Combustion and pollutant emission characteristics of coal in a pressurized fluidized bed under O2/CO2 atmosphere
2015-05-08DuanYuanqiangDuanLunboHuHaihuaZhaoChangsui
Duan Yuanqiang Duan Lunbo Hu Haihua,2 Zhao Changsui
(1School of Energy and Environment, Southeast University, Nanjing 210096, China)(2Jiangxi Electric Power Design Institute, Nanchang 330096, China)
Combustion and pollutant emission characteristics of coal in a pressurized fluidized bed under O2/CO2atmosphere
Duan Yuanqiang1Duan Lunbo1Hu Haihua1,2Zhao Changsui1
(1School of Energy and Environment, Southeast University, Nanjing 210096, China)(2Jiangxi Electric Power Design Institute, Nanchang 330096, China)
The pressurized combustion experiments of bituminous coal and lignite under air and O2/CO2atmospheres were conducted to study the influences of pressure and atmosphere on combustion and the CO, NO, SO2release process. Two indices, the maximum concentration and the total emission, were applied to quantitatively evaluate the influence of several different operating parameters such as pressure, atmosphere and temperature on the formation of NO and SO2during coal combustion in the fluidized bed. The experimental results show that the releasing profiles of CO, NO and SO2during coal combustion under a pressurized oxy-fuel atmosphere are similar to those under a pressurized air atmosphere, and the curves of measured gas components are all unimodal. Under the oxy-fuel condition, pressure increasing from 0.1 to 0.7 MPa can cause the inhibition of NO and SO2emission. The elevation of temperature can lead to an increase in the maximum concentration and the total production of NO and SO2, and the increase under atmospheric pressure is higher than that under high pressure.
pressurized oxy-fuel combustion; fluidized bed; SO2emission; NO emission
Among all the carbon capture utilization and storage (CCUS) technologies, oxy-fuel combustion is one of the most promising ways to achieve the zero-emission goal. Fuel burns with the mixture of pure oxygen and recycle flue gas, making the CO2concentration reach more than 90% (dry basis) in the flue gas. This is conducive to CO2separation from flue gas, and the coal combustion pollutants such as NOxand SOxcan also be removed together. However, the bottleneck of oxy-fuel combustion is of low net efficiency and high cost. Researchers[1-2]have found that if a conventional power station converts from air combustion into oxy-fuel combustion, it will lead to a nearly 10% to 12% drop in its efficiency.
Pressurized oxy-combustion technology was first proposed by Fassbender[3]. Research shows that pressurized oxy-combustion technology has several advantages over atmospheric oxy-combustion. In the pressurized system, the work loss due to the pressure fluctuation among the compression and purification unit (CPU), air separation unit (ASU) and boiler can be substantially reduced. Also, the dew point of flue gas increases at high pressure, then the latent heat of water vapour in the flue gas can be recovered easily.
Pressurized oxy-fuel fluidized bed combustion (FBC) technology is a combination of pressurized oxy-combustion and FBC technology, and it is still in its initial stage. Present studies are mainly focused on economic analysis and numerical simulation, while only a small number concerns experimental work[4]. A lab-scale bubbling bed was used by the Institute for Chemical Processing of Coal in Poland to study NO emissions under air and O2/CO2atmospheres[5], and the results show that pressurized combustion can inhibit the formation of NO and SO2. Researchers from the Delft University of Technology[6]conducted some exploratory studies on a 1.6 MW pressurized fluidized bed, and they found that NO emission level depended mainly on fuel-N and outlet oxygen concentration. Li et al.[7]carried out the experimental research of pressurized oxy-FBC in China. Also, the influence of pressure on fluidization velocity of bed material under thermal state was analyzed, and an equation about critical fluidization velocity was proposed.
For better understanding of SO2and NO generation under the pressurized O2/CO2atmosphere, a lab-scale pressurized fluidized bed was designed and built at Southeast University. Experimental and theoretical analyses have been done to clarify how the pressure and atmosphere affect coal combustion and SO2/NO emission. This work will contribute to a deep understanding of pressurized oxy-FBC technology.
1 Experiment
Tab.1shows the ultimate and proximate analysis of the bituminous coal and lignite used in the experiment. The particle size of coal ranges from 0.45 to 0.6 mm. Silica sand (particle size: 0.25 to 0.35 mm; true density: 2 600 kg/m3) is used as bed material, giving a static bed height of 0.3 m.
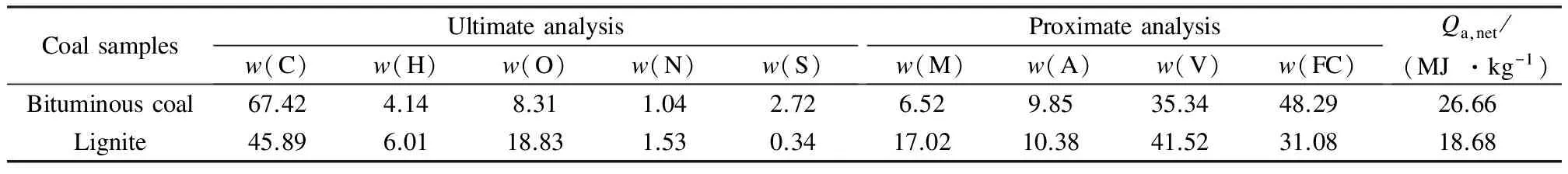
Tab.1 Ultimate and proximate analysis of bituminous coal and lignite on air-dry basis %
Experiments were conducted on a lab-scale pressurized oxy-fuel FBC system, as shown in Fig.1, which consists of the bubbling fluidized bed combustor, gas distribution, feeding systems, temperature and pressure controlling system, flue gas cooling system, and a gas analyzer (ecom-J2KN, produced by rbrMesstechnik GmbH, Germany). The bubbling bed combustor is 1.3 m high, 50 mm in inner diameter, and made of 310S stainless steel.
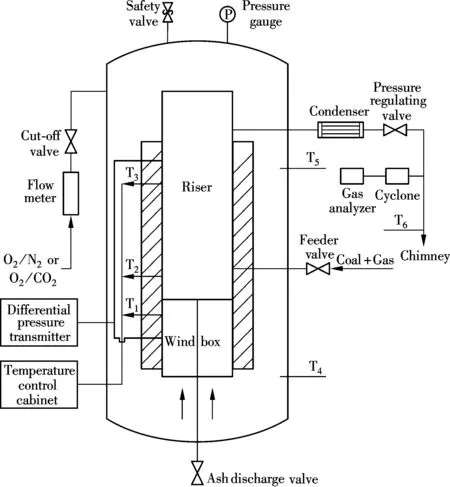
Fig.1 Schematic diagram of pressurized oxy-fuel fluidized bed combustion system
Before each experiment, streams of process gases (O2, CO2and air) were measured and mixed before being supplied into the vessel. The gas flow was turned off when the pressure in the vessel met the working condition, and then the electric heater was turned on around the fluidized bed furnace. The pressure regulating valve was opened slightly when the temperature reached the working states, and the bed material mixed and maintained the fluidization state under the drive of differential pressure inside and outside the vessel. The flowrate and velocity were controlled by changing the valve opening according to the value of differential pressure transmitters. A specific quantity of coal (4, 7 and 10 g) was injected into the dense zone with the N2stream. A gas analyzer was used to analyze and record the composition of flue gas. The analysis process lasted 120 s and this time covered the whole combustion processes of coal particles in the bubbling bed. The coal combustion and pollutant emission characteristics were invested under air and 21%O2/79%CO2atmospheres with the operation pressure ranges from 0.1 to 0.7 MPa. The operation temperature of the dense zone was 750 to 850 ℃, and each experimental condition was repeated 3 times to minimize unreliability in the experiments.
2 Results and Discussion
2.1 Analysis of typical experimental conditions
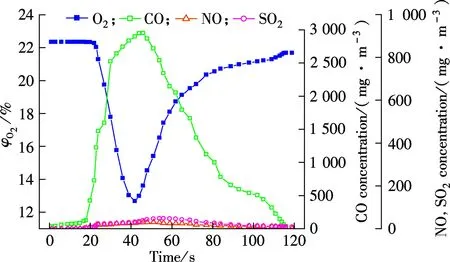
Fig.2 shows the release profiles of O2, CO, NO and SO2during coal combustion in the pressurized air and oxy-fuel atmosphere (0.7 MPa, 850 ℃) respectively, as typical experimental conditions.
It is clear from Fig.2 that the release of the main exhaust gas compounds (O2, CO, NO and SO2) during coal combustion under a pressurized oxy-fuel atmosphere are similar to that under a pressurized air atmosphere, and the curves of measured gas components are all unimodal. The oxygen concentration curve decreases first and then increases whether under air or O2/CO2atmospheres. This is because coal is heated by the bed material and the high temperature gas when it comes into the dense zone, and the combustion reaction of coal consumes large amounts of oxygen leading to a drop of oxygen concentration in flue gas. Since a batch feeding mode is used in the experiments, that is, each time a certain quality of coal particles is injected into the chamber, and at the end of combustion reaction, the oxygen consumption rate is reduced gradually, resulting in a slow rise in oxygen concentration.
As shown in Fig.2, for the gaseous pollutants CO, NO and SO2in two atmospheres, their concentration curves increase first and then decrease. The coal combustion in the fluidized bed can be divided into two successive processes, volatile combustion and char combustion. Generally, the typical time of devolatilization and particle mixing in the fluidized bed are at the “second” level, while the burnout time of char is much longer, which means that char combustion process accounts for the vast majority of the coal combustion time in a fluidized bed. In the heating process, coal is pyrolyzed and releases a large number of volatile matters containing C, N, and S, such as CH4, C2H4, CO, HCN, NH3, H2S and CS2[8].

(a)
(b)
Then, part of volatile substances will react with oxygen to generate CO, NO and SO2. As the combustion reaction proceeds, the char combustion process will also produce these three kinds of gas, resulting in the rise of pollutant concentrations. According to the same reason for the rise of oxygen concentration at the end of the combustion process, as the reaction goes on, the combustible content of the coal will be gradually reduced; resulting in the slow formation rate and the decrease of the concentrations of CO, NO and SO2.
It is clear from Fig.2 that the maximum amount of each pollutant under two atmospheres are quite different. Compared with pressurized air combustion, the maximum concentration of CO in pressurized oxy-fuel combustion is much higher, about 3 000 mg/m3. This means that the CO concentration in the flue gas in a 21%O2/79%CO2atmosphere is much higher than that in a 21%O2/79%N2atmosphere. The peak values of NO and SO2under a pressurized air are about 150 and 315 mg/m3, while those under a pressurized oxy-fuel atmosphere are 35 and 50 mg/m3, respectively. So it can be concluded that when the oxygen is at the same level, the NO and SO2concentrations in the flue gas under a pressurized O2/CO2atmosphere are lower than those under a pressurized O2/N2atmosphere.
2.2 Effect of pressure on O2/CO2combustion pollutant release process
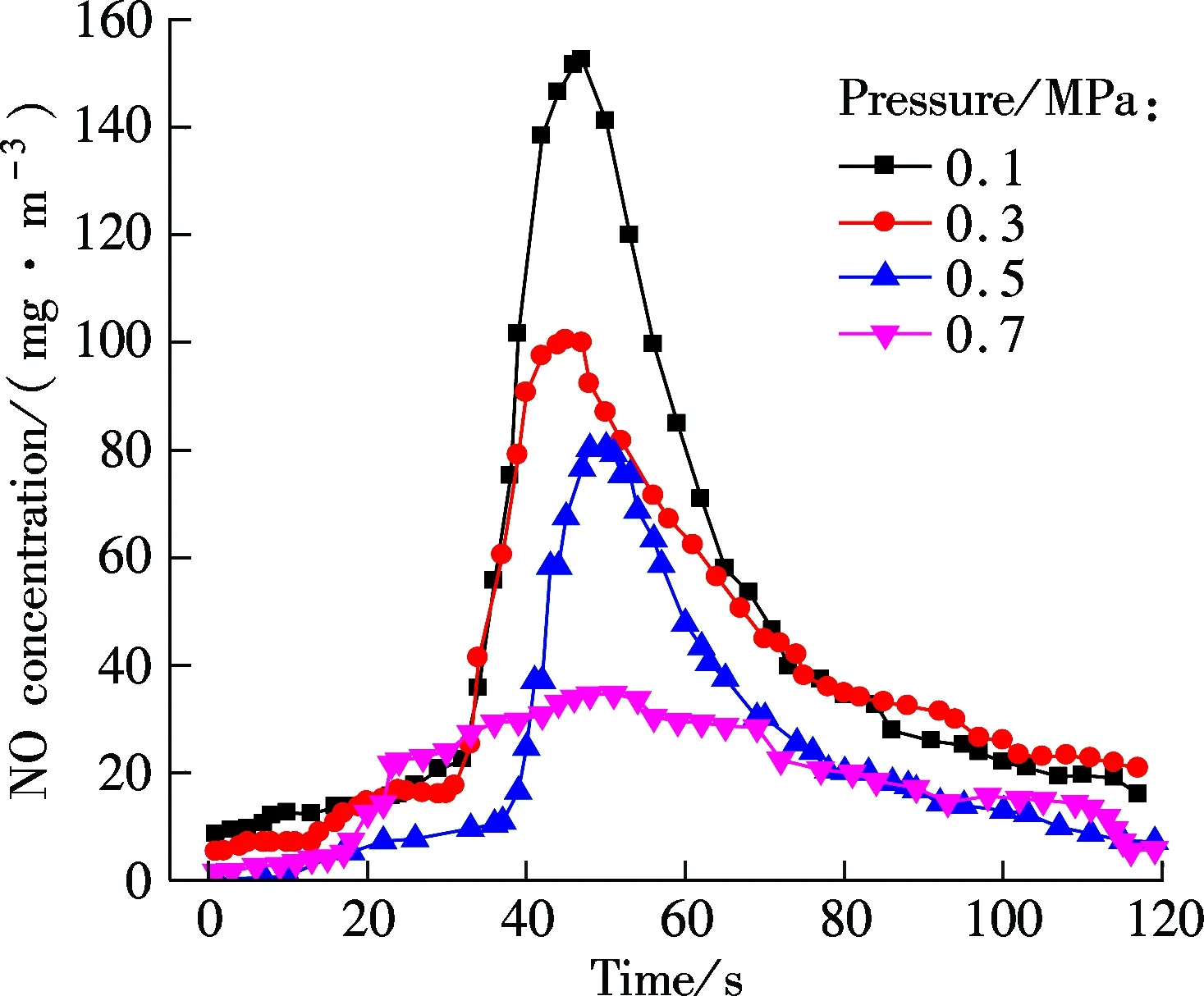
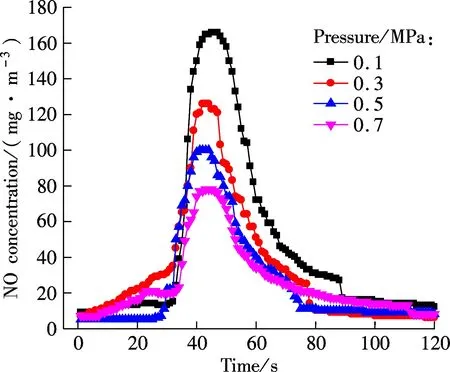
Fig.3 shows the NO concentration curves of bituminous coal and lignite at various pressures during oxy-fuel combustion, respectively. Figs.4 to 6 show the SO2emission curves of bituminous coal at various pressures during oxy-fuel combustion. For easy comparison, the total emission amount of SO2at 850 ℃ with a coal feed of 4 g is regulated as a baseline. Due to the low content of sulphur in Yunnan lignite, its SO2emission will not be analyzed in this study. For Figs.3 and 4, the temperature of the riser is maintained at around 850 ℃, and the amount of coal fed is 7 g. It is clear from Fig.3 that the NO concentration of both types of coal has a significant reduction when the pressure increases from 0.1 to 0.7 MPa.
(a)
(b)
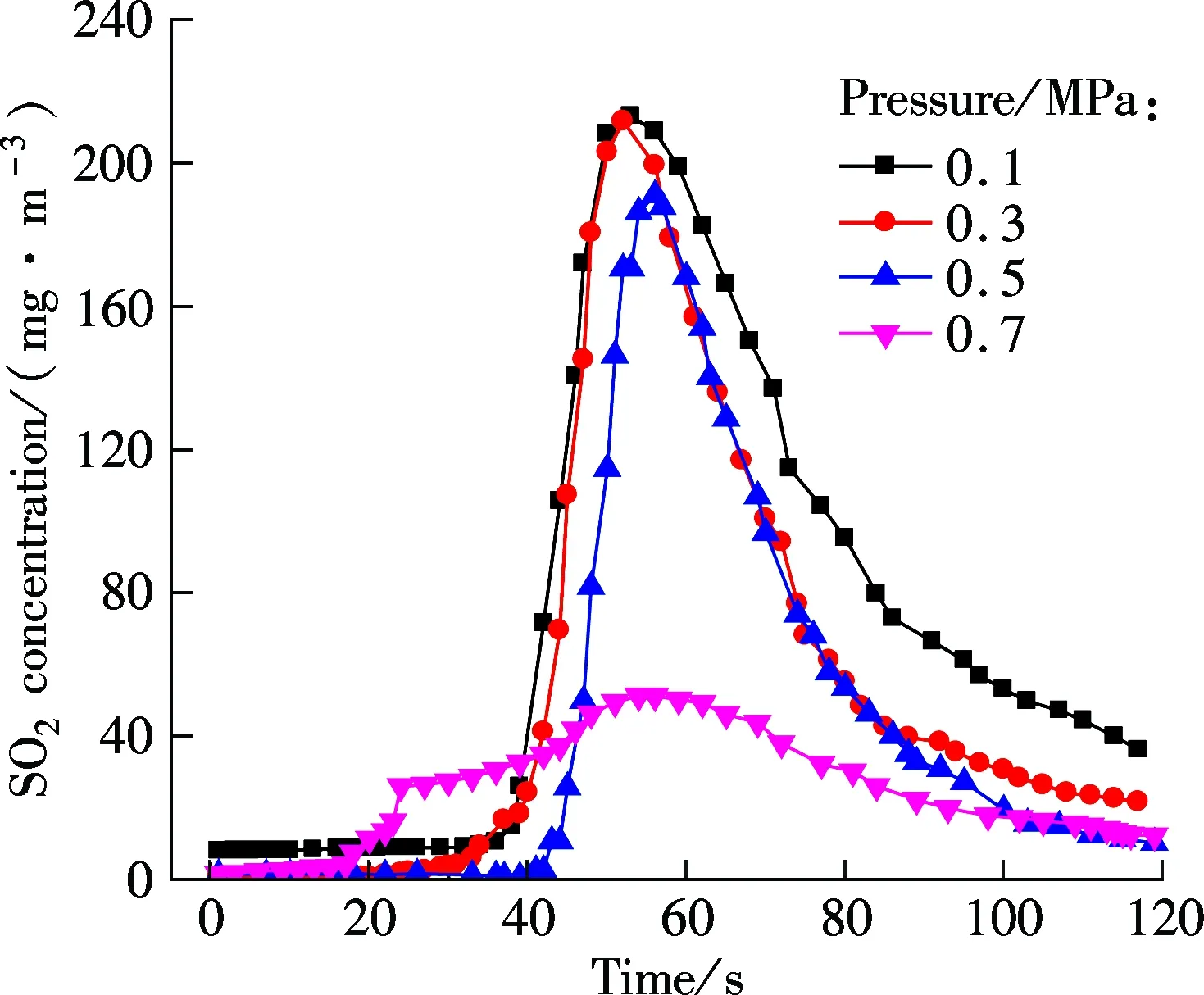
Fig.4 SO2 concentration curves for bituminous coal under various pressures
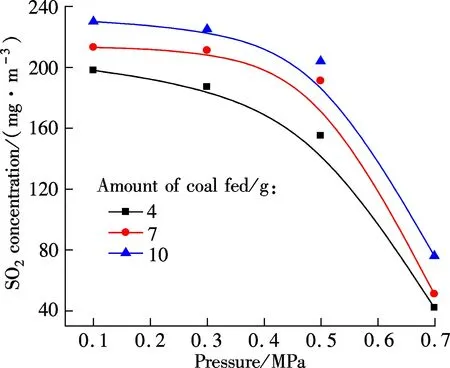
Fig.5 Relationship between the maximum concentration of SO2 and pressure for bituminous coal (O2/CO2)
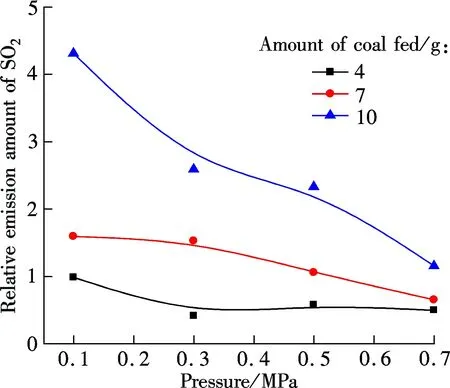
Fig.6 Relationship between SO2 emission and pressure for bituminous coal (O2/CO2)
According to the formation mechanism of NO, the NO generated during coal combustion can be divided into three types, thermal-NO, prompt-NO and fuel-NO. The nitrogen molecules from air oxidized at high temperature is called the thermal-NO, and it depends highly on the combustion temperature. Due to the relatively low temperature of fluidized bed combustion, usually fuel-NO makes up 90% of the total NO. In this section, experiments were conducted under the O2/CO2atmosphere with the dense zone temperature less than 900 ℃, so almost all of the NO measured by the gas analyzer can be considered to be fuel-NO.
The influence of pressure on NO emission can be divided into the following two aspects: on the one hand, the pressure will affect the NO formation during the combustion process[9], and on the other hand, the pressure also affects NO reduction[10]. It can be known from Fig.3 that the amount of NO generated in a fluidized bed during oxy-coal combustion decreases when the operation pressure increases. The oxygen partial pressure increases with the increase in the total pressure if the gas composition is constant, and the high oxygen partial pressure can speed up the oxidation reaction of fuel-N and increase the conversion rate of fuel-N to NO. However, the CO2partial pressure also increases with the increase in the total pressure, and the high CO2partial pressure promotes the formation reaction of CO[11-13], as expressed by the following reactions:
CO2+C→2CO
(1)
CO2+H→CO+OH
(2)
2CO2→2CO+O2
(3)
Also, the existence of the high concentration of CO promotes the NO/CO/char reduction reactions:
NO+CO→0.5N2+CO2
(4)
NO+C→0.5N2+CO
(5)
In addition, the high pressure increases the diffusion resistance of NO from internal to external of char, and thus prolongs the residence time of NO inside the char. Therefore , it makes it easier for NO to be heterogeneously reduced by char. The influence of pressure on the NO emission under the O2/CO2atmosphere is a combined effect of both fuel-N oxidation reaction and NO reduction reaction; therefore, the low NO emissions under high pressure is due to the fact that the NO consumption rate is greater than the formation rate.
The influence of pressures on SO2concentration can be divided into two parts. From 0.1 to 0.5 MPa, SO2concentration has a slight decline. However, when the pressure reaches 0.7 MPa, the SO2concentration decreases significantly. During oxy-fuel combustion, many process parameters such as atmosphere, temperature, excess oxygen ratio and fuel affect the SO2release, and the most important one is the sulfur content in the fuel[14]. Ref.[11] studied SO2emission during oxy-fuel combustion in the fluidized bed at various pressures, and it showed that the pressure has little influence on SO2emission. However, it must be mentioned that in that paper the maximum operation pressure is 0.45 MPa, and to some extent their findings are similar to the results of this study when the pressure ranges from 0.1 to 0.5 MPa. Also, Bo[15]pointed out that increasing pressure can decrease SO2emission. CT5000B sulphur analyzer is used to analyze the sulfur content in fly ash for bituminous coal under various pressures, and the result is shown in Tab.2. It can be demonstrated that the sulfur content in fly ash will increase as the pressure increases, and the increase is particularly clear when the pressure changes from 0.5 to 0.7 MPa. This phenomenon indicates that high pressure enhances the sulfur retention ability of ash under the O2/CO2atmosphere. In summary, the effect of pressure on SO2emission under the O2/CO2atmosphere will appear only at high pressures (>0.5 MPa).

Tab.2 Sulfur content in fly ash for bituminous coal under various pressures
2.3 Effect of atmosphere on pollutants’ release process
A number of studies[4,14]showed that due to the difference in combustion atmosphere, there is a significant difference in N and S release and conversion between oxy-coal combustion and typical air combustion. Compared with atmospheric O2/CO2combustion, the existence of high concentration and a high partial pressure of CO2will have a great impact on coal combustion and gaseous pollutant formation.
Fig.7 is the NO concentration curves for bituminous coal under two different atmospheres at 0.1 and 0.7 MPa. The maximum values of NO concentration are high at an atmospheric pressure under the O2/CO2and air atmosphere, about 150 and 250 mg/m3, respectively. When the pressure reaches 0.7 MPa, the peak values decrease to 35 and 150 mg/m3. Compared with air atmosphere, NO release concentration under the O2/CO2atmosphere at different pressures is relatively low. The reason is that when the O2concentration is constant, the replacement of N2by CO2is beneficial to the generation of CO. Then, the high concentration of CO can stimulate the homogeneous reduction reaction of NO, and a large amount of NO is reduced to N2.
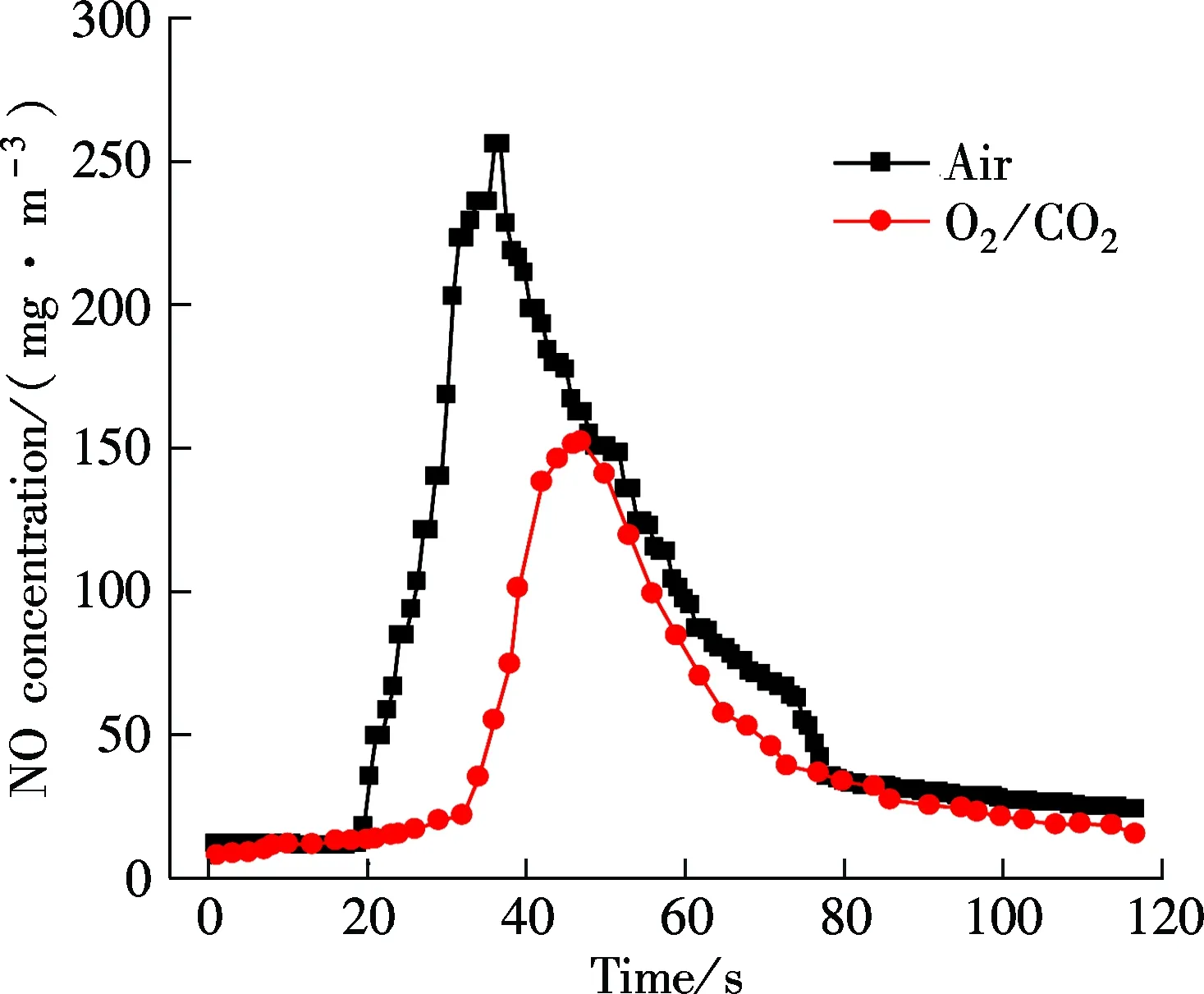
(a)

(b)
Fig.8 and Fig.9 are the SO2concentration for bituminous coal under two different atmospheres at 0.1 and 0.7 MPa, respectively. The total emission amount of SO2during air combustion at 0.1 MPa is set to be the baseline value. The maximum values of SO2concentration are 210 and 630 mg/m3, respectively. When pressure reaches 0.7 MPa, the peak values are about 50 and 320 mg/m3. It is clear that the SO2release concentration during oxy-fuel combustion at different pressures is lower than that during air combustion. Sulfur in coal usually presents in three main types, pyrite, organic and inorganic sulfur. Due to differences in the combustion atmosphere, the high heat capacity of CO2can lower the particle temperature by absorbing more heat generated during combustion, resulting in the low conversion rate of S to gas phase SO2and the enhancement on sulfur retention ability of solid ash[16].
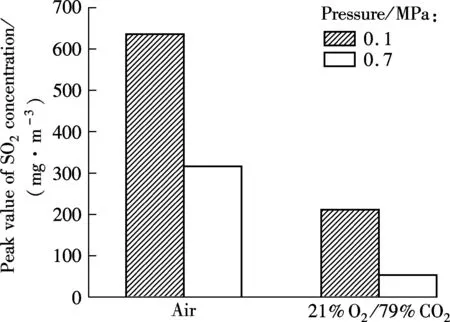
Fig.8 Relationship between the maximum concentration of SO2 and atmosphere for bituminous coal

Fig.9 Relationship between SO2 production and atmosphere for bituminous coal
3 Conclusions
Experiments on the combustion of bituminous coal and lignite were conducted in a lab-scale pressurized fluidized bed system, and the influences of pressure and atmosphere on CO, NO and SO2release process were focused upon. Some conclusions can be drawn as follows:
1) The release of the main exhaust gas compounds (O2, CO, NO and SO2) during coal combustion under a pressurized oxy-fuel atmosphere are similar to those under a pressurized air atmosphere under the baseline condition (0.7 MPa,850 ℃), and the curves of measured gas components are all unimodal.
2) Under the oxy-fuel condition, the increase in pressure can result in the inhibition of NO and SO2emissions, following that as the total pressure increases, both the maximum concentration and the total emissions of NO and SO2decrease.
3) Compared with air combustion, the maximum concentration and the total production of NO and SO2in oxy-fuel combustion are lower.
4) The elevation of temperature can lead to an increase in the maximum concentration and the total production of NO and SO2, and the increase under atmosphere is stronger than that under pressure.
[1]Hong J, Field R, Gazzino M, et al. Operation pressure dependence of the pressurized oxy-fuel combustion power cycle [J].Energy, 2010, 35(12): 5391-5399.
[2]Hong J, Chaudhry G, Brisson J G, et al. Analysis of oxy-fuel combustion power cycle utilizing a pressurized coal combustor [J].Energy, 2009, 34(9): 1332-1340.
[3]Fassbender A. Power system with enhanced thermodynamic efficiency and pollution control: US Patent, 6196000 [P]. 2001-03-06.
[4]Chen L, Yong S Z, Ghoniem A F. Oxy-fuel combustion of pulverized coal: characterization, fundamentals, stabilization and CFD modeling [J].ProgressinEnergyandCombustionScience, 2012, 38(2): 156-214.
[5]Lasek J A, Janusz M, Zuwala J, et al. Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures [J].Energy, 2013, 62(1): 105-112.
[6]Andries J, Becht J G M, Hoppesteyn P D J. Pressurized fluidized bed combustion and gasification of coal using flue gas recirculation and oxygen injection [J].EnergyConversionandManagement, 1997, 38(Sup): S117-S122.
[7]Li H Y, Yan W P, Wang C B, et al. Experimental study on minimum fluidization velocity at elevated pressure and high temperature [J].ProceedingsoftheCSEE, 2011, 13(32): 8-15. (in Chinese)
[8]Strezov V, Lucas J, Strezov L. Experimental and modelling of the thermal regions of activity during pyrolysis of bituminous coals [J].JournaloftheAnalyticalandAppliedPyrolysis, 2004, 71(1): 375-392.
[9]Lu Y, Jahkola A, Hippinen I, et al. The emissions and control of NOxand N2O in pressurized fluidized bed combustion [J].Fuel, 1992, 71(6): 693-699.
[10]Lin S Y, Suzuki Y, Hatano H. Effect of pressure on NOxemission from char particle combustion [J].Energy&Fuels, 2002, 16(3): 634-639.
[11]Janusz A L, Krzysztof G, Marcin J, et al. Pressurized oxy-fuel combustion: a study of selected parameters [J].Energy&Fuels, 2012, 26(11): 6492-6500.
[12]Okazaki K, Ando T. NOxreduction mechanism in coal combustion with recycled CO2[J].Energy, 1997, 22(2): 207-215.
[13]Hu Y Q, Kobayashi N, Hasatani M. Effects of coal properties on recycled-NOxreduction in coal combustion with O2/recycled flue gas [J].EnergyConversionandManagement, 2003, 44(14): 2331-2340.
[14]Toftgaard M B, Brix J, Jensen P A, et al. Oxy-fuel combustion of solid fuels [J].ProgressinEnergyandCombustionScience, 2010, 36(5): 581-625.
[15]Bo L. Fluidized bed combustion: mixing and pollutant limitation [J].ProgressinEnergyandCombustionScience, 1998, 24(1): 31-61.
[16]Croiset E, Thambimuthu K V. NOxand SO2emissions from O2/CO2recycle coal combustion [J].Fuel, 2001, 80(14): 2117-2121.
煤在增压流化床O2/CO2气氛下的燃烧及污染物排放特性
段元强1段伦博1胡海华1,2赵长遂1
(1东南大学能源与环境学院, 南京 210096)
(2中国电建集团江西省电力设计院, 南昌 330096)
在空气和O2/CO2气氛下进行烟煤和褐煤的燃烧实验以考察压力和气氛对煤燃烧以及CO, NO, SO2析出过程的影响.采用排放峰值与排放总量2个指标来评估压力、气氛和温度等操作参数对煤在增压流化床富氧燃烧过程中NO及SO2生成的影响.结果表明:煤在增压富氧燃烧时CO, NO和SO2析出规律和增压空气燃烧时的规律相似,各组分气体均呈现单峰析出;O2/CO2气氛下,压力从0.1 MPa提高到0.7 MPa会抑制NO和SO2生成;随着温度的升高,NO和SO2的排放峰值和总量均增大,常压时两者增加的幅度要高于加压时.
增压富氧燃烧;流化床;SO2排放; NO排放
TK16
Foundation items:The National Natural Science Foundation of China (No.51206023), the National Key Basic Research Program of China (973 Program) (No.2011CB707301-3), the Fundamental Research Funds for the Central Universities.
:Duan Yuanqiang, Duan Lunbo, Hu Haihua, et al. Combustion and pollutant emission characteristics of coal in a pressurized fluidized bed under O2/CO2atmosphere[J].Journal of Southeast University (English Edition),2015,31(2):188-193.
10.3969/j.issn.1003-7985.2015.02.005
10.3969/j.issn.1003-7985.2015.02.005
Received 2015-01-11.
Biographies:Duan Yuanqiang (1990—),male, graduate; Duan Lunbo (corresponding author), male, doctor, associate professor, duanlunbo@seu.edu.cn.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Adaptive modulation in MIMO optical wireless communication systems
- An improving energy efficiency cooperation algorithm based on Nash bargaining solution in selfish user cooperative networks
- Performance analysis of an O2/CO2 power plantbased on chemical looping air separation
- Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
- A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
- Applicability of Markov chain-based stochastic modelfor bubbling fluidized beds
