5)1.224(2)O(5)—N(4)1.250(1)O(4)—C(5)1.256(2)N(3)—O(3)1.229(2)O(5)—N(6)1.232(2)N(3)—O(2)1.225(2)O(6)—N(6)1.235(2)N(3)—C(5)1.463(2)O(7)—N(7)1.232(2)O(7)—N(5)1.226(1)O(8)—N(7)1.254(2)N(5)—C(9)1.452(2)N(5)—C(4)1.462(2)N(5)—O(8)1.224(2)N(6)—C(6)1.453(1)IMI·TNRbondangle/(°)4-AT·TNRbondangle/(°)C(6)—O(4)—H(4)O106(2)C(3)—O(1)—H(1)O104(2)O(3)—N(3)—O(2)123.7(1)O(2)—N(5)—O(3)123.8(1)O(3)—N(3)—C(5)117.9(1)O(2)—N(5)—C(4)118.1(1)O(2)—N(3)—C(5)118.4(1)O(3)—N(5)—C(4)118.1(1)O(7)—N(5)—C(9)118.3(1)O(5)—N(6)—O(6)123.4(1)O(7)—N(5)—O(8)122.2(1)O(5)—N(6)—C(6)118.8(1)C(9)—N(5)—O(8)119.5(1)O(6)—N(6)—C(6)117.8(1)N(5)—C(9)—C(8)116.8(1)O(7)—N(7)—O(8)121.6(1)N(5)—C(9)—C(4)120.9(1)O(7)—N(7)—C(8)120.1(1)C(8)—C(9)—C(4)122.3(1)O(8)—N(7)—C(8)118.3(1)N(3)—C(5)—C(4)115.8(1)O(1)—C(3)—C(4)118.1(1)
Table 3 Hydrogen bond lengths and bond angles for IMI·TNR
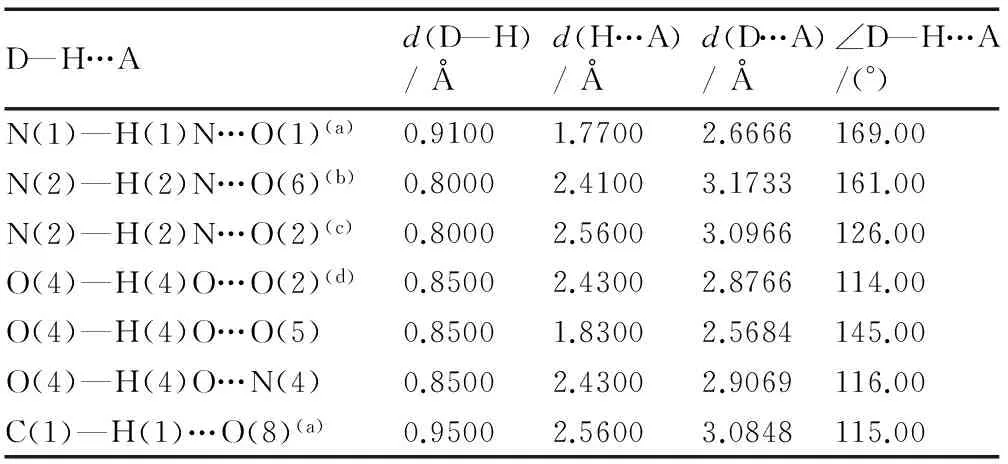
D—H…Ad(D—H)/Åd(H…A)/Åd(D…A)/Å∠D—H…A/(°)N(1)—H(1)N…O(1)(a)0.91001.77002.6666169.00N(2)—H(2)N…O(6)(b)0.80002.41003.1733161.00N(2)—H(2)N…O(2)(c)0.80002.56003.0966126.00O(4)—H(4)O…O(2)(d)0.85002.43002.8766114.00O(4)—H(4)O…O(5)0.85001.83002.5684145.00O(4)—H(4)O…N(4)0.85002.43002.9069116.00C(1)—H(1)…O(8)(a)0.95002.56003.0848115.00
Note: Symmetry operators: (a) 1-x,1/2+y,1/2-z; (b) -1+x,y,z;(c) -x,1/2+y,1/2-z; (d) 1+x,y,z.
Table 4 Hydrogen bond lengths and bond angles for 4-AT·TNR

D—H…Ad(D—H)/Åd(H…A)/Åd(D…A)/Å∠D—H…A/(°)O(1)—H(1)O…O(5)(a)0.94002.39002.9419117.00O(1)—H(1)O…O(8)0.94001.75002.5843147.00O(1)—H(1)O…N(7)0.94002.39002.9307116.00N(2)—H(2)N…O(4)(a)0.90001.79002.6255153.00N(2)—H(2)N…O(5)(a)0.90002.40003.0317127.00N(4)—H(4)A…O(6)(b)0.96002.19003.1443173.00N(4)—H(4)B…O(3)0.91002.53003.0263114.00
Note: Symmetry operators: (a) -1+x,1+y,z; (b) -1+x,1+y,-1+z.
3.2 Thermal decomposition
The thermal behavior, DSC and TG-DTG curves of IMI·TNR and 4-AT·TNR at a linear heating rate of 10 ℃·min-1, recorded in a nitrogen atmosphere separately, are given in Fig.2 and Fig.3.
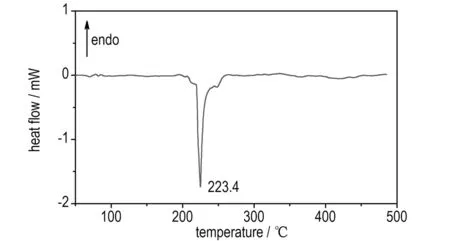
Fig.2b shows that there are three exothermic peaks (the first and last small exothermic peaks are overshadowed in the middle of quickly sharp exothermic peak) with the main peak temperature of 223.4 ℃ of IMI·TNR, and there is the mass loss of 62.5% corresponding to this temperature range in Fig.2a. The mass of the final residue is 6.5% at 500 ℃.

a. TG-DTG curve
b. DSC curve
Fig.2 TG-DTG and DSC curves of IMI·TNR in a nitrogen atmosphere at heating rate of 10 ℃·min-1
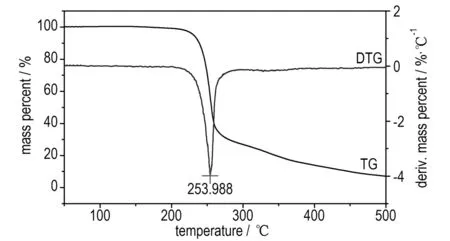
Moreover, Fig.3b exhibits two sharp peaks. One is endothermic melting peak, and another is rapidly decomposed peak. The first endothermic process starts from 195.2 ℃ and gained a peak temperature at 205.7 ℃. Following is an exothermic process, which indicates that the product immediately decomposes after melting. The decompose temperature ranges from 230.8 ℃ to 297.3 ℃ with the peak temperaturevat 259.8 ℃. Fig.3a shows that the compound loses mass 70%inthisprocess,andremains7.5%finally.Aftertherapiddecomposition the products of the two compounds are H2O, CO2, N2and a small amount of residue.
a. TG-DTG curve

b. DSC curve
Fig.3 TG-DTG and DSC curves of 4-AT·TNR in a nitrogen atmosphere at a heating rate of 10 ℃·min-1
3.3 Energy of combustion and enthalpy of formation
We used Kissinger′s method[25]and Ozawa′s method[26]to study the kinetic parameters of the rapidly exothermic process of title compounds, based on the DSC curves obtained under the condition of static air at heating rates of 5, 10, 15 ℃· min-1and 20 ℃· min-1. The peak temperatures (Tp) of the exothermic process at different heating rates, the apparent activation energy(Ea), the pre-exponential factor (A) and the linear correlation coefficient of two compounds were determined and listed in Table 5 and Table.6. The calculated results with two methods, are similar and all in the normal range (40-400 kJ·mol-1)[27].
Table 5 Peak temperatures of the first main exothermic stage at different heating rates and kinetic parameters for IMI·TNR with different method
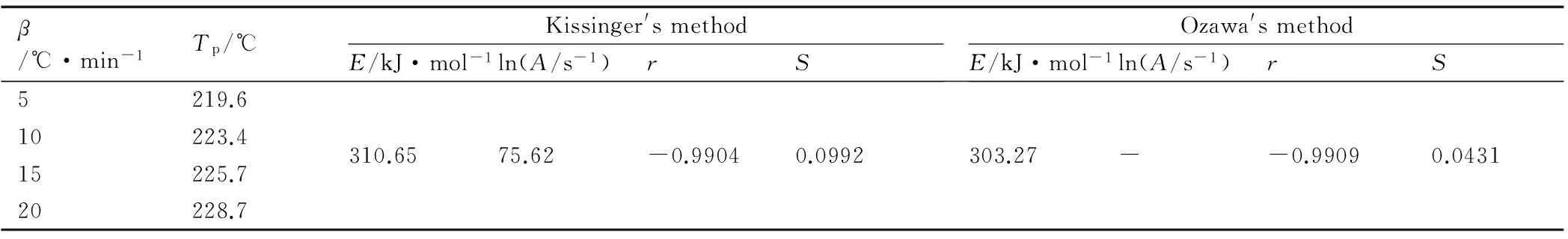
β/℃·min-1Tp/℃Kissinger'smethodE/kJ·mol-1ln(A/s-1)rSOzawa'smethodE/kJ·mol-1ln(A/s-1)rS5219.610223.415225.720228.7310.6575.62-0.99040.0992303.27--0.99090.0431
Note:βis the heating rate,ris the linear correlation coefficient.
Table 6 Peak temperatures of the first main exothermic stage at different heating rates and kinetic parameters for 4-AT·TNR with two method
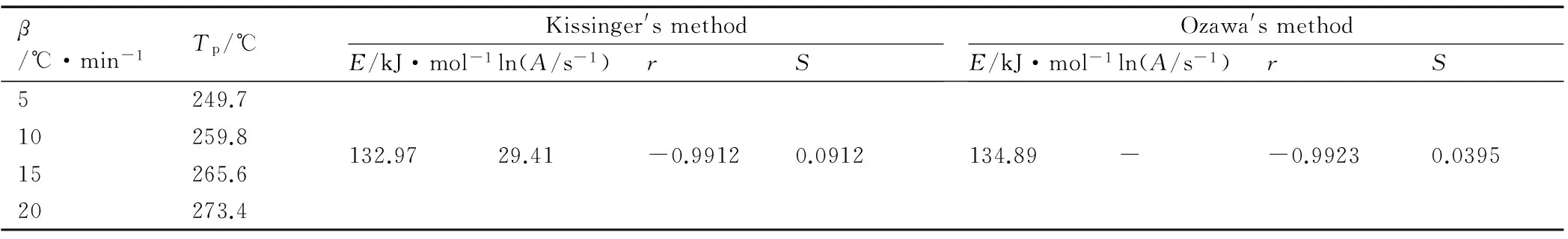
β/℃·min-1Tp/℃Kissinger'smethodE/kJ·mol-1ln(A/s-1)rSOzawa'smethodE/kJ·mol-1ln(A/s-1)rS5249.710259.815265.620273.4132.9729.41-0.99120.0912134.89--0.99230.0395
3.4 Calculation of the Thermal Explosion Properties
According to the formula group[28]as follow, the corresponding critical temperatures of thermal explosion (Tb), entropies of activation (ΔS≠), enthalpies of activation (ΔH≠), and free energies of activation (ΔG≠) of the decomposition reaction are obtained, and listed in Table 7.
Tpi=Tp0+aβ+bβ2+cβ2+dβ2
ΔH≠=E-RT
ΔG≠=ΔH≠-TΔS≠
Among them,a,b,canddare constant coefficients, andTpiis the peak temperature of the exothermic process at different heating rates. ThekBis the Boltzmann constant, 1.381×10-23J·K-1andhis the Planck constant, 6.626×10-34J·s,T=Tp0andA=Ak(Kissinger′s method).
Table 7 CalculatedTb, ΔS≠, ΔH≠, and ΔG≠
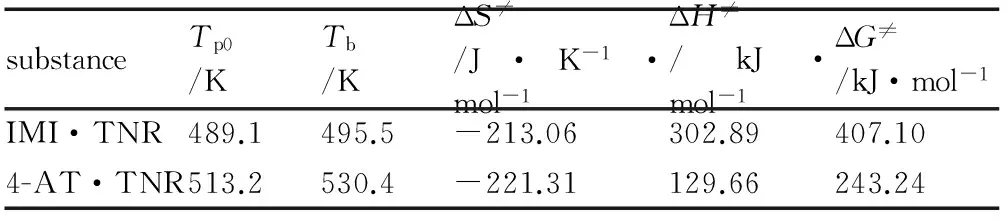
substanceTp0/KTb/KΔS≠/J·K-1·mol-1ΔH≠/kJ·mol-1ΔG≠/kJ·mol-1IMI·TNR489.1495.5-213.06302.89407.104-AT·TNR513.2530.4-221.31129.66243.24
3.5 Physicochemical properties
The impact and friction sensitivities as well as the flame sensitivity were determined on the basic of the China National Military Standard (CNMS)[29-31]. The impact sensitivities for title compounds, RDX, HMX and TNT are shown in Table 8. The results show that the title compounds are insensitive to friction sensitivity (misfire under the condition of pressure 3.92 MPa, hammer angle 90°) and flame sensitivity (do not fire when the distance between agents and the black powder pellet<6 cm). Meanwhile, they misfire in the impact sensitivities measurement even the drop height was above 80 cm. It reveals that the two compounds have low impact sensitivity, friction sensitivity and flame sensitivity.
Table 8 Physicochemical properties of IMI·TNR, 4-AT·TNR, RDX, HMX and TNT

substanceTm/℃Td/℃ρ/g·cm-3ΔUc/kJ·kg-1ΔHc/kJ·kg-1ΔHf/kJ·mol-1OB/%N/%Si/%Sf/%SF/cmIMI·TNRDec.2231.78-14329-14366.6-42.18-68.9622.36///4-AT·TNR2052601.77-11313-11356.3-409.69-55.8929.78///RDX[28]Dec.2301.91-9600//-21.637.848076±8/HMX[28]Dec.2871.82-9880//-21.637.84100100/TNT[28,32]813001.65-15220//-74.018.504-84-6/
Note:Tmis the melting point (peak).Tdis the peak temperature.ρis the calculated density. ΔUcis the energy of combustion. ΔHcis the enthalpy of combustion of cation. ΔHfis the molar enthalpy of formation.OBis the oxygen balance (O-2C-H/2-Z) ×1600/M;O, the number of oxygen atoms;C, the number of carbon atoms;H, the number of hydrogen atoms;Z, the number of metal atoms;M, the molecular mass of the compound.Nis the nitrogen content.Sidenotes the impact sensitivity, firing rate with 10.0 kg drop hammer.Sfdenotes the friction sensitivity, firing rate at the pressure of 3.92 MPa with a 1.5 kg hammer from 90° angle.SFdenotes the flame sensitivity, the maximum height of 100% ignition.
Compared with RDX, HMX and TNT, some physicochemical properties of the twotitle compounds are shown in Table 8. Obviously, physicochemical properties of IMI·TNR and 4-AT·TNR (Td=223, 260 ℃, ΔUc=-14329, -11313 kJ·kg-1,ρ=1.77, 1.78 g·cm-3) are both not lower than RDX (Td=230 ℃, ΔUc=-9600 kJ·kg-1) and close to HMX (Td=287 ℃, ΔUc=-9880 kJ·kg-1), whose densities are even higher than that of TNT (ρ=1.65 g·cm-3).
3.6 Calculation of Detonation Parameters
In accordance with the Brinkley-Wilson rule[28], the detonation reaction equations of title compounds are given in Scheme 3.
Using method of literature[33-34], the heat of detonation (QV), detonation temperature (TB), detonation pressure (pCJ), detonation velocity (D) of two materials were calculated, and results are shown in Table 9. Compared with conventional explosives, explosion heat and detonation temperature of IMI·TNR are close to RDX (QV=1266.08 kJ·mol-1,TB=3700 K), while explosion pressure and detonation velocity of 4-AT·TNR are comparable to that of TNT (pCJ=19.1 GPa,D=6.92 km·s-1)[28].
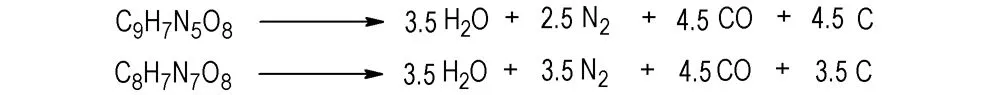
Scheme 3 Detonation reaction equations of the title compounds
Table 9 Detonation parameters of the title compounds
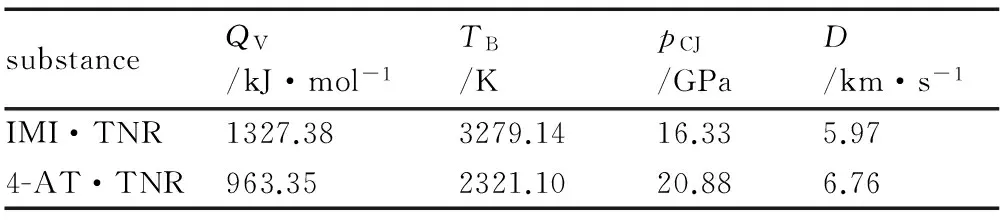
substanceQV/kJ·mol-1TB/KpCJ/GPaD/km·s-1IMI·TNR1327.383279.1416.335.974-AT·TNR963.352321.1020.886.76
4 Conclusions
Two styphnate salts, with IMI and 4-AT cations were preparedwith a ratio 1∶1 in water solution. As characterized by X-ray diffraction, IMI·TNR is monoclinic, space groupP21/cwith a density of 1.779 g·cm-3and 4-AT·TNR is triclinic, space groupP-1 with a density of 1.772 g·cm-3. IMI·TNR and 4-AT·TNR are stabilized by a variety of hydrogen bonds in their crystals. In addition, the high decompose point are 224.4 ℃ and 259.8 ℃, and their activation energies are 306.96 kJ·mol-1and 133.93 kJ·mol-1. The sensitivity measuring shows that the two compounds are insensitive energetic materials confirming with their calculated results of detonation parameters. Compared with conventional explosives, heat of detonation and detonation temperature of IMI·TNR are close to RDX (QV=1266.08 kJ·mol-1,TB=3700 K), while the detonation pressure and detonation velocity of 4-AT·TNR are comparable to that of TNT (pCJ=19.1 GPa,D=6.92 km·s-1).
[1] Steinhauser G, Klapötke T M.“Green” pyrotechnics: a chemists′ challenge[J].AngewChemIntEd, 2008, 47: 3330-3347.
[2] Klapötke T M, Sabaté C M. Bistetrazoles: Nitrogen-rich, high-performing, insensitive energetic compounds[J].ChemMater, 2008, 20: 3629-3637.
[3] WU Bi-dong, ZHANG Tong-lai, TANG Shi-min, et al. The environmentally friendly energetic salt (ATZ)(TNPG) based on 4-Amino-1,2,4-triazole (ATZ) and trinitrophloroglucinol (TNPG)[J].ZAnorgAllgChem, 2012, 638(14): 2347-2352.
[4] ZHANG Jian-guo, WANG Kun, LI Zhi-min, et al.Synthesis, crystal structure and thermal decomposition of a novel environmentally friendly energetic cesium compound,[Cs-2(HTNR)(OH)(H2O)](n)[J].Maingroupchemistry. 2011, 10: 205-213.
[5] Talawar M B, Sivabalan R, Mukundan T, et al.Environmentally compatible next generation green energetic materials (GEMs)[J].JournalofHazardousMaterials, 2009, 161: 589-607.
[6] Huynh M H, Hiskey M A, Meyer T J, et al. Green primaries: environmentally friendly energetic complexes[J].PNAS, 2006, 103: 5409-5412.
[7] Klapötke T M, Sabaté C M, Welch J M. Alkaline earth metal salts of 5-nitro-2H-tetrazole: prospective candidates for environmentally friendly energetic applications[J].EurJInorgChem, 2009: 769-776.
[8] Klapötke T M, Magdalena R, Véronique S. Preparation of energetic poly (azolyl) borates as new environmentally benign green-light-emitting species for pyrotechnics[J].ZAnorgAllgChem, 2013, 639(14): 2433-2443.
[9] Dippold A A, Klapötke T M, Winter N. Insensitive nitrogen-rich energetic compounds based on the 5,5′-Dinitro-3,3′-bi-1,2,4-triazol-2-ide anion[J].EurJInorgChem, 2012: 3474-3484.
[10] FENG Jin-ling, ZHANG Jian-guo, ZHANG Tong-lai, et al. Synthesis, crystal structure, thermal behavior and sensitivity of [Mn(AZT)2(H2O)4] (HTNR)2·4H2O[J].ActaPhysChimSin, 2010: 2410-2416.
[11] CUI Yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Synthesis, structural investigation and thermal analyses of a novel coordination compound[Cd(DAT)6](HTNR)2·3.5H2O (DAT=1,5-diaminotetrazole, H2TNR=styphnic acid)[J].JournalofMolecularStructure, 2008, 889: 177-185.
[12] Poturovic S, Lu Dong-Mei, Heeg M J, et al. Synthesis and structural characterization of heavier group 1 methyl tetrazolate complexes: new bridging coordination modes of the tetrazolate ligand[J].Polyhedron, 2008, 27: 3280-3286.
[13] XU Cheng, BI Fu-qiang, FAN Xue-zhong, et al. One-pot synthesis of 2-nitro-4,5-dicyano-1H-imidazole[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2011, 19(6): 743-744.
[14] HE Yun, FAN Gui-juan, ZHANG Guang-quan, et al. Review on synthesis and reactivity of 5-amino-3-nitro-1,2,4-trizole[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2012, 20(6): 715-720.
[15] WU Jin-ting, ZHANG Jian-guo, YIN Xin, et al. Synthesis, characterization, and thermal analysis of two energetic ionic salts based on 3,4-diamino-1,2,4-triazole (DATr)[J].ZAnorgAllgChem, 2013, 639, (12-13): 2354-2358.
[16] FENG Jin-ling, ZHANG Jian-guo, LI Zhi-min, et al. Synthesis, crystal structure and properties of a novel high-nitrogen energetic complex[Co(AZT)2(H2O)4](HTNR)2·4H2O[J].ActaChimicaSinica, 2010, 24: 2493-2499.
[17] XIA Yun-xia, WANG Ping, SUN Jie, et al. Crystal structure of energetic compound 4-amino-1,2,4-triazolium picrate[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2010, 18(1): 4-6.
[18] MA Gui-xia, ZHANG Tong-lai, SHAO Bing, et al. Crystal structure and thermal decomposition mechanism of[Mn(SCZ)3](PA)2H2O[J].ChineseJStructChem, 2004, 23: 445-451.
[19] TANG Zhan, YANG Li, QIAO Xiao-jing, et al. Synthesis, crystal structure, thermal decomposition and sensitivity properties of (AIM)(HTNR) and (AIM)(PA).ChemResChineseUniversities, 2012, 28(1): 4-8.
[20] CUI Yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Synthesis, crystal structure, thermal decomposition and sensitivity properties of [Zn(AZT)4(H2O)2](PA)2·4H2O and [Zn(AZT)2(H2O)4](HTNR)2·4H2O[J].ChineseJournalofChemistry, 2008, 26: 2021-2028.
[21] Klapötke T M, Sabaté C M. 1,2,4-triazolium and tetrazolium picrate salts: “On the Way” from nitroaromatic to azole-based energetic materials[J].EurJInorgChem, 2008: 5350-5366.
[22] Sheldrick G M. SHELXS 97, program forcrystal structure solution[CP].UniversityofGöttingen, Germany, 1997.
[23] Sheldrick G M. SHELXL 97, program for crystal structure refinement from diffraction data[CP].UniversityofGöttingen, Germany, 1997.
[24] Frank H A, Kennard O, Watson D G.Tables of bond lengths determined by X-Ray and neutron diffraction. part I .bond lengths in organic compounds[J].JChemSocPerkinTransⅡ, 1987: s1-s19.
[25] Kissinger H E. Reaction kinetics in differential thermal analysis[J].AnalChem, 1957, 29: 1702.
[26] Ozawa T. A new method of analyzing thermogravimetric data[J].ChemSocJpn, 1965, 38: 1881-1886.
[27] Han D G, Gao Z D, Gao P L. Physical chemistry (second edition)[M]. Beijing: Higher Education Press, 2009:355-387.
[28] OU Yu-xiang. Explosives[M].Beijing: Beijing Institute of Technology Press, 2006: 145, 202, 218.
[29] GJB 772A-1997. Method 601.2. Beijing: Commission of science technology and industry for national defense, 1997: 191-200.
[30] GJB 772A-1997. Method 602.1. Beijing: Commission of science technology and industry for national defense, 1997: 207-213.
[31] GJB 772A-1997. Method 604.1. Beijing: Commission of science technology and industry for national defense, 1997: 221.
[32] Lui Z T, Lao Yun-liang. Initiating explosive experimental[M]. Beijing: Beijing Institute of Technology Press, 1995: 238-239.
[33] WANG Jun, DONG Hai-shan, LI Jin-shan, et al. Empirical calculation of the explosion parameters of nitrodiazole explosives[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2012, 20(5): 541-544.
[34] WANG Jun, JING Mei, ZHANG Xiao-yu, et al. Empirical calculation of the explosion parameters of nitrodiazole explosives (Ⅱ)[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2013, 21(5): 609-611.
杂志排行
含能材料的其它文章
- 《含能材料》2015年(第23卷)总目次
- Crystal Structure and Enthalpy of Combustion of AEFOX-7
- Anisotropic Thermal Expansion in Nitroguanidine Crystal
- Impact Sensitivity in Respect of the Crystal Lattice Free Volume and the Characteristics of Plasticity of Some Nitramine Explosives
- The Empirical Nitrogen Equivalent Equations for Predicting the Detonation Velocity and Detonation Pressure of CHNO Explosives with Approaching the Results of Kamlet-Jacobs Equations
- Atomization of Gelled Propellant Simulant with Carbon Particles
