A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
2015-05-08TangHongjianDuanYufengZhuChunZhouQiangSheMinCaiLiang
Tang Hongjian Duan Yufeng Zhu Chun Zhou Qiang She Min Cai Liang
(Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, China)
A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
Tang Hongjian Duan Yufeng Zhu Chun Zhou Qiang She Min Cai Liang
(Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, China)
A novel carbon trap sampling system for gas-phase mercury measurement in flue gas is developed, including the high efficient sorbents made of modified biomass cokes and high precision sorbent traps for measuring particle-bound and total vapor-phase mercury in flue gas. A dedusting device is installed to collect fine fly ash for reducing the measurement errors. The thorough comparison test of mercury concentration in flue gas is conducted between the novel sampling system and the Ontario hydro method (OHM) in a 6 kW circulating fluidized bed combustor. Mercury mass balance rates of the OHM range from 95.47% to 104.72%. The mercury breakthrough rates for the second section of the sorbent trap are all below 2%. The relative deviations in the two test cases are in the range of 15.96% to 17.56% under different conditions. The verified data suggest that this novel carbon trap sampling system can meet the standards of quality assurance and quality control required by EPA Method 30B and can be applied to the coal-fired flue gas mercury sampling system.
mercury sorbent trap; coal-fired flue gas; mercury sampling unit
Coal-fired power plants are the largest source of anthropogenic mercury emissions. According to the global mercury assessment[1], approximately 484 t of mercury in the air originated from coal-fired power plants in 2010, accounting for 24% of total anthropogenic mercury emissions. Mercury pollution has been of considerable concern due to its high toxicity, which is widely known to cause neurological, accumulative and permanent damage in humans[2].
Actually, research on the mechanism of mercury speciation and emission can help to effectively control mercury pollution from coal-fired flue gas. Therefore, it is necessary to develop novel sampling and monitoring technologies to measure mercury in flue gas with accuracy and expedience. The present monitoring technologies for industrial application can be divided into two categories[3]. They are the artificial sampling and analyzing method and the mercury continuous emission monitoring system (Hg-CEMS).
The Ontario hydro method (OHM) is recognized to be the standard and reference method for artificial mercury measurements in flue gas[4]. However, its operational complexity and errors tendency in solution preparation, digestion and analysis are not negligible. The Hg-CEMS can continuously sample the stack gases and provide an indication of mercury concentration in the flue gas. However, the high cost and difficult equipment maintenance have significantly restricted its wide application[5]. In recent years, a sorbent trap method known as EPA Method 30B has been gradually accepted internationally[6]for its easy operation, high precision and low cost. Nevertheless, the sorbent traps can be blocked by the ash in the flue gas easily. Another remarkable problem is the high cost of the activated carbons used in the sorbent traps. Therefore, it is highly imperative to develop a low cost and highly effective mercury sorbent to serve the carbon traps based on a broad potential utilization in coal-fired power plants. A novel self-developed carbon trap sampling system for gas-phase mercury measurements in flue gas based on the EPA Method 30B is introduced in this paper, focusing on the preparation of high efficient sorbents derived from modified biomass cokes and the innovative design of the dedusting device. Experimental tests for mercury measurement are conducted in a 6 kW circulating fluidized bed (CFB) combustor. The comparative results between the novel sampling system and the OHM were analyzed to evaluate the performance of this novel sampling system for flue gas mercury measurements.
1 The Mercury Carbon Trap Sampling System
The novel self-developed carbon trap sampling system adheres to the technical requirements of the quality accuracy and quality control (QA/QC) of EPA Method 30B. The entire sampling system consists of five parts (see Fig.1), namely, the dedusting device, the carbon sorbent trap, water removal, the flow controller, and the temperature controller.
1.1 Mercury carbon trap
The whole carbon sorbent trap is made of Pyrex, which
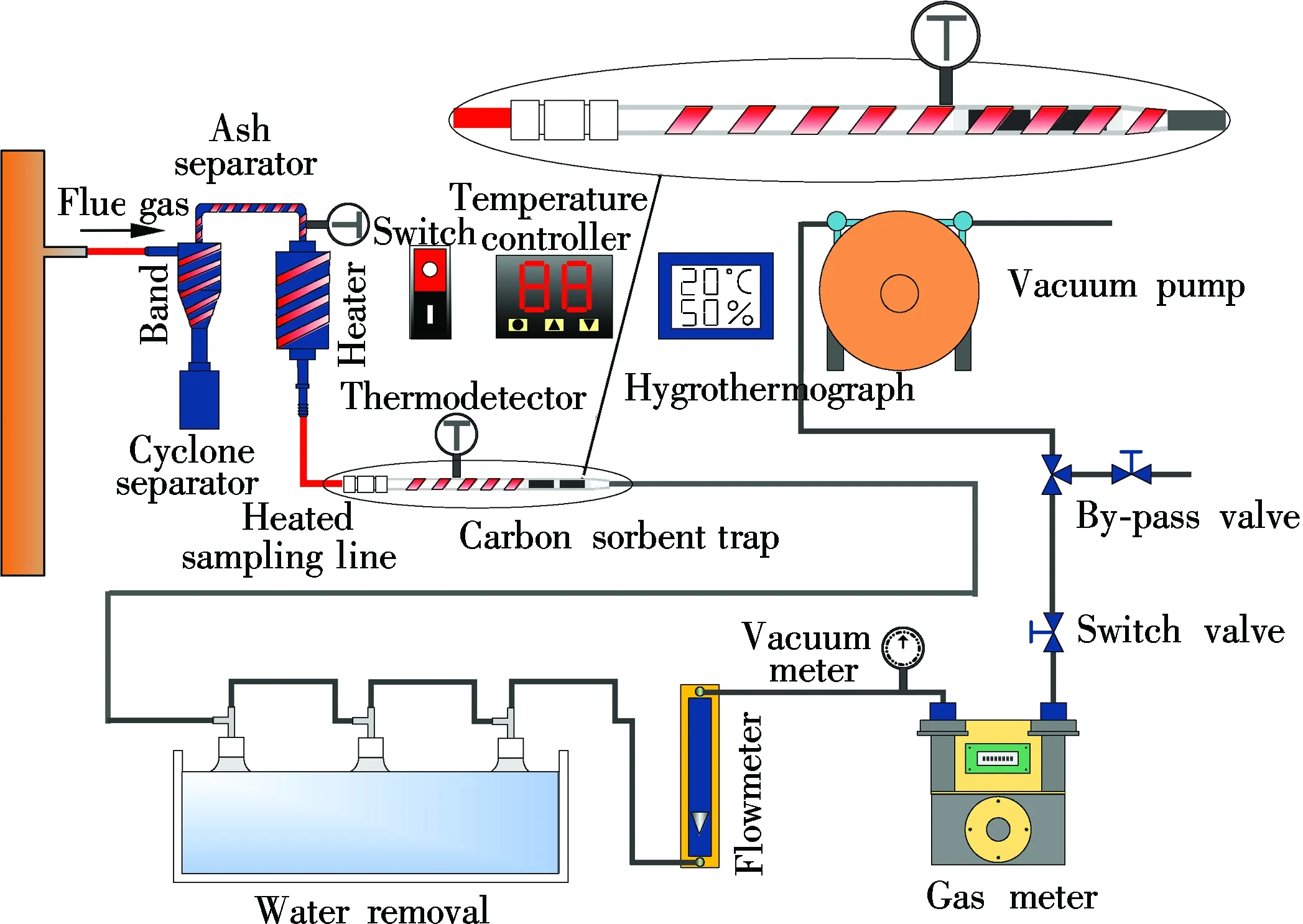
Fig.1 Schematic diagram of carbon trap sampling system
is a type of heat resisting glass and has mercury adsorption free on the tube surface. The standard size of 200-mm length and 10-mm outside diameter of the sorbent trap is adapted, as shown in Fig.2. For easy installation and disassembly, a connector made of Teflon is used between the carbon sorbent trap and the front sampling nozzle.
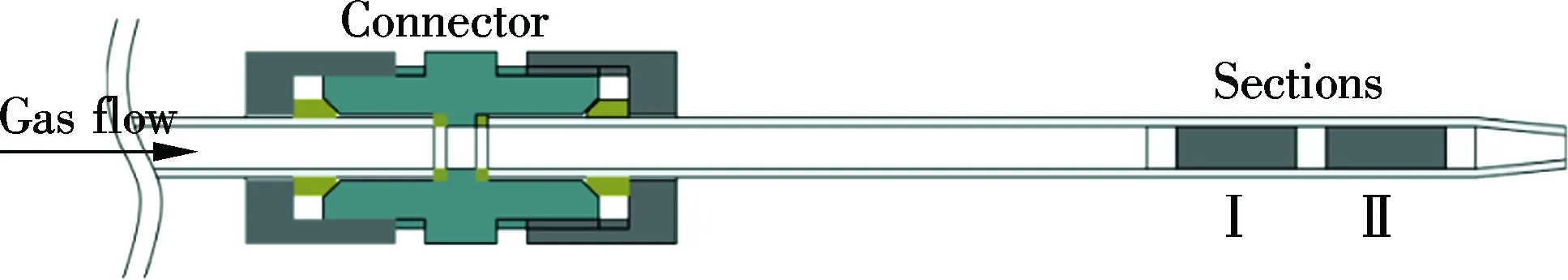
Fig.2 Carbon sorbent trap for mercury measurement
This sorbent trap consists of two separated sorbent sections which are insulated by the fiberglass. Section Ⅰ represents the absorption part and Section Ⅱ is the breakthrough part. Each section is loaded with 500 mg sorbents.
1.2 Dedusting device
The sorbent traps can be blocked by the ash in the flue gas[7]. A dedusting device is equipped to prevent the ash from jamming the sorbent traps so that the sampling can be conducted in flue gas with the ash concentration. As illustrated in Fig.3, the dedusting device contains a cyclone separator and filter. During the sampling, the large particle ash (PM 10) in the flue gas is separated by the cyclone, while the remaining ash (PM 2.5) is screened by the filter. This preprocessed gas guarantees a measurement which is accurate and reliable.
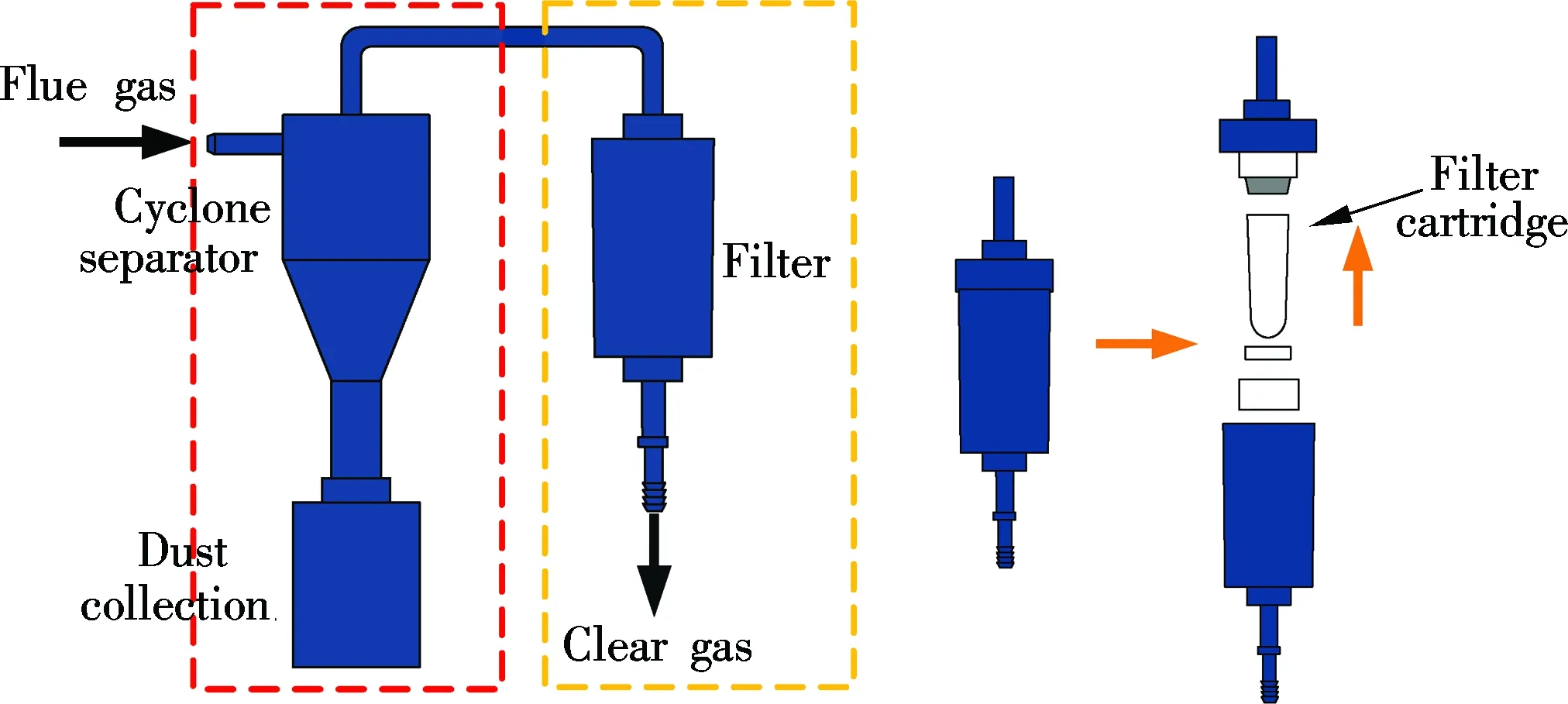
Fig.3 Schematic diagram of dedusting device
1.3 Control of temperature and flow rate
The sampling line is heated electrically and coupled with the PID temperature control ensuring that the entire dedusting, sampling and adsorption process are conducted under a stable temperature around 120 ℃ for preventing the steam and gas-phase mercury in the flue gas from condensing. The isokinetic sampling and flow regulation are operated by the flow meter, gas meter and vacuum pump, restricting the sampling flow error within 2%[6].
2 Quality Accuracy and Quality Control
In order to ensure the precision of sampling results, strict adherence is required to the EPA standard regulations of QA/QC[6]. The gas tightness should be checked before and after the sampling test. The negative pressure of the leak check must be 50.662 kPa[8]minimum. In detail, before sampling, the gas leak flow should be less than 4% of the setting flow, while after sampling, the gas leak flow should be less than 4% of the average flow.
2.1 Performance of sorbents
Since the commonly used sorbent trap is of high cost due to the consumption of the activated carbons, it is nessary to develop highly efficient but low cost sorbents. An innovative carbon sorbent made of modified biomass coke is proposed in this study for replacing the activated carbons, which performs as efficiently as commercial activated carbon[9].
Three kinds of modified biomass sorbents are tested on the fixed bed adsorption systems to verify their adsorption capacity for mercury (see Fig.4). The performance test is conducted in the simulated flue gas at 150 ℃, which has similar components to the coal fired flue gas. The tested sorbents are placed on the adsorption column homogeneously. The gas-phase mercury can be adsorbed by the sorbents when the simulated flue gas passes through.
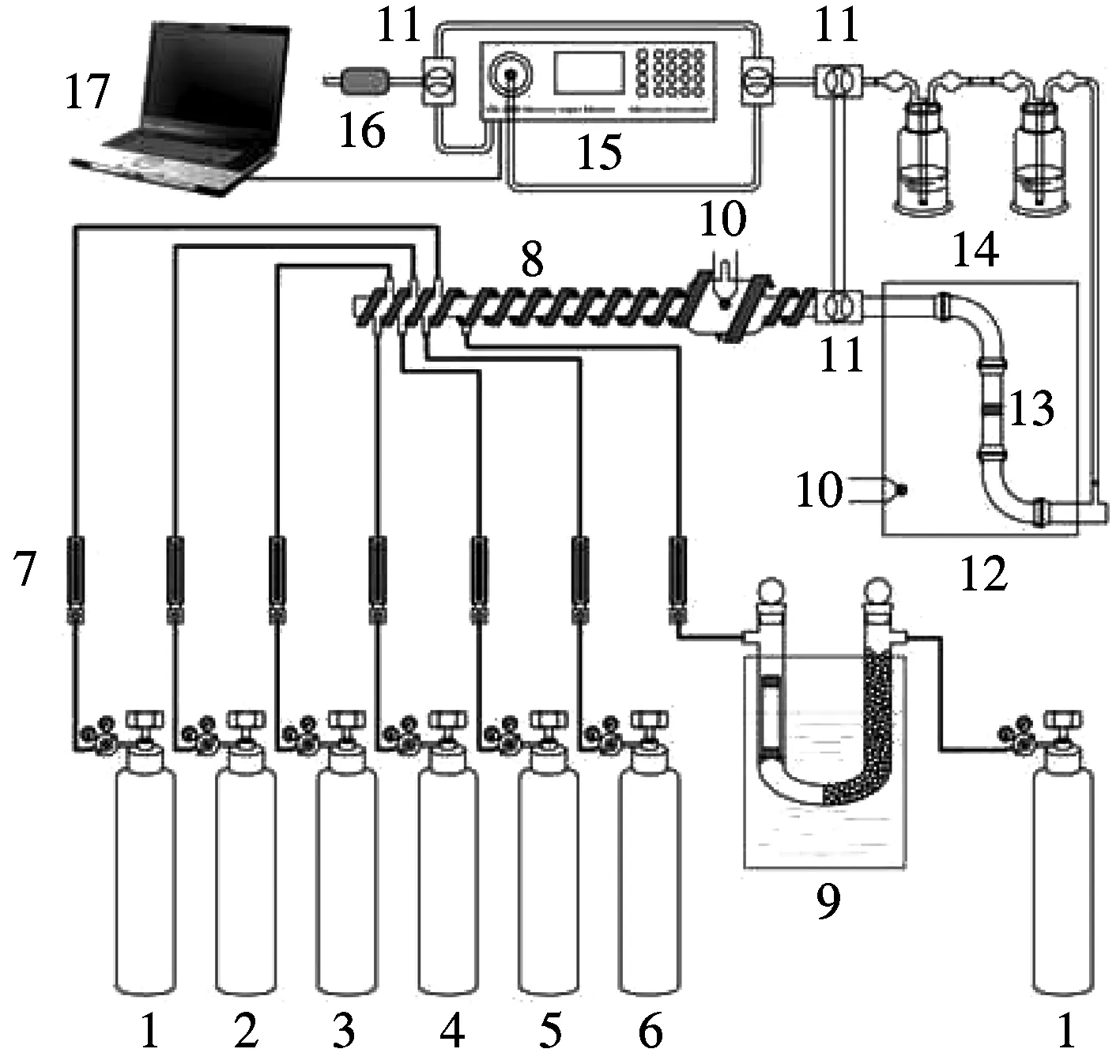
1 to 6—Simulated flue gas; 7—Flow meter; 8—Electrical heating belts; 9—Mercury permeation device; 10—Thermocouple; 11—Teflon triple valve; 12—Hot box; 13—Adsorption column; 14—Absorption liquid; 15—Mercury analyzer; 16—Activated carbon column; 17—Computer
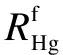
(1)
(2)
2.2 Quantity of adsorbed Hg mass
Fig.5 indicates the definition of mercury mass in each section of the trap. According to the protocols[12]of EPA Method 30B, the mass of mercury adsorbed in Section Ⅰ (m1) should be the sum of P1, S1and P2, and mercury in Section Ⅱ (m2) should be the sum of S2and P3. S1and S2mean the sorbents while P1, P2and P3represent the interval layers of fabric filter.
m1=mP1+mS1+mP2
(3)
m2=mS2+mP2
(4)
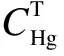
(5)

Fig.5 Schematic diagram of sorbent trap
2.3 Breakthrough rate
To evaluate the precision of the carbon trap, the breakthrough rate (RB) of Section Ⅱ is defined by
(6)
A valid sample[13]conducted by the carbon trap requires that if the Hg concentration is more than 1 μg/m3, RB must be within 10%, and if the Hg concentration is less than or equal to 1 μg/m3, RB must be within 20%. Otherwise, the sample data are invalid.
2.4 Relative deviation
The relative deviation (RD) is evaluated by comparing the measuring results of sorbent traps with the simultaneous results obtained by the OHM, which is accepted as the reference method for mercury measurements in flue gas. RD is calculated by
(7)
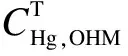
3 Results and Discussion
3.1 Adsorption ability of sorbents
Three kinds of sorbents (AC-HBr, AC-KI and HC-PHBr) are selected for the adsorption ability test. AC-HBr and AC-KI are two kinds of activated carbons modified by HBr and KI, respectively, while RHC-PHBr is a kind of biomass (rice husk) coke chemically treated by phosphorus activation and HBr modification.
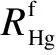
The data in Tab.1 suggest that these tested sorbents passed the restrictions ofRHg≤120% andBHg≤5%, indicating that when the simulated gas flows through the adsorption column, gas-phase mercury is adsorbed by the sorbents completely and immediately. Moreover, the modified biomass cokes perform perfectly as the activated carbons for mercury adsorption. The adsorption ability test suggests that the modified sorbents possess an outstanding mercury adsorption property and capacity, and are competent for the Method 30B sorbent traps measurement, which should be recognized for its significant contribution to reducing the high sampling cost.
3.2 Hg mass balance by OHM
The OHM is adopted as the reference method during the comparison test. To ensure the validity,the Hg mass balance by the OHM is also examined under different conditions, as shown in Tab.2.
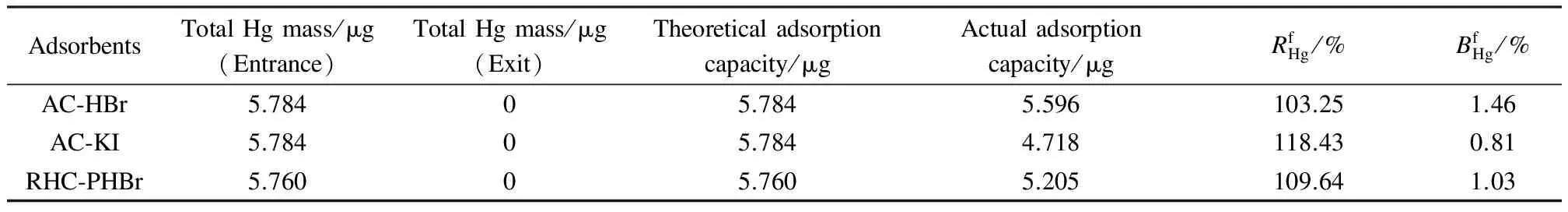
Tab.1 Hg equilibrium test for sorbent

Tab.2 Measuring results by the OHM
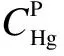
3.3 Comparison test between sorbent trap and OHM
In order to evaluate this novel mercury carbon trap sampling system, a thorough comparison test of measurements on mercury speciation concentration in flue gas is conducted between the carbon trap sampling system and the OHM in a 6 kW CFB combustor. Different types of coal are used in the four sampling runs. During the comparison test, great attention is paid to evaluating the practicability and precision of this novel sampling system.
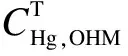

Fig.6 Comparison of sorbent trap and OHM
The RB and RD were calculated for deep discussion on the measuring results by sorbent traps, as shown in Tab.3.
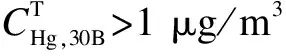

Tab.3 Measuring results by sorbent trap
4 Conclusions
1) The fixed-bed Hg adsorption tests indicate that the modified biomass cokes (RHC-PHBr) perform perfectly as the activated carbons (AC-HBr, AC-KI) for mercury adsorption. All the modified sorbents are feasible for Method 30B sorbent traps.
2) The gas-phase mercury is completely absorbed by the sorbent traps during the sampling. The novel sorbent traps have consistent results with the reference method OHM. Both the relative deviation and breakthrough rate of sorbent traps show negligible fluctuation with the variations of the mercury concentration in the flue gas and meet the requirements of QA/QC of EPA Method 30B.
3) This novel carbon sorbent trap sampling system possesses high precision and reliability and it can be applied to the coal-fired flue gas mercury sampling.
[1]UNEP Chemicals. Global mercury assessment 2013 sources, emissions, releases and environmental transport[EB/OL]. (2013-01)[2015-02-02].http://www.unep.org/PDF/PressReleases/GlobalMercuryAssessment2013.pdf.
[2]Myers G J, Davidson P W. Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research [J].EnvironmentalHealthPerspectives, 1998, 106(Sup3): 841-847.
[3]Yang Xianghua. Mercury emission analysis and prediction in a pulverized coal-fired boiler system of power plant [D]. Nanjing: School of Energy and Environment, Southeast University, 2006. (in Chinese)
[4]ASTM D6784—02 Standard test method for elemental, oxidized, particle-bound, and total mercury in flue gas generated from coal-fired stationary sources (Ontario-hydro method) [S]. Philadelphia, Pennsylvania, USA: American Society for Testing and Materials International, 2008.
[5]Zhang Jie. Online monitoring technology for mercury emission into air from coal-fired power plants and its application [J].HuadianTechnology, 2011, 33(7):72-76. (in Chinese)
[6]EPA Method 30B. Determination of total vapor phase mercury emissions from coal-fired combustion sources using carbon sorbent traps [S]. Washington DC: United States Environmental Protection Agency, 2008.
[7]Zhong Li, Xiao Ping, Jiang Jianzhong, et al. Study on several measuring methods of mercury emission from coal-fired power plants[J].ProceedingsoftheCSEE, 2012, 32(Sup1): 158-163. (in Chinese).
[8]Zhang Disheng, Zhou Gang. Dual independent mercury sampling method based on the adsorption principle [J].TheAdministrationandTechniqueofEnvironmentalMonitoring, 2013, 25(2): 47-49, 56. (in Chinese)
[9]Fuente-Cuesta A, Diaz-Somoano M, Lopez-Anton M A, et al. Biomass gasification chars for mercury capture from a simulated flue gas of coal combustion [J].JournalofEnvironmentalManagement, 2012, 98: 23-28.
[10]Zheng Y. Mercury removal from cement plants by sorbent injection upstream of a pulse jet fabric filter [D]. Lyngby, Denmark: Department of Chemical and Biochemical Engineering, Technical University of Denmark, 2012.
[11]Lee S S, Lee J Y, Keener T C. Novel sorbents for mercury emissions control from coal-fired power plants [J].JournaloftheChineseInstituteofChemicalEngineers, 2008, 39(2): 137-142.
[12]Sorbent trap analysis procedure [EB/OL]. (2008-10-28) [2015-01-12]. http://www.ohiolumex.com/download/SOP_Sorbent_Trap_Testing_102808.pdf.
[13]Dennis L Laudal. Conducting a RATA of continuous mercury monitors using EPA Method 30B [J].FuelProcessingTechnology, 2009, 90(11): 1343-1347.
碳管法燃煤烟气汞浓度取样装置研制
汤红健 段钰锋 朱 纯 周 强 佘 敏 蔡 亮
(东南大学能源热转换及其过程测控教育部重点实验室, 南京 210096)
自主研制了包括内置吸附剂和两段式碳吸附管在内的整套新型碳管法烟气汞浓度取样装置,以期实现燃煤烟气中颗粒汞和气相总汞浓度的精确测量.在6 kW燃煤循环流化床装置上同时采用碳管法与安大略标准法(OHM)进行烟气中汞浓度取样.结果表明,碳管法所得汞平衡率均处于95.47%~104.72%之间.不同工况下碳吸附管第2段穿透率始终低于2%,且与相同工况下OHM测试结果的相对偏差在15.96%~17.56%之间,均小于20%.结果表明,所研制的碳吸附管干法烟气汞浓度取样装置符合美国EPA质量保证和质量控制(QA/QC)标准,可应用于实际燃煤烟气汞浓度的取样测试.
汞吸附管;燃煤烟气;汞取样装置
X511
Foundation items:The National Natural Science Foundation of China (No.51376046, 51076030), the National Science and Technology Support Program of China (No.2012BAA02B01), the Fundamental Research Funds for the Central Universities, the Scientific Innovation Research of College Graduates in Jiangsu Province (No.CXZZ13_0093, KYLX_0115, KYLX_018).
:Tang Hongjian, Duan Yufeng, Zhu Chun, et al. A novel carbon trap sampling system for coal-fired flue gas mercury measurement[J].Journal of Southeast University (English Edition),2015,31(2):244-248.
10.3969/j.issn.1003-7985.2015.02.015
10.3969/j.issn.1003-7985.2015.02.015
Received 2015-01-11.
Biographies:Tang Hongjian (1991—), male, graduate; Duan Yufeng (corresponding author), male, doctor, professor, yfduan@seu.edu.cn.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Adaptive modulation in MIMO optical wireless communication systems
- An improving energy efficiency cooperation algorithm based on Nash bargaining solution in selfish user cooperative networks
- Performance analysis of an O2/CO2 power plantbased on chemical looping air separation
- Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
- Applicability of Markov chain-based stochastic modelfor bubbling fluidized beds
- Composite bioabsorbable vascular stents via 3D bio-printingand electrospinning for treating stenotic vessels
