Impact Sensitivity in Respect of the Crystal Lattice Free Volume and the Characteristics of Plasticity of Some Nitramine Explosives
2015-05-10SvatoplukZemanMarcelaJUNGOVQiLongYAN
Svatopluk Zeman, Marcela JUNGOV, Qi-Long YAN
(Institute of Energetic Materials, Faculty of Chemical Technology, University of Pardubice, CZ-532 10 Pardubice, Czech Republic)
1 Introduction
Impact sensitivity may result from a combination of three fundamental sensitivities[1]: molecular, crystalline and environmental. The first two of these have been extensively studied for individual energetic materials (EMs) by means of the NMR chemical shifts of the key atoms in the reaction centers and by means of the heats of fusion for such compounds[2-4].
Concerning the crystals of organic poly-nitro compounds, very important facts were obtained[5]and recently verified on the basis of an X-ray crystallographic study of some polynitroarenes[6-8]and ofcis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole (BCHMX)[9]. Particularly in nitramine crystals, the oxygen atoms of nitro groups, by their dipole-dipole interactions, contact the oxygen and nitrogen atoms of nitro groups in neighboring nitramine molecules in the crystal[10-13], which is the decisive factor governing the crystal structure of such EMs. A very important finding from the referenced studies, which still needs further investigation, is that non-binding inter-atomic distances between oxygen atoms inside all of the nitro groups in these poly-nitro compounds are shorter than those corresponding to the intermolecular contact radii for oxygen in carbonyl or nitro groups; this distance is especially short inside the most reactive nitro groups. These facts led our colleague Vávra to conduct his research on free spaces in crystals of EMs and of their influence on the sensitivity of the energetic compounds described[14-15]; as a continuation of these two papers a new study was published[16]on the basis of which we explore the relationships in this paper between free spaces in crystals on the one hand, and the bulk and shear moduluses of some attractive nitramines, on the other.
2 Data Sources
2.1 Nitramine Explosives
From the group of individual nitramines, the following compounds were taken into consideration: bis-(2,2,2-trinitroethyl)nitramine(HOX), 1,4-dinitro-1,4-diazabutane(EDNA), 1,3,3-trinitroazetidine(TNAZ), 1,4-dinitroimidazole(1,4-DNI), 1,3,5-trinitro-1,3,5-triazinane(RDX), 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX),cis-1,3,4,6-tetranitrooctahydroimidazo[4,5-d]-imidazole(BCHMX),trans-1,4,5,8-tetranitrodecahydro-pyrazino[2,3-b]-pyrazine(TNAD), -2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(HNIW), 2,4,6,N-tetranitro-N-methylaniline(TETRYL). Besides these nitramines, plastic bonded explosives(PBXs), bonded by 9 % wt. Viton A and filled by RDX, HMX, BCHMX and HNIW[17-18], are also a topic of interest in this paper; these PBXs are designated in Table 1 and in the Figures as RDX-9V, HMX-9V, BCHMX-9V and HNIW-9V. The last of these was prepared from technical grade HNIW, with an impact sensitivity of 4.2 J[17-18].
2.2 Impact Sensitivity
The impact sensitivity of the nitramines and PBXs studied, shown in Table 1, have been taken from respectable literature[17-23]: they were obtained by means of a standard impact tester with an exchangeable anvil (Julius Peters), detection of the 50% probability of initiation being based on acoustic detection (Bruceton method)[17-23]. The sensitivity is expressed as drop energy,Edr.
2.3 Bulk and Shear Moduluses
The tendency of an object to deform in all directions when uniformly loaded in all directions, i.e. volumetric stress, is described as a bulk modulus,K, and it is the inverse of compressibility. Shear modulus,G, is connected with deformation ofthe shape at constant volume (it represents a resistance to plastic deformation). The ratio of bulk modulus to shear modulus, K·G-1, can be used as an indication of the extent of the plasticity range for a given material[24]; a high value for this ratio is associated with malleability and a low one with brittleness.
Table 1 Bulk and shear modulus′s, their ratio, impact sensitivities expressed as drop energy,Edr, and crystal lattice free volume, ΔV, of all nitramine explosives studied

nitraminecodedesignationformulamodulusK/GPaG/GParef.ratio/K·G-1impactsensitivityEdr/Jref.ΔV[16]/Å3RDX6.903.3[25]2.095.6[20]46β-HMX7.704.2[26]1.836.4[20]49ε-HNIWpure10.307.4[27]1.3913.4[22]86ε-HNIWtechnical4.2 3.4[23,16,34]BCHMX7.152.68[28]2.673.0[9,19]321)TNAD10.807.75[29]1.398.6[31]611)HOX1.2[20]73EDNA8.3[20]29TNAZ6.9[19,32]371,4-DNI13.5[16,34]34TETRYL7.8[33]57RDX-9V F23114.902.4[25]2.0410.6[17-18]HMX-9V F23117.803.9[30]2.010.3[17-18]BCHMX-9V F23116.602.30[28]2.875.3[17-18]HNIWtechn.-9V F23119.805.0[27]1.966.9[17-18]
Note: 1) The value calculated by means of line A in Fig.1.
For the nitramines RDX, HMX, BCHMX, TNAD and HNIW, the literature contains different values for their modules which depend on the temperature and specification methods. Therefore, we used the results of the molecular dynamic simulations (MDS) by Xiao[25-30]et al. from Nanjing University in Sci. & Technology in order to have a uniform approach. The second problem, i. e. the missing values of both these moduluses for PBXs with the Viton A binder, we have solved similarly; in the first approximation we used again the MDS results for the analogous PBXs, bonded by the high poly-fluorinated polymer F2311and the corresponding values were taken along the crystalline surface (010)[25-30].
2.4 Free Space per Molecule ina Crystal Lattice
Calculation of this ΔVvalue is described in the literature, papers[14-16]. It represents a difference between the effective volume (ratio of molecular weight and density) and the intrinsic molecular volume; this last one is the product of molecular weight and packing coefficient divided by density-in paper[16]its authors describe in detail a method for obtaining this value in which they work on an isolated molecule. In this study, the ΔVvalues are taken from the literature[16], see in Table 1.
3 Discussion
On the basis of the same approach as that in paper[16], we have found relationships between impact sensitivity (drop energy,Edr) and the ΔVvalues of the nitramines studied in the sense of Fig.1. Only one relationship for these nitramines is presented in paper[16]. What are the reasons for the difference between Fig.1 and paper[16].
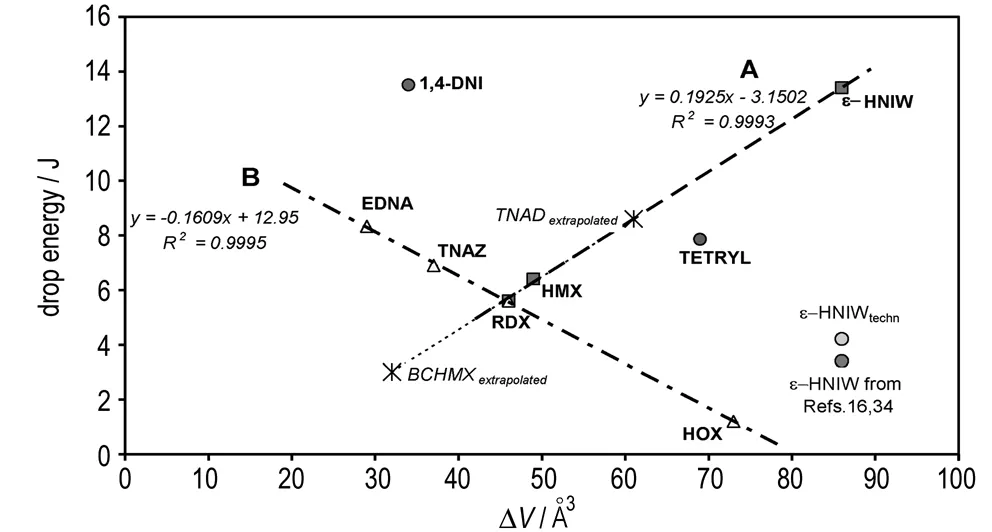
Fig.1 Relation between impact sensitivity (expressed as drop energy,Edr) and free space per molecule in crystal lattice, ΔV; the ΔVvalues for TNAD and BCHMX were calculated by means of the relationship for line A
The first problem is the impact sensitivity ofε-HNIW. Many publications have reported thatε-HNIW has a high sensitivity to impact, greater than that of both RDX and HMX[16, 34]. Although Ou Yuxiang et al.[22]have described pureε-HNIW with an impact sensitivity of 13.2 J and for the rest of its pure polymorphs found values forα-HNIW of 10.1 J,β-HNIW of 11.9 J andγ-HNIW of 12.2 J, all these values are still mostly ignored in the literature. The correctness of these values has also been verified by the molecular-structural relationships of the impact sensitivity of nitramines[3, 36-37]. In the last five years, however, the sensitivity of technical grade HNIW is the main focus of crystal engineering for this particular substance[23, 35]; the result of this isε-HNIW with reduced sensitivity (RS-ε-HNIW, RS-CL-20) and with impact sensitivity from 8 to 12 J[23, 35, 38-40]. By incorporation of the impact sensitivity of pureε-HNIW into theEdr-ΔVrelationship from paper[16], the new relationship, i.e. Fig.1, appears.
Line A in Fig.1 represents a group of “genuine” nitramines, i. e. compounds with intermolecular interaction of the same kinds; these interactions are typified by Scheme 1.

Scheme 1 Scheme of several possible interactions in the HMX molecular crystal-dipole-dipole interaction contacts between oxygen atoms and their interaction with nitrogen and hydrogen atoms in neighboring nitramine molecules (obtained by means of X-ray spectroscopy in the conditions described in Ref.[9])
Despite the relatively low number of points, the correlation in the sense of group A is very good. Data for Tetryl and 1,4-DNI do not correlate with this line. Molecules of Tetryl are arranged in its crystal in such a way that nitro groups in the 4-position of the picryl group face each other and the oxygen atoms of the neighboring molecules make the short contact with each other[41]. It is clear that this interaction, together with the bulkier picryl group in the Tetryl molecule, represents another type of intermolecular interaction in comparison with those in Scheme 1. In the 1,4-DNI molecule one nitro group is bonded onto the aza nitrogen atom which is a part of a hetero-aromatic system; during heating of 1,4-DNI this nitro group is shifted onto the carbon atom in position 2 thus forming 2,4-DNI[42-43]. The crystallographic study of the planar molecule of 1,4-DNI shows that hydrogen atoms in the 2 and 3 positions form hydrogen bonds with neighboring molecules in its crystal[43]. This fact is also different from interactions in the sense of Fig.2. Data for both types ofε-HNIW do not correlate with line A due to the fact that their impact sensitivity logically does not correspond to their molecular structure (they have defected crystals). The ΔVvalues for TNAD and BCHMX were calculated using the relationship for line A.
The nitramines collected along line B have different kinds of intermolecular interactions from those for the “genuine” cyclic nitramines assembled around the line A. The HOX molecule exists in two crystal modifications[44]; the relatively bulky 2,2,2-trinitroethyl groupings in its molecule are not mutually equivalent as far as the steric strain is concerned[44]. The bulkiness of these groupings must decrease the nitramino grouping′s interactions in the HOX crystal which contains just a single such group in each molecule (this supposition corresponds to a relatively low melting point, i.e. at 95-95.5 ℃). These groupings have also a strong electron withdrawing effect the result of which is a higher initiation reactivity for the HOX molecules. It is similar in the case of TNAZ molecules[45]; here electron withdrawing of geminal dinitro groupings should be lower in comparison with the geminal trinitro analogs but also here the nitramino groupings are disadvantaged as far as intermolecular interactions are concerned (its melting point is 103-104 ℃). The EDNA molecule has the nearly planar CN—HNO2group and is symmetrical[46]. The packing in an EDNA crystal appears to be based predominantly on dipole-dipole interaction[46]. RDX has features common to the other members of the “line B” group, and methylene-nitraminic groupings in its molecule, the N—N bond of which is one trigger in the initiation (reaction center) of the compounds studied[1-4,9,19,36]. The difference in the intermolecular interactions of nitramino and nitroparaffinic groupings also results from differences in the electron density movements (from the manner and degree of their polarization) in these groupings:

Scheme 2 Electron density movement in nitramino and nitroparaffinic groupings-the first one gives a markedly higher polarized grouping with more intensive intermolecular interaction
It has been shown that the configuration in the reaction center of the molecule plays a decisive role in the initiation reactivity of energetic materials[2-4].
Concerning the intermolecular interactions and their relationship with impact[2-4,21]and friction[47-49]sensitivities it must be stated that all these relationships are not characterized by unequivocal equations; there are several characterizations possible, depending on the quality of the key groupings in the molecule and the conformation of the molecule[2-4, 47-49]. The same is valid for the N—N bond dissociation energies[50]. Relationships in Fig.1 correspond to these observations[2-4, 47-49]and are thus quite in line with expectations.
Plastic properties also have a considerable significance in the impact sensitivity of energetic materials (they are also a result of intermolecular interactions). These properties are concerned mainly with uniaxial compression (bulk modulus,K) and shear slide with a fixed volume (shear modulus,G). The relationships between both these moduluses and the impact sensitivity of the explosives studied are shown in Fig.2 and Fig.3. Despite the low number of data points, it can be seen that data for PBXs based on HNIW and BCHMX might correlate with curves for pure nitramines; this is not new news since analogous correlations are commonplace in the case of relationships between impact sensitivity and performance[17, 51]. Substitution of the impact sensitivity in Fig.2 and Fig.3 by the ΔVvalues gives similar relationship for pure nitramines; also here, for shear modulus, the data for HNIW do not correlate. Action of forces in the crystal lattices with globular nitramine molecules should be in several details different from those in nitramines with roughly planar molecules.

Fig.2 Approximate relationships between impact sensitivity and bulk modulus (inversed compressibility) of the energetic materials studied
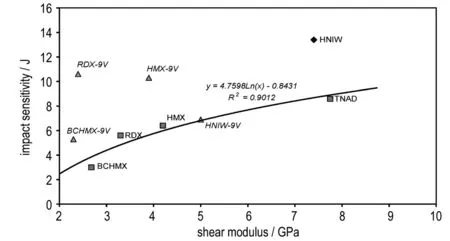
Fig.3 Approximate relationships between impact sensitivity and shear modulus (deformation of shape at constant volume) of the energetic materials studied
In metallurgy, the dimensionless ratio, K·G-1, is used for characterization of the malleability of metals[24]; the ratio indicates the extent of the plasticity range and a low value is expected for brittle materials, in which plastic flow is relatively difficult[24-25, 27]. The K·G-1ratio has been used also for characterization of the plasticity of PBXs[25-28]. The relationship between this ratio and impact sensitivity of the explosives studied is shown in Fig.4. Here again, data for pureε-HNIW lies outside those for the pure nitramine groups. The data correlate well with those of the PBXs studied; as has already been mentioned, data correlation for pure nitramines with data for PBXs based on them are routine in “impact sensitivity-performance” relationships[17, 51].A similar relationship to the K·G-1ratio exists in the case of the ΔVvalues as is documented by Fig.5. Concerning the relationship of the data for pureε-HNIW with those of other pure nitramines in Fig.4 and Fig.5, what has been mentioned for Fig.3 is valid here.
From the above-mentioned fact, the unusual behavior ofε-HNIW is evident. Its incorporation into polymeric matrices might be connected with a change in its sensitivity due to the physical instability of itsε-modification in polar matrices[52-54]. It might also be due to the relatively high ΔVvalue of HNIW. But this is a new subject which needs specific attention.

Fig.4 Relationships between impact sensitivity and the K·G-1ratio of the energetic materials studied
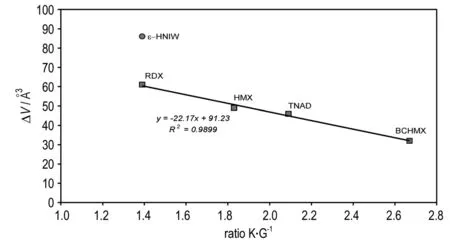
Fig.5 Relationships between the free space per molecule in crystal lattice and K·G-1ratio of the nitramines studied
4 Conclusions
The relationship between the free space per molecule in crystal lattice, ΔV, and impact sensitivity (here a drop energy,Edr) is, very broadly, inversely proportional[16]. Analysis of this relationship for nitramines has shown that it is not so uniquely characterized, i.e. it is not given only by their own ΔVvalues but fundamentally by the kind and intensity of intermolecular forces in the nitramine crystals (thereby the configuration of the reaction center in molecule can also be influenced). Relationships of impact sensitivity with the bulk (K) and shear (G) moduluses of these nitramines and their PBXs resemble somewhat the relationships between this sensitivity and performance, concerning the composition of corresponding groups of such explosives. The closest linear correlation exists between theEdror ΔVvalues and the dimensionless K·G-1ratio which indicates the plasticity range. Relationships of theEdrand/or ΔVvalues towards the shear modulus or to the K·G-1ratio pointed out an unusual behavior ofε-HNIW to which the published morphological instability of this particular HNIW version might also be related.
[1] Delpuech A., Cherville J., Relation entre la Structure Electronique et la Sensibilité au Choc des Explosifs secondaries Nitrés. Critère Moleculaire de Sensibilité[J].IPropellantsExplos,1978, 3:169-175.
[2] Zeman S.New aspect of initiation reactivity of energetic materials demonstrated on nitramines[J].JHazardMater, 2006, 132: 155-164.
[3] Zeman S.Study of the Initiation Reactivity of Energetic Materials[M]. Chapter 8 in: Armstrong R W, Short J M, Kavetsky R A, Anand D K Energetics Science and Technology in Central Europe, CECDS, University of Maryland, College Park, Maryland, 2012:131-167.
[4] Zeman S. Sensitivity of High Energy Compounds [M]. Klapoetke T. (Ed), High Energy Density Materials, Series: Structure & Bonding, 125, Springer, New York, 2007: 195-271.
[5] Eckhardt C J, Gavezzotti A. Computer simulations and analysis of structural and energetic features of some crystalline energetic materials[J].JPhysChemB, 2007, 111: 430-3437.
[7] Zeman S, Roháĉ M, Friedl Z, et al. Crystallography and structure-property relationships of 2,2″,4,4′,4″,6,6′,6″-octanitro-1,1′:3′,1″-terphenyl (ONT)[J].Propellants,Explosives,Pyrotechnics, 2010, 35: 130-135.
[8] Zeman S, Roháĉ M, Friedl Z, et al.Crystallography and structure-property relationships in 2,2′,2″,2‴,4,4′,4″,4‴,6,6′,6″,6‴-dodecanitro-1,1′,:3′,1″:3″,1‴-quaterphenyl (DODECA)[J].Propellants,Explos,Pyrotech, 2010, 35:339-346.
[10] Atovmyan LO, Golovina N I, Zolotoy AB, et al. Structure and packing of primary and secondary nitro amines[J].ZhOrgKhim, 1998, 24(9): 1848.
[11] Krebs B, Mandt J, Cobbledick R E, et al. The structure ofN,N-Dimethylnitramine[J].ActaCryst, 1979, B35: 402-404.
[12] Filhol A, Bravic G, Rey-Lafon M, et al.X-Ray and Neutron Studies of a Displacive Phase Transition inN,N-Dimethylnitramine (DMN)[J].ActaCryst, 1980: B36, 575.
[13] Turley J W. A Refinement of the crystal structure of n,n-dinitroethylenediamine[J].ActaCryst, 1968, B24: 942-946.
[14] Pospíšil M, Vávra P, Concha M C, et al. A possible crystal volume factor in the impact sensitivities of some energetic compounds[J].JMolModel, 2010, 16(5): 895-901.
[15] Pospíšil M, Vávra P, Concha M C, et al Sensitivity and the Available Free Space per Molecule in the Unit Cell[J].JMolModel, 2011, 17: 2569-2574.
[16] Politzer P, Murray J S. Impact sensitivity and crystal lattice compressibility/free space[J].JMolModel, 2014, 20:2223; DOI 10.1007/s00894-014-2223-7.
[17] Elbeih A, Zeman S, Jungova M,, et al. Attractive Nitramines and Related PBXs[J].Propellants,Explosives,Pyrotechnics, 2013, 38(3): 379-385.
[18] Elbeih A, Jungová M, Zeman S, et al. Explosive strength and impact sensitivity of several pbxs based on attractive cyclic nitramines[J].Propellants,Explosives,Pyrotechnics, 2012, 37(3): 329-334.
[19] Atalar T, Jungová M, Zeman S. A new view of relationships of the N—N bond dissociation energies of cyclic nitramines. part ii. relationships with impact sensitivity[J].JEnergMater, 2009, 27: 200-216.
[20] Storm C B, Stine J R, Kramer J F. Sensitivity Relationships in Energetic Materials[M]. Bulusu S N (Ed), Chemistry and Physics of Energetic Materials, Kluwer Acad. Publs., Dordrecht, 1990, 605-639.
[21] Zeman S, Krupka M. New aspects of impact reactivity of polynitro compounds. part iii. impact sensitivity as a function of the intermolecular interactions[J].Propellants,Explosives,Pyrotechnics, 2003, 28: 301-307.
[22] Ou Y, Wang C, Pan Z, et al Sensitivity of hexanitrohexaazaisowurtzitane[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 1999, 7: 100-108.
[23] Elbeih A, Husárová A, Zeman S. Path to hniw with reduced impact sensitivity[J].CentralEurJEnergetMater, 2011, 8(3): 173-182.
[24]Pugh S F. Relation between the elastic moduli and the plastic properties of polycrystalline pure metals[J].PhilosMag, 1954, 45: 823-843.
[25] Zhu W, Xiao J, Zhu W, et al.Molecular dynamics simulation of RDX and RDX-based plastic-bonded explosives [J].JHazardMater, 2009, 164: 1082-1088.
[26] Xiao J, Huang H, Li J, et al Computation of interface interactions and mechanical properties of HMX-based PBX with estane 5703 from atomic simulation[J].JMaterSci, 2008, 43: 5685-5691.
[27] Xu X J, Xiao H M, Xiao J J, et al. Molecular dynamic simulation for pure CL-20 and CL-20-based PBXs[J].JPhysChemB, 2006, 110: 7203-7207.
[28] Qiu L, Xiao H. Molecular Dynamic study of binding energies, mechanical properties, and detonation performances of bicyclo-HMX-based PBXs[J].JHazardMater, 2009, 164: 329-336.
[29] Qiu L, Zhu W H, Xiao J J, et al. Molecular dynamic simulation of trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecaline-based polymer-bonded explosives[J]. JPhysChemB, 2007, 111: 1559-1566.
[30] Ma X, Zhao F, Ji G, et al. Computational study of structure and performance of four constituents hmx-based composite material[J].JMolStruct,Theochem, 2008, 851: 22-29.
[31] Willer R L. Synthesis and Characterization of High-Energy Compounds. I. Trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin (TNAD)[J].Propellants,Explosives,Pyrotechnics, 1983, 8: 65-69.
[32] Simpson R I, Garza R G, Foltz M F, et al. Characterization of TNAZ[R]. Rep UCRL-ID-119572, Laewrence Livermore Lab, 1994.
[33] Kamlet M J, Adolph H G. The relationship of impact sensitivity with structure of organic high explosives. part ii. polynitroaromatic explosives[J].Propellants,Explosives,Pyrotechnics, 1979, 4: 30-34.
[34] Rice B M, Hare J J. a quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules[J].JPhysChem, 2002, A106: 1770-1783.
[35] Chen H, Li L, Jin S, et al. Effect of additives on hniw crystal morphology and impact sensitivity[J].Propellants,Explosives,Pyrotechnics, 2012, 37: 72-82.
[36] Jungova M, Zeman S, Yan Q L. Recent advances in the study of the initiation of nitramines by impact using their15N NMR chemical shifts[J].CentEurJEnerget.Mater, 2014, 11(3): 285-294.
[37] Zeman S, Yan Q L, Vlcek M. Recent advances in the study of the initiation of energetic materials using characteristics of their thermal decomposition part i. cyclic nitramines[J].CentEurJEnergetMater, 2014, 11(2): 173-189.
[38] H Chen, S Chen, J Liu, et al. Preparation of the spheroized hniw crystals. Faming Zhuanli Shenqing Gongkai Shuomingshu 2010, CN 101624394, A 20100113[P].
[39] Doo K B.Spherical high-density 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane and preparation thereof [P]. Korean Patent KR 224043 B1, Dong Woon Specialty Chemical Co, Ltd, S Korea, 1999.
[40] Elbeih A, Husarova A, Zeman S. Process for Preparing {epsilon}-2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane with Reduced Impact Sensitivity. Czech Rep. Pat. 2013, CZ 303686 B6 20130306 [P]
[41] Zhulkhistova N E, Prezdo W W, Bykova A S.Molecular and crystal structures of 2,4,6-trinitro-N-methyl-N-nitroaniline [J],Crystall.Reports, 2002, 47: 65-68.
[42] Cao D, Wang J, Liu H. Synthesis and thermal decomposition of 1,4-DNI, and 2,4-DNI[C]∥Proceedings of the 37thInt. Annual Conf ICT, Karlsruhe, 2006: 175/1-175/5.
[43] Zhang J, Lan T, Li M,et al. Preparation and crystal structure of 1,4-dinitroimidazole[J].YingyongHuagong, 2012, 41(9): 1664-1666.
[44] Atovmyan L O, Gafurov R G, Golovina N I, et al. Crystal and molecular structure of two modifications of bis-(2,2,2-trinitroethyl)nitramine[J].Zhur.Strukt.Khim. 1980, 21(6): 35-141.
[45] Archibald T G, Gilardi R G, Baum K,et al. Synthesis and x-rays crystal structure of 1,3,3-trinitroazetidine[J].JOrgChem, 1990, 55: 2920-2924.
[46] Turley J W. A refinement of the crystal structure ofN,N′-dinitroethylenediamine[J].ActaCrystall,Sect.B:StructuralCrystallographyandCrystalChemistry, 1968, 24(Pt. 7): 942-6.
[47] Jungová M, Zeman S, Husarová A. Friction Sensitivity of Nitramines. Part I: Comparison with Impact Sensitivity and Heat of Fusion[J].ChinJEnerget.Mater(HanNengCaiLiao),2011, 19(6): 603-606.
[48] Friedl Z, Jungová M, Zeman S, et al. Friction sensitivity of nitramines. part IV: links to surface-electrostatic potentials[J].Chin.J.Energet.Mater. (HannengCailiao),2011, 19(6): 613-615.
[49] Jungová M, Zeman S, Husarová A. New aspect of friction sensitivity of nitramines (in Persian)[J].JEnergticMater(Tehran), 2012, 1(14): 13-18.
[50] Atalar T, Zeman S. A new view of relationships of the N—N bond dissociation energies of cyclic nitramines. part I. relationships with heats of Fusion[J].JEnergMater, 2009, 2(3): 186-199.
[51] Elbeih A, Zeman S, Jungova M, et al. Effect of different polymeric matrices on some properties of plastic bonded explosives[J].Propellants,Explos,Pyrotech, 2012, 37(6): 676-684
[52] Torry S, Cunliffe A. Polymorphism and solubility of CL-20 in plasticizers and polymers[C]∥Proc 31stInt Annual Conf ICT, Karlsruhe, 2000, 107/1-107/12.
[53] Pelikán V, Zeman S, Yan Q L, et al. Concerning the shock sensitivity of cyclic nitramines incorporated into a polyisobutylene matrix[J].CentEurJEnergMater, 2014, 11(2): 219-235.
[54] Pu Z, Xu J J, Guo X, Jiao Q, et al. Effect of addictives on polymorphic transition of CL-20 in castable systems[J].JThermalAnalCalorim, 2014, 117(2): 1001-1008.
杂志排行
含能材料的其它文章
- Synthesis and Characterization of Two New Energetic Polyamino and Nitro Pyridine Derivatives
- 《含能材料》2015年(第23卷)总目次
- Non-isothermal Decomposition Kinetics,Specific Heat Capacity and Adiabatic Time-to-explosion of Cu(pn)2(FOX-7)2
- Facile Synthesis and Crystal Structure of 3,4-Bis(1H-5-tetrazolyl)furoxan
- Synthesis and Properties of N,N-Bis((3,5-dinitro-1H-1,2,4-triazol-1-yl)methyl) nitramine
- Comparison with Molecular Surface Electrostatic Potential and Thermal Reactivity of Nitramines
