磁共振形态学半定量评分对新生儿细菌性脑膜炎出院结局的评估价值
2015-05-04杨鸣姝帕米尔乔中伟
杨鸣姝 王 莉 翟 倩 帕米尔 周 剑 曹 云 乔中伟
·论著·
磁共振形态学半定量评分对新生儿细菌性脑膜炎出院结局的评估价值
杨鸣姝1王 莉1翟 倩2帕米尔1周 剑1曹 云2乔中伟1
目的 分析MRI形态学半定量评分对新生儿细菌性脑膜炎出院结局的评估价值。方法 收集复旦大学附属儿科医院2011年7月至2013年12月NICU收治的出院诊断为新生儿细菌性脑膜炎的病例,采用基于大脑损伤MRI形态学分析的半定量评分,对头颅MRI图像进行回顾性分析。MRI形态学评价包括脑室扩大、脑室旁白质容积丢失、脑白质囊性病灶、内囊后肢髓鞘化异常、皮质信号异常、颅内脑外间隙异常、基底节信号异常、脑白质非囊性信号异常、脑室内出血、脑室积脓、脑膜异常强化、室管膜异常强化和脑脓肿。将上述13项评分归纳为脑白质异常(WMA)、脑灰质异常(GMA)和非脑实质异常(NPA)。同时采集患儿出生孕周、发病时间、MRI检查时间、发病至MRI检查间隔时间和出院结局。按照出生孕周分为早产儿组和足月儿组,再按照出院结局分为预后良好和预后不良亚组,在各组内比较亚组之间时间因素、MRI单项评分和综合评分的差异。结果 63例新生儿细菌性脑膜炎病例进入分析(早产儿组18例,足月儿组45例)。MRI单项评分构成预后良好和预后不良亚组间差异有统计学意义的指标:早产儿组中有脑室扩大(P=0.012)和脑室旁白质容积丢失(P=0.004);足月儿组有脑室扩大(P=0.002)、脑室旁容积丢失(P=0.040)、颅内脑外间隙异常(P=0.005)和脑室内出血(P=0.038)。MRI综合评分中,早产儿组WMA评分(P=0.001)和NPA评分(P=0.039)、足月儿组NPA评分(P=0.018)在预后不良和预后良好亚组之间分布差异有统计学意义。足月儿组和早产儿组内不同预后亚组的各时间因素差异未发现统计学意义或临床意义。结论 新生儿细菌性脑膜炎MRI脑室扩大和脑室旁白质容积丢失预示早产儿出院不良结局;脑室扩大、脑室旁白质容积丢失、颅内脑外间隙异常和脑室内出血预示足月儿出院不良结局。WMA评分高预示早产儿出院不良结局,NPA评分高预示早产儿和足月儿出院不良结局。
新生儿; 细菌性脑膜炎; 磁共振成像; 脑损伤; 出院结局

新生儿细菌性脑膜炎脑损伤形式多样,与不良预后密切相关[9~12]。常规MRI和弥散加权成像可以敏感地发现脑膜炎时不同部位的脑损伤[12~14],可为新生儿细菌性脑膜炎的诊断、治疗及预后判定提供客观依据。但目前尚无基于MRI的新生儿细菌性脑膜炎脑损伤的统一分类标准,对各种MRI影像学异常与预后的关系尚无系统地阐述;且上述文献中报道MRI检查时的病程长短不一,未对可能与MRI检查结果有关的时间因素进行具体分析。有文献报道新生儿颅脑MRI较晚检查的阳性发现才与预后相关[15,16],早期MRI检查对预后判断的价值值得商榷。
笔者在临床中发现新生儿细菌性脑膜炎MRI表现以大脑异常为主,故本文采用基于大脑损伤MRI形态学表现的半定量评分系统,对复旦大学附属儿科医院(我院)NICU收治的新生儿细菌性脑膜炎病例的头颅MRI图像进行回顾性分析。初步探讨与出院时预后有关的MRI形态学表现,同时分析出生孕周、发病时间、MRI检查时间是否可能影响MRI结果的判断。
1 方法
1.1 纳入标准 回顾性收集我院2011年7月1日至2013年12月31日NICU住院患儿出院诊断中含“新生儿脑膜炎”或“化脓性脑膜炎”的病历。我院新生儿细菌性脑膜炎的诊疗常规主要参考第4版《实用新生儿学》[8],诊断标准主要依据临床表现,脑脊液常规、生化和培养结果。
1.2 剔除标准 ①出院诊断中含以下的病例:先天性畸形或综合征、先天性TORCH感染、病毒性脑膜炎、神经梅毒、结核性脑膜炎、真菌性脑膜炎、新生儿窒息、新生儿缺氧缺血性脑病和代谢性脑病;②不是因为脑膜炎死亡的病例;③出生史不详的病例(如弃婴);④过期产儿;⑤病历记载发病时足月儿日龄>28 d,早产儿纠正胎龄>40周;⑥在发病后未在我院行头颅MRI检查者。
1.3 分组考虑 根据患儿出生孕周分为早产儿组和足月儿组。根据出院时的预后结局行亚组分析,包括预后良好亚组和预后不良亚组。满足以下一项及以上者定义为预后不良[17~19]:①出院时存在神经系统症状或体征(抽搐、肌张力异常、颅神经瘫痪);②视觉、听觉神经电生理检查异常;③全身运动(GMs)评估异常;④脑软化;⑤脑内脓肿;⑥需手术治疗的脑积水。不含上述任何一种情况者定义为预后良好。
1.4 观察及截取指标 本文以新生儿细菌性脑膜炎出院时为结局,分析MRI形态学评分的价值,与MRI检查结果可能有关的时间因素。故截取以下资料用于本文分析:①与新生儿细菌性脑膜炎有关的临床表现,脑脊液常规、生化和培养结果;②MRI评分;③MRI评分与时间因素(早产儿以纠正胎龄计,足月儿以生后周龄计)的关系,新生儿出生孕周,发病时间(需要说明,发病时间以病史明确记录为准,如果从病史中不能明确提取发病时间,以距腰椎穿刺阳性结果最近的相关症状出现时间为发病时间),MRI检查时间, 发病至MRI检查间隔时间。行多次MRI检查者,选取首次MRI检查结果进入本文分析。
1.5 MRI检查 ①MRI设备为德国西门子Avanto 1.5T;②MRI常规序列包括轴位TSE T2加权成像、轴位SE/FLASH T1加权成像、矢状位SE T1加权成像、轴位tirm dark-fluid T2加权成像;轴位DWI采用单次激发EPI技术,b值为0和1000 s·mm-2;增强MRI为外周静脉注射钆双胺(浓度0.5 mmol·mL-1,剂量0.2 mL·kg-1)后进行SE/FLASH T1加权序列扫描。③MRI检查前30 min予10%水合氯醛0.5 mL·kg-1口服镇静。
1.6 MRI评分原则


1.7 质量控制 收集进入分析病例的MRI图像,由1名放射科主治医生在不知晓患儿出院结局的情况下集中独立阅片及评分,由1名放射科副主任医师复核。
2 结果
2.1 一般情况 63例进入本文分析,病例具体纳入和排除流程见图1。男43例(68.2%),女20例,出生孕周27.4~41.0(37.8±3.1)周。早产儿18例,男14例,女4例;出生孕周27.4~36.9(33.6±2.7)周。足月儿45例,男29例,女16例;出生孕周37.3~41.0(39.5±0.9)周。32例(50.8%)脑脊液培养或血培养细菌学阳性;其中19例(30.2%)为脑脊液培养细菌学阳性(早产儿9例,足月儿10例),22例(34.9%)为血培养细菌学阳性(早产儿9例,足月儿13例),7例(11.1%)为脑脊液培养与血培养细菌学均阳性且病原菌一致(早产儿2例,足月儿5例),2例(3.2%)脑脊液培养与血培养细菌学均阳性但病原菌不一致(早产儿和足月儿各1例)。
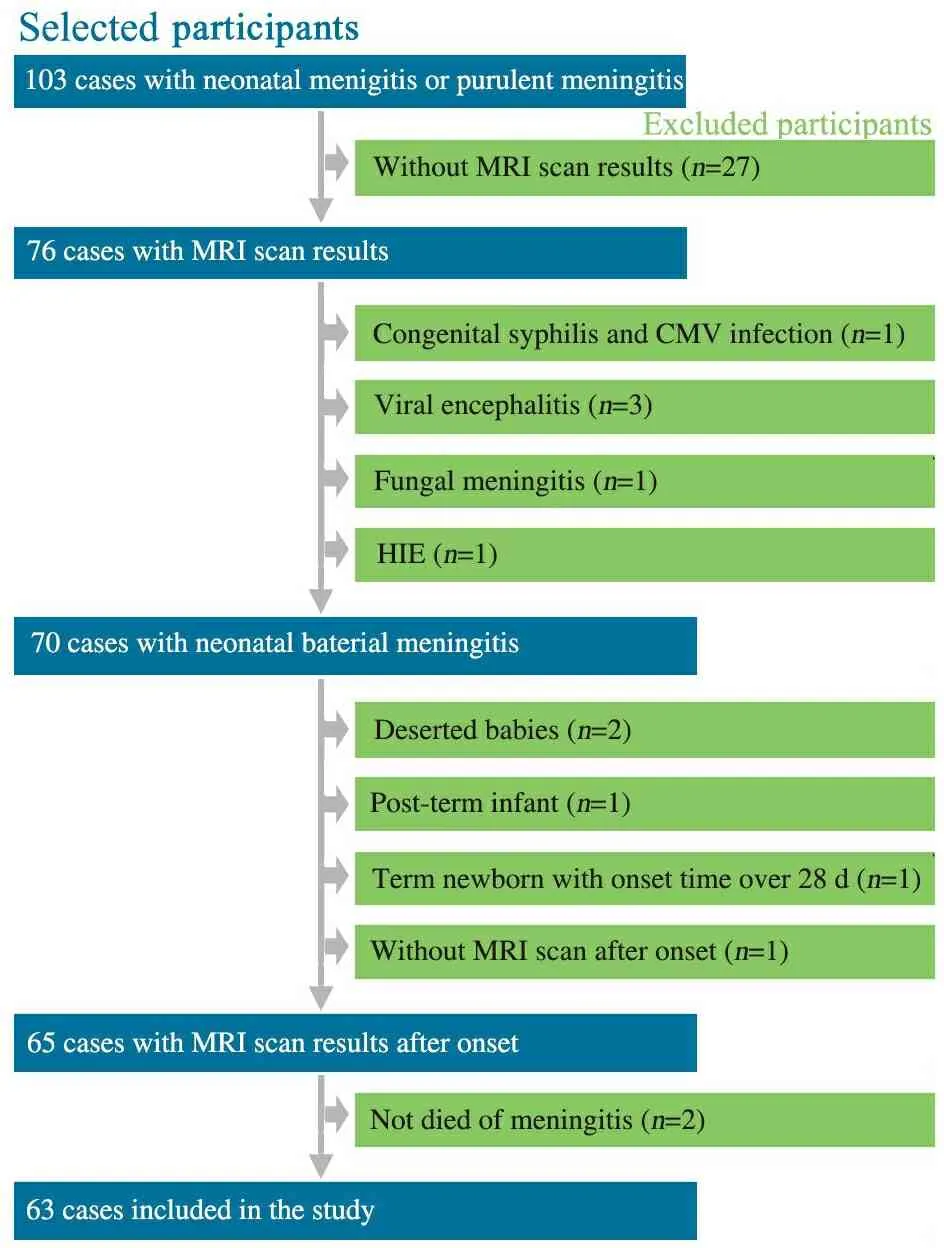
1 病例纳入和排除流程图
Fig 1 Flow chart of inclusion and exclusion of patients
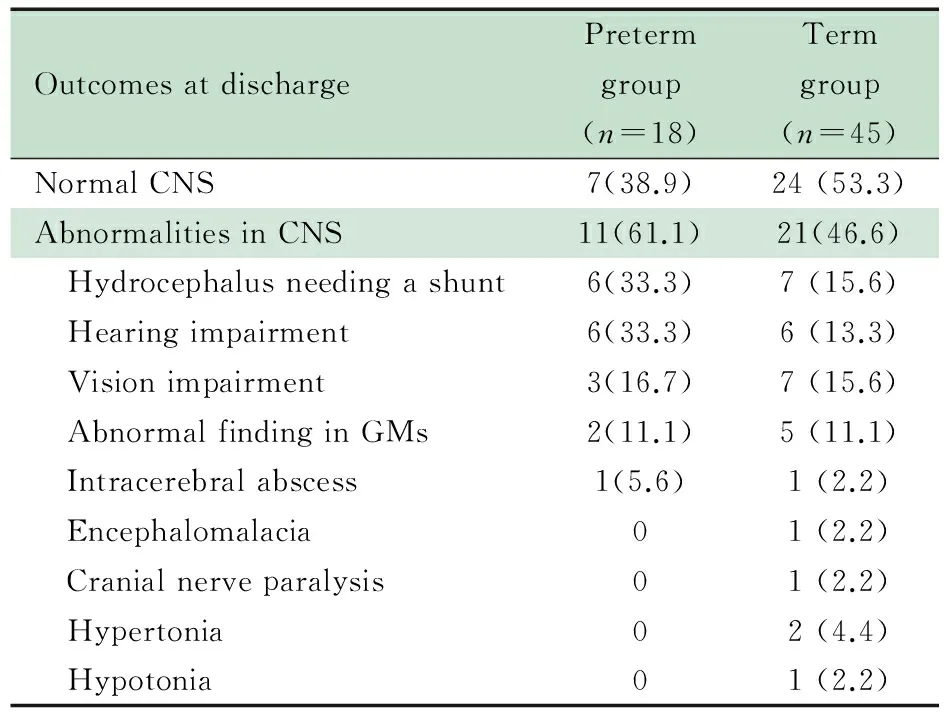
2.2 出院结局情况 表1显示,早产儿组出院时预后良好7例,预后不良11例;足月儿组出院时预后良好24例,预后不良21例。
表1 63例新生儿细菌性脑膜炎出院时结局[n(%)]
Tab 1 Outcomes at discharge of 63 cases with neonatal bacterial meningitis[n(%)]
OutcomesatdischargePretermgroup(n=18)Termgroup(n=45)NormalCNS7(38.9)24(53.3)AbnormalitiesinCNS11(61.1)21(46.6) Hydrocephalusneedingashunt6(33.3)7(15.6) Hearingimpairment6(33.3)6(13.3) Visionimpairment3(16.7)7(15.6) AbnormalfindinginGMs2(11.1)5(11.1) Intracerebralabscess1(5.6)1(2.2) Encephalomalacia01(2.2) Cranialnerveparalysis01(2.2) Hypertonia02(4.4) Hypotonia01(2.2)
Notes GMs: general movements; CNS: central nervous system
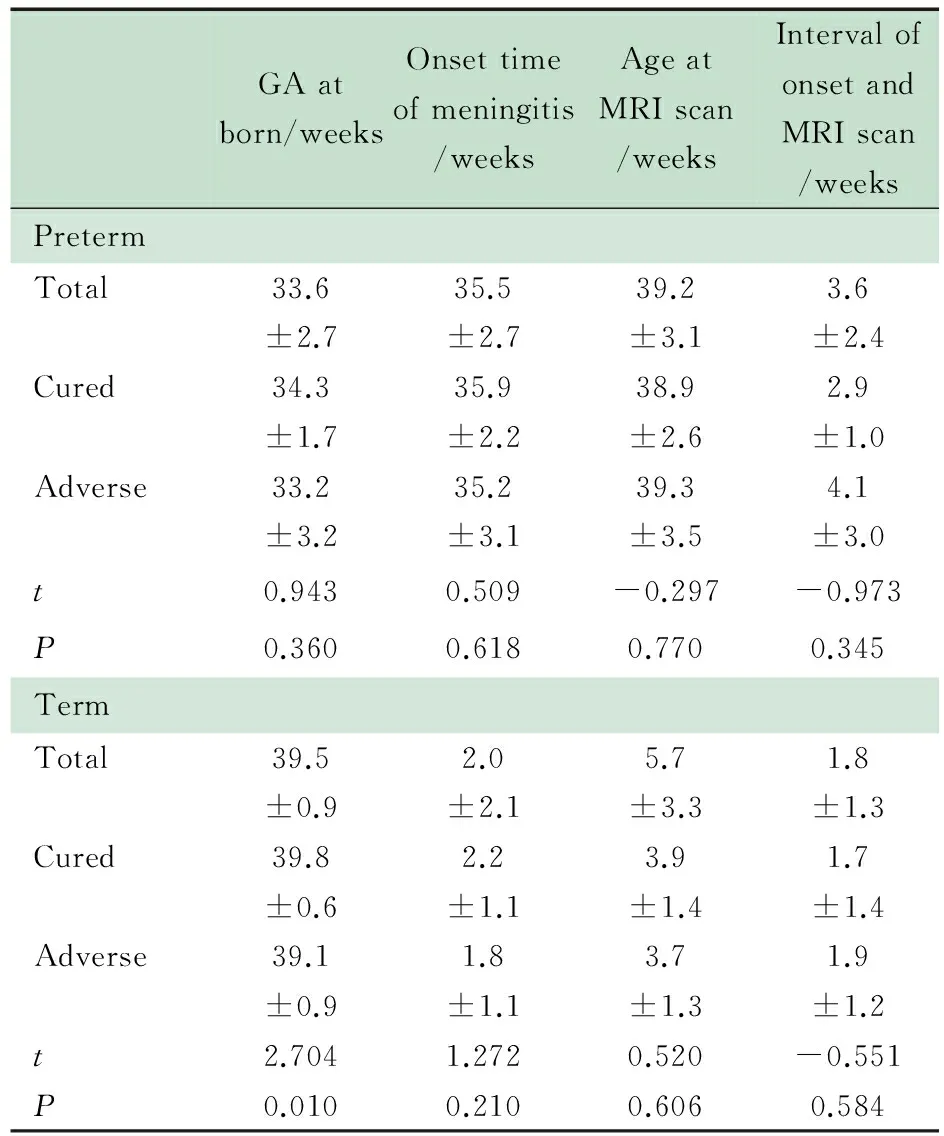
2.3 与MRI检查结果可能有关的时间因素 表2显示,足月儿组预后良好和预后不良亚组间出生孕周差异有统计学意义(P=0.010);发病时间、MRI检查时间、发病至MRI

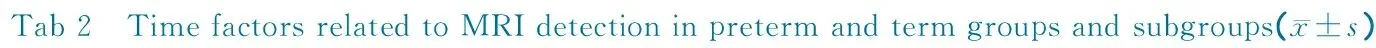
GAatborn/weeksOnsettimeofmeningitis/weeksAgeatMRIscan/weeksIntervalofonsetandMRIscan/weeksPretermTotal33.6±2.735.5±2.739.2±3.13.6±2.4Cured34.3±1.735.9±2.238.9±2.62.9±1.0Adverse33.2±3.235.2±3.139.3±3.54.1±3.0t0.9430.509-0.297-0.973P0.3600.6180.7700.345TermTotal39.5±0.92.0±2.15.7±3.31.8±1.3Cured39.8±0.62.2±1.13.9±1.41.7±1.4Adverse39.1±0.91.8±1.13.7±1.31.9±1.2t2.7041.2720.520-0.551P0.0100.2100.6060.584
Notes GA: gestational age. The ages of oneset time of meningitis and MRI scan were presented as corrected geatational ages in preterm group while in term group they were presented as chronological ages
检查间隔时间在早产儿组和足月儿组中的预后良好亚组、预后不良亚组之间差异均无统计学意义(P均>0.05)。
2.4 单项评分结果
2.4.1 早产儿组 表3显示,MRI单项评分中分值最高的4项为脑室扩大(33分)、内囊后肢髓鞘化异常(29分)、脑室旁白质容积丢失(26分)和脑白质囊性病变(26分)。预后良好和预后不良亚组间脑室扩大、脑室旁白质容积丢失分值构成差异有统计学意义(P分别为0.012和0.004)。余各单项分值构成在两亚组间差异无统计学意义。
2.4.2 足月儿组 表3显示,MRI单项评分中分值最高的3项为脑室扩大(66分)、颅内脑外间隙异常(63分)、脑室积脓(56分)。预后良好和预后不良亚组间脑室扩大、脑室旁白质容积丢失、颅内脑外间隙异常和脑室内出血分值构成差异有统计学意义(P分别为0.002, 0.040, 0.005和0.038)。余各单项分值构成两亚组间差异无统计学意义。
2.5 综合评分结果 表4显示,早产儿组预后良好和预后不良亚组相比,WMA和NPA评分差异有统计学意义(P分别为0.001和0.039)。 预后不良亚组WMA、NPA评分中位数均为9分,预后良好亚组WMA、NPA评分中位数均为6分,预后不良亚组均高于预后良好亚组;两亚组GMA评分差异无统计学意义。足月儿组预后良好和预后不良亚组相比,NPA评分差异有统计学意义(P=0.018),预后不良亚组NPA评分中位数(8分)高于预后良好亚组(6分);WMA和GMA评分差异无统计学意义。
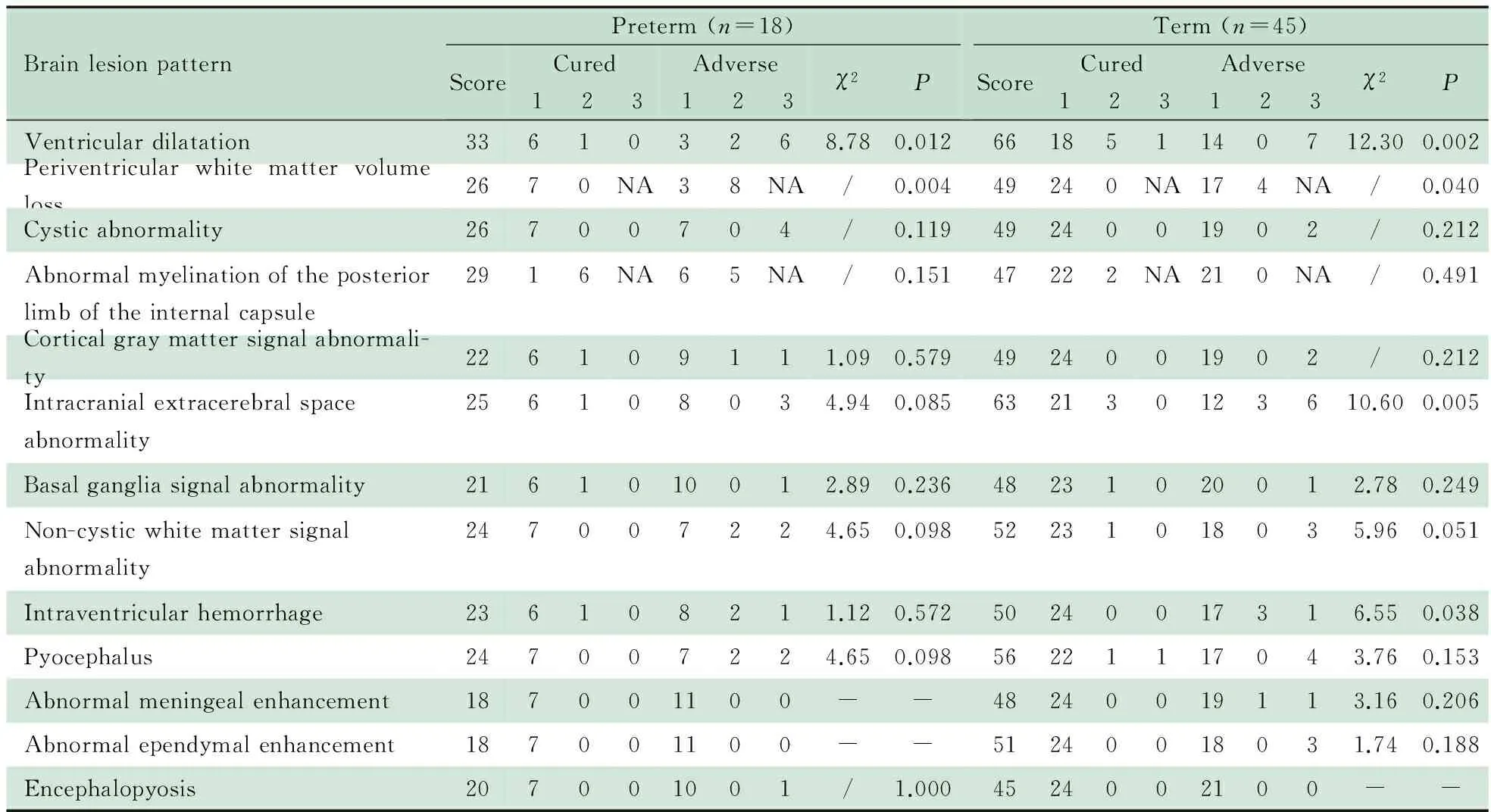
表3 MRI单项评分与出院时预后的关系
Notes "/": Chi-square test of the brain lesion pattern was Fisher′s exact test without 2 value. "-" represented no case presenting this brain lesion pattern.NA: the item was described as “with” or “without” the character and just had score 1 and score 2 without score 3. Score represented the total score of this brain lesion pattern
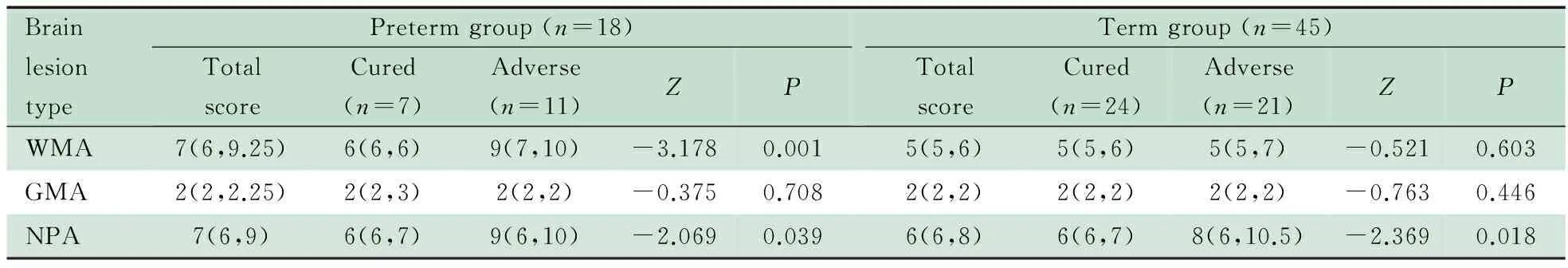
表4 早产儿组和足月儿组MRI综合评分结果[M(P25,P75)]
NotesP: The significance of the difference of the distribution about scores between two subgroups; WMA: white matter abormality; GMA: gray matter abormality; NPA: non-brain-parenchyma abormality
3 讨论
新生儿、婴儿期脑膜炎中以细菌性脑膜炎最常见[5,29],后遗症发生率高[7,8]。目前对于新生儿细菌性脑膜炎预后的研究,无论是大型回顾性队列研究[30~32]还是特殊病原菌的个例报道[33, 34],其中涉及到影像学表现的报道有限;而专注于新生儿脑膜炎影像学表现的研究,则多仅关注脑白质或非脑实质、某一征象或某一MRI技术在该病诊断和预后中的价值[9,14,25],并未对脑膜炎脑损伤MRI表现类型进行全面评价。此外,上述部分研究报道中对于脑膜炎病例的选择标准较宽泛,将细菌性脑膜炎、病毒性脑膜炎、真菌性脑膜炎或所有新生儿期不同病原的脑膜炎同时纳入研究,并非针对细菌性脑膜炎进行分析。因此,鉴于新生儿细菌性脑膜炎的高发病率和高致残率,本研究按照新生儿细菌性脑膜炎的诊断标准[8],通过严格的病例筛选,采用涵盖脑白质、脑灰质和非脑实质多种脑损伤表现的MRI评分系统,以期全面分析该疾病大脑损伤MRI表现与预后的关系。鉴于本研究为长期随访研究的初步结果,本文以出院时结局作为预后判断,对早产儿和足月儿MRI表现与预后进行相关分析。
既往研究中发现MRI检查结果可能与患儿出生孕周、发病时间、MRI检查的时间有关[16,35],因此在进行MRI表现与预后关系分析中,需要首先研究这些时间因素对MRI表现结果的影响。本文结果显示足月儿组出生孕周在预后良好与不良亚组间差异有统计学意义,(39.1±0.9)周vs(39.8±0.6)周;两亚组间差值为0.7周,此差异在临床实际工作中并无临床意义。其余时间因素的分析结果在早产儿和足月儿组中的预后良好和不良亚组间差异均无统计学意义。表明本研究中患儿出生孕周、发病时间、MRI检查时间和发病至MRI检查间隔时间对两亚组的MRI评分干扰不大。
本研究结果提示新生儿细菌性脑膜炎MRI脑室扩大和脑室旁白质容积丢失与早产儿出院不良结局有关(P<0.05)。而其他单项评分如颅内脑外间隙、脑白质非囊性信号异常、脑室积脓在早产儿预后良好和预后不良亚组间差异虽无统计学有意义(P值在0.05~0.1),但鉴于本研究早产儿样本量较小,尚无法明确这些单项评分与出院结局的关系。也有文献报道,早产儿中最常见的炎症相关性病变是脑白质损伤,其特点为局灶性、囊性、脑室周围白质软化、弥漫性坏死或兼而有之[1],这些形态学的表现与本研究发现相似。其可能的发病机制是早产儿血脑屏障发育不完善,某些炎性介质更容易通过血脑屏障进入脑实质并触发“瀑布效应”样连锁反应而导致脑实质损伤;早产儿脑成熟度低(少突胶质细胞前体对缺血的易感性较强)、免疫系统不完善,致使其脑膜炎时脑白质损伤更为明显[1, 8]。另有研究发现早产儿斑点状白质病变、脑室扩大与长期预后中的智力、心理运动发育延迟,动作发育延迟以及脑瘫显著相关[36]。尽管本研究尚未总结长期预后,但出院结局显示早产儿脑膜炎若出现MRI脑室扩大和脑室旁白质容积丢失征象,说明出院时不良预后是存在的。
足月儿组的结果显示脑室扩大、脑室旁白质容积丢失、颅内脑外间隙异常和脑室内出血预示出院结局不良,差异有统计学意义(P<0.05);而脑白质非囊性信号异常分值构成在两亚组间差异非常接近有统计学意义(P=0.051);余MRI单项评分在两亚组间差异均无统计学意义。有文献报道,足月儿B组链球菌脑膜炎可以出现脑室旁白质局灶性异常信号且预后差[9];大肠杆菌脑膜炎的足月儿可出现弥漫性白质异常信号伴严重的脑室扩大、脑萎缩,后期需要外科手术治疗脑积水[37]。这些研究结果均说明足月儿脑膜炎的脑白质损伤、脑室和脑外间隙的脑脊液系统受累所导致的脑损伤与其不良预后有关。足月儿脑室和脑外间隙的脑脊液系统受累的特征更接近于婴儿和儿童脑膜炎[38],可能与足月儿相对于早产儿而言其血脑屏障和脑膜血管发育相对成熟,作为第一屏障,更易受累有关。有文献报道婴儿和儿童脑膜炎MRI所见的脑膜弥散加权异常高信号预示不良预后[39],与本文观察到的特征相类似。这也与成人细菌性脑膜炎研究中发现的硬膜下积脓与出院时不良结局相关的结果一致[40]。
大脑白质、灰质与非脑实质在结构与功能上具有各自的特点,在发生脑膜炎损伤后可能会引起不同的神经功能障碍。本研究对MRI的各单项评分进行汇总,归纳为WMA、GMA、NPA三项综合评分,旨在分析三者在新生儿细菌性脑膜炎脑损伤中的发生情况、严重程度,分析其与出院结局的关系。
MRI综合评分结果显示,WMA评分高预示早产儿出院不良结局。WMA评分包含脑室扩大、脑室旁白质容积丢失、内囊后肢髓鞘化异常、脑白质囊性病变和脑白质非囊性信号异常。如前所述,这些单项均是早产儿脑白质损伤的形态学表现[1]。与脑室扩大和脑室旁白质容积丢失单项评分相比,WMA评分同样显示早产儿脑白质损伤与预后不良相关,早产儿预后不良亚组WMA评分中位数高于预后良好亚组。因此采用WMA评分可以对预后判断进行半定量分析。足月儿预后良好亚组与预后不良亚组的WMA评分中位数均为5分(正常分),四分位区间为5~6分,显示本研究中整个足月儿组的患儿脑白质损伤程度轻,与出院时预后无显著相关(P=0.603)。
MRI综合评分中的NPA评分高预示早产儿和足月儿出院时不良结局。NPA包括脑室扩大、颅内脑外间隙异常、脑室内出血、脑室积脓、室管膜和脑膜异常强化。在早产儿组单项评分中,并未发现颅内脑外间隙信号异常和脑室积脓在预后良好和预后不良亚组间差异有统计学意义;而足月儿组单项评分中,未发现脑室积脓、室管膜和脑膜异常强化在预后良好和预后不良亚组间差异有统计学意义。联合这些与脑膜和脑脊液系统有关的损伤类型,提升了亚组之间的差异,预后不良亚组的NPA评分均高于预后良好亚组。
早产儿组和足月儿组的预后良好亚组与预后不良亚组的GMA评分中位数均为2分,显示新生儿细菌性脑膜炎时脑灰质损伤程度轻,与出院预后无显著相关(P值分别为0.708和0.446)。
MRI形态学上脑室扩大表现,在新生儿脑膜炎中,既可能是脑实质损伤后的被动扩大,也可能是脑脊液循环受阻或脑脊液分泌异常增多所致的脑室扩大。虽然在本文病例中没有直接证据来说明脑室扩大直接原因,但是文献报道脑膜炎患者脑室扩大的主要原因是炎症累及基底池时,纤维素粘附于蛛网膜上,致使基底池处脑脊液循环受阻,从而发生脑积水;同时由于蛛网膜颗粒的纤维化和粘连致使脑脊液吸收障碍,进一步加重脑积水[41]。当然也有研究认为早产儿脑室扩大是由于脑室旁白质丢失而导致,因此将其归为WMA的范畴[15,16,20]。
需要说明的是,本研究的设计无法回答脑室内出血与脑室扩大是否由感染直接导致或属于伴发征象,本文研究结果只是反映了脑室内出血与足月儿细菌性脑膜炎出院结局不良有关,当然本组病例中的脑室内出血亦可能是导致脑室扩大的因素之一。
本研究不足之处:①以出院时神经系统评估为结局指标,和临床最终结局存在不同,可能会影响MRI评分在临床的应用价值;②样本量局限,特别是判断某些单项评分与预后的关系时统计效能不高;③由于MRI增强检查需要增加患儿镇静时间,而MRI平扫已经可以提供新生儿科医生所关心的脑损伤改变,所以临床实际工作中只有对怀疑脑脓肿的病例才进行头颅增强MRI检查;本研究中仅6.3%(4/63)的患儿进行了头颅增强MRI检查,确诊脑脓肿1例,这使得本研究中对于脑膜、室管膜异常强化征象的观察受到限制,判断其与出院结局的关系时会存在局限。
[1]Strunk T, Inder T, Wang X, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis, 2014, 14(8): 751-762
[2]Counsell SJ, Tranter SL, Rutherford MA. Magnetic resonance imaging of brain injury in the high-risk term infant. Semin Perinatol, 2010, 34(1): 67-78
[3]Bonifacio SL, Glass HC, Peloquin S, et al. A new neurological focus in neonatal intensive care. Nat Rev Neurol, 2011, 7(9): 485-494
[4]Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol, 2012, 71(4): 444-457
[5]Holt DE, Halket S, de Louvois J, et al. Neonatal meningitis in England and Wales: 10 years on. Arch Dis Child Fetal Neonatal Ed,2001, 84(2): F85-F89
[6]Gaschignard J, Levy C, Romain O, et al. Neonatal Bacterial Meningitis: 444 Cases in 7 Years. Pediatr Infect Dis J, 2011, 30(3): 212-217
[7]Stevens JP, Eames M, Kent A, et al. Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed, 2003, 88(3): F179-F184
[8]邵肖梅, 叶鸿瑁, 丘小汕. 实用新生儿学. 第4版. 北京: 人民卫生出版社, 2011: 347-351
[9]Hernandez MI, Sandoval CC, Tapia JL, et al. Stroke patterns in neonatal group B streptococcal meningitis. Pediatr Neurol, 2011, 44(4): 282-288
[10]Malik GK, Trivedi R, Gupta A, et al. Quantitative DTI assessment of periventricular white matter changes in neonatal meningitis. Brain Dev, 2008, 30(5): 334-341
[11]Rollins NK, Morriss MC, Evans D, et al. The role of early MR in the evaluation of the term infant with seizures. Am J Neuroradiol, 1994, 15(2): 239-248
[12]Jan W, Zimmerman RA, Bilaniuk LT, et al. Diffusion-weighted imaging in acute bacterial meningitis in infancy. Neuroradiology, 2003, 45(9): 634-639
[13]Mader I, Schoning M, Klose U, et al. Neonatal cerebral infarction diagnosed by diffusion-weighted MRI: pseudonormalization occurs early. Stroke, 2002, 33(4): 1142-1145
[14]Malik GK, Yadav A, Trivedi R, et al. Temporal alterations in brain water diffusivity in neonatal meningitis. Acta Paediatr, 2009, 98(9): 1426-1432
[15]Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. Am J Neuroradiol, 2003, 24(8): 1661-1669
[16]Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr, 2005, 147(5): 609-616
[17]Roine I, Weisstaub G, Peltola H. Influence of malnutrition on the course of childhood bacterial meningitis. Pediatr Infect Dis J, 2010, 29(2): 122-125
[18]Fluegge K, Siedler A, Heinrich B, et al. Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics, 2006, 117(6): e1139-e1145
[19]Levent F, Baker CJ, Rench MA, et al. Early outcomes of group B streptococcal meningitis in the 21st century. Pediatr Infect Dis J, 2010, 29(11): 1009-1012
[20]Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med, 2006, 355(7): 685-694
[21]Shroff MM, Soares-Fernandes JP, Whyte H, et al. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics, 2010, 30(3): 763-780
[22]Inder TE, Wells SJ, Mogridge NB, et al. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr,2003, 143(2): 171-179
[23]Wong AM, Zimmerman RA, Simon EM, et al. Diffusion-weighted MR imaging of subdural empyemas in children. AJNR Am J Neuroradiol, 2004, 25(6): 1016-1021
[24]Tsuchiya K, Osawa A, Katase S, et al. Diffusion-weighted MRI of subdural and epidural empyemas. Neuroradiology, 2003, 45(4): 220-223
[25]Han KT, Choi DS, Ryoo JW, et al. Diffusion-weighted MR imaging of pyogenic intraventricular empyema. Neuroradiology, 2007, 49(10): 813-818
[26]Mohan S, Jain KK, Arabi M, et al. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am, 2012, 22(4): 557-583
[27]Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr, 1978, 92(4): 529-534
[28]Jansson AK, Enblad P, Sjolin J. Efficacy and safety of cefotaxime in combination with metronidazole for empirical treatment of brain abscess in clinical practice: a retrospective study of 66 consecutive cases. Eur J Clin Microbiol Infect Dis, 2004, 23(1): 7-14
[29]Jaremko JL, Moon AS, Kumbla S. Patterns of complications of neonatal and infant meningitis on MRI by organism: a 10 year review. Eur J Radiol, 2011, 80(3): 821-827
[30]Okike IO, Johnson AP, Henderson K L, et al. Incidence,etiology, and outcome of bacterial meningitis in infants aged <90 days in the United Kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis, 2014, 59(10): e150-e157
[31]Lin MC, Chi H, Chiu NC, et al. Factors for poor prognosis of neonatal bacterial meningitis in a medical center in Northern Taiwan. J Microbiol Immunol Infect, 2012, 45(6): 442-447
[32]de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: sequelae at 5 years of age. Eur J Pediatr,2005, 164(12): 730-734
[33]Vaz MC, Ferreira M, Ferreira MM, et al. Sepsis, meningitis and cerebral abscesses caused by Citrobacter koseri. BMJ Case Rep, 2012
[34]Sonntag J, Kaczmarek D, Brinkmann G, et al. Complicating neonatal Escherichia coli meningitis. Z Geburtshilfe Neonatol, 2004, 208(1): 32-35
[35]Zhang J(张静),Mao J, Li J, et al. MRI findings of neonatal purulent meningitis caused by different pathogenic bacteria. Chin J Contemp Pediatr(中国当代儿科杂志), 2012,14(7): 489-495
[36]de Bruine FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology, 2011, 261(3): 899-906
[37]Shah DK, Daley AJ, Hunt RW, et al. Cerebral white matter injury in the newborn following Escherichia coli meningitis. Eur J Paediatr Neurol, 2005, 9(1): 13-17
[38]Oliveira CR, Morriss MC, Mistrot JG, et al. Brain magnetic resonance imaging of infants with bacterial meningitis. J Pediatr, 2014, 165(1): 134-139
[39]Kawaguchi T, Sakurai K, Hara M, et al. Clinico-radiological features of subarachnoid hyperintensity on diffusion-weighted images in patients with meningitis. Clin Radiol, 2012, 67(4): 306-312
[40]Jim KK, Brouwer MC, van der Ende A, et al. Subdural empyema in bacterial meningitis. Neurology, 2012, 79(21): 2133-2139
[41]Chatterjee S, Chatterjee U. Overview of post-infective hydrocephalus. Childs Nerv Syst, 2011, 27(10): 1693-1698
(本文编辑:张崇凡)
Predicting the outcomes at discharge of neonatal bacterial meningitis: a semi-quantitative MRI-based-score system analysis
YANGMing-shu1,WANGLi1,ZHAIQian2,PAMi-er1,ZHOUJian1,CAOYun2,QIAOZhong-wei1
(1DepartmentofRadiology; 2DepartmentofNeonatology,Children′sHospitalofFudanUniversity,Shanghai201102,China)
QIAO Zhong-wei,E-mail:qiaozhwei@163.com;CAO Yun,E-mail:yuncaochina@hotmail.com
ObjectiveTo investigate the value of a semi-quantitative MRI-based-score system in predicting outcomes at discharge in infants with neonatal bacterial meningitis.MethodsNewborns with a final diagnosis of neonatal meningitis were included in this study. The severities of thirteen patterns of brain injuries on cranial MR images of the infants were graded, which included ventricular dilatation, periventricular white matter volume loss, cystic abnormality, abnormal myelination of the posterior limb of the internal capsule, cortical gray matter signal abnormality, intracranial extracerebral space abnormality, basal ganglia signal abnormality, non-cystic white matter signal abnormality, intraventricular hemorrhage, pyocephalus, abnormal meningeal enhancement, abnormal ependymal enhancement and encephalopyosis. Except for encephalopyosis, the frequencies of white matter abnormality (WMA), gray matter abnormality (GMA), and non-brain-parenchyma abnormality (NPA) were also calculated. Four time factors and outcomes at discharge were collected from the clinical history of each patient. The patients were divided into preterm and term groups followed by cured and adverse outcome subgroups. The severity and spectrum of different part of cerebral injuries and time factors were compared between two subgroups of each group by using chi-square test, Mann-WhitneyUtest and studentt-test. ResultsSixty-three newborns including 18 preterm infants and 45 term ones were recruited in this study. There were significant differences in the scores of ventricular dilatation and periventricular white matter volume loss between cured and adverse outcome subgroups in preterm group (Pvalue was 0.012 and 0.004, respectively). There were significant differences in the scores of ventricular dilatation, periventricular white matter volume loss, intracranial extracerebral space abnormality, and intraventricular hemorrhage between cured and adverse outcome subgroups in term group (Pvalue was 0.002, 0.040, 0.005 and 0.038, respectively). There were significant differences in the scores of WMA, NPA in preterm group and NPA in term group between two outcome subgroups (Pvalue was 0.001, 0.039 and 0.018, respectively). There was no significant difference with clinical value in time factors between outcome subgroups whether in preterm or term group.ConclusionVentricular dilatation and periventricular white matter volume loss could predict adverse outcomes at discharge in preterm infants with neonatal bacterial meningitis. Ventricular dilatation, periventricular white matter volume loss, intracranial extracerebral space abnormality, and intraventricular hemorrhage could predict adverse outcomes at discharge in term infants with neonatal bacterial meningitis. Regarding to the significance of WMA, GMA and NPA types, high NPA scores could predict adverse outcomes at discharge in both preterm and term infants, while WMA scores could predict adverse outcomes at discharge in preterm ones.
Newborn; Bacterial meningitis; Magnetic resonance imaging; Brain injury; Outcomes at discharge
复旦大学附属儿科医院 1 放射科, 2 新生儿科 上海,201102
乔中伟,E-mail:qiaozhwei@163.com;曹云,E-mail:yuncaochina@hotmail.com
10.3969/j.issn.1673-5501.2015.02.005
2015-01-13
2015-03-25)
