α2-肾上腺素能受体激动剂B-TH933抑制LPS处理的心肌细胞释放TNF-α*
2015-04-27朱琳欣杨多猛唐翔诩吕秀秀李红梅严玉霞戚仁斌陆大祥王华东暨南大学医学院临床医学系0级本科班病理生理学系国家中医药管理局三级科研实验室生物化学系广东广州506
朱琳欣,杨多猛,唐翔诩,王 媛,吕秀秀,李红梅,严玉霞,戚仁斌,陆大祥,王华东△(暨南大学医学院临床医学系0级本科班,病理生理学系,国家中医药管理局三级科研实验室,生物化学系,广东广州506)
α2-肾上腺素能受体激动剂B-TH933抑制LPS处理的心肌细胞释放TNF-α*
朱琳欣1▲,杨多猛2▲,唐翔诩2,王媛2,吕秀秀2,李红梅2,严玉霞3,戚仁斌2,陆大祥2,王华东2△
(暨南大学医学院1临床医学系2011级本科班,2病理生理学系,国家中医药管理局三级科研实验室,3生物化学系,广东广州510632)
[摘要]目的:观察α2-肾上腺素能受体激动剂B-HT933对脂多糖(LPS)刺激的心肌细胞产生肿瘤坏死因子α(TNF-α)的影响,并初步分析其作用机制。方法:分离培养SD大鼠乳鼠心肌细胞。利用免疫荧光染色观察心肌α(2A)-肾上腺素能受体的分布; B-HT933和/或LPS处理心肌细胞一定时间后,用ELISA方法检测细胞培养液中TNF-α的含量、实时荧光定量PCR测定心肌细胞TLR4和TNF-α mRNA的表达、Western blot分析心肌细胞中相关信号分子的磷酸化水平。结果:免疫荧光染色证实乳鼠心肌细胞中存在α(2A)-肾上腺素能受体。LPS以剂量和时间依赖的方式刺激心肌细胞产生TNF-α,0.1 μmol/L的B-HT933处理能显著抑制LPS诱导的TNF-α mRNA的表达和TNF-α蛋白的产生。而且,BHT能抑制LPS诱导的心肌细胞IκBα的磷酸化。结论:乳鼠心肌细胞上存在α(2A)-肾上腺素能受体,其激动剂B-HT933可能通过抑制心肌细胞IκBα的磷酸化来减少LPS诱导的TNF-α产生。
[关键词]乳鼠;心肌细胞;脂多糖;α2-肾上腺素能受体
[修回日期]2015-08-25
▲并列第1作者
脓毒症是危重病患者死亡的主要原因,尽管临床治疗措施不断改进,脓毒症患者的死亡率仍高达25%~30%,如果发生脓毒症性休克,其死亡率可超过50%[1]。文献报道,约50%的脓毒症患者伴有心功能障碍,心功能障碍的发生是脓毒症患者死亡的重要促进因素[2]。虽然多种因素参与了脓毒症性心功能障碍的发生机制,包括内皮细胞功能障碍、心肌钙代谢紊乱、心肌线粒体功能失调和心肌细胞凋亡等,但是Toll样受体4(Toll-like receptor 4,TLR4)激活导致肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)等炎症细胞因子的产生在脓毒症性心功能障碍中发挥重要作用[2]。研究发现,心肌细胞上存在TLR4[3],TLR4的激动剂革兰氏阴性细菌的细胞壁成分脂多糖(lipopolysaccharide,LPS)与脓毒症心功能障碍的发生机制有关,LPS可刺激心肌细胞产生TNF-α[4],TNF-α可直接抑制心肌细胞的功能[5],给予TNF-α抗体阻断TNF-α的作用可以显著改善脓毒症患者的心功能[6]。显然,深入研究LPS诱导心肌细胞TNF-α产生的调节机制对理解脓毒症心肌损伤具有重要意义。另一方面,脓毒症时循环中儿茶酚胺浓度显著上升[7],儿茶酚胺激活心肌β1-肾上腺素能受体可增强LPS诱导的TNF-α生成,加重心肌损伤[8]。有研究表明,胎鼠和成体大鼠心肌细胞上存在α2-肾上腺素能受体[9-10],理论上,心肌细胞α2-肾上腺素能受体活化也可能影响心肌功能。然而,α2-肾上腺素能受体激活是否能影响LPS诱导的心肌细胞释放TNF-α尚缺乏研究。因此,本研究首先检测乳鼠心肌细胞α2A-肾上腺素能受体的表达,在此基础上,进一步观察α2-肾上腺素能受体激动剂BHT933对LPS刺激的心肌细胞产生TNF-α的影响,并分析其作用机制。
材料和方法
1主要试剂
戊巴比妥钠购自国药集团化学试剂有限公司; DMEM培养基、青霉素、链霉素、PBS液及胎牛血清均购自HyClone;含EDTA的0.125%的胰酶购自Gibco;大肠杆菌LPS(O55∶B5)、6-乙基-5,6,7,8-四氢-4H-17唑(4,5-d)氮杂卓-2-胺盐酸[6-ethyl-5,6,7,8-tetrahydro-4H-oxazolo(4,5-d) azepin-2-amine dihydrochloride,B-HT933]和4’,6-二脒基-2-苯基吲哚(4’,6-diamidino-2-phenylindole,DAPI)均购自Sigma;抗心肌肌钙蛋白I (cardiac troponin I,cTnI)抗体、抗α2A-肾上腺素能受体抗体购自Abcam; Alexa FluorDyes标记的荧光II抗购自Invitrogen; TNF-α的酶联免疫吸附分析(enzyme-linked immunosorbent assay,ELISA)试剂盒购自R&D; RNAiso Plus购于TaKaRa; real-time PCR逆转录试剂盒和SYBR Green试剂购自Roche; PCR引物设计和合成由上海生工生物工程有限公司提供;抗核因子κBα抑制物(inhibitor of nuclear factor-κBα,IκBα)抗体、抗磷酸化IκBα (p-IκBα)抗体、抗细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)抗体、抗磷酸化ERK(p-ERK)抗体、抗c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)抗体、抗磷酸化JNK(p-JNK)抗体、抗p38丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)抗体和抗磷酸化p38 MAPK(p-p38)抗体均购自CST。
2方法
2.1乳鼠心肌细胞的培养与处理取出生1~3 d SPF级SD大鼠乳鼠[购自南方医科大学实验动物中心,合格证号为SCXX(粤) 2011-0015],麻醉后用75%乙醇消毒8~10 s,取出心脏,将心室心肌组织剪碎,加入心脏组织量5~10倍体积的胰酶(0.125%),37℃消化7 min。丢弃第一次上清,再次加入等量的胰酶消化5 min,收集细胞上清液于离心管内,并加入等体积的含10%胎牛血清的DMEM终止消化。重复收集细胞,将收集的细胞与DMEM混合,800 r/min,4℃离心7 min,获得细胞沉淀。用含10%胎牛血清的DMEM悬浮细胞,200目筛网过滤,将滤过的细胞在37℃、5% CO2培养箱中培养2 h,纯化心肌细胞。最后,将5.5×108cells/L接种于培养板,培养40 h。然后进行如下处理:①用免疫荧光染色观察心肌细胞α2A-肾上腺素能受体的分布;②观察B-HT933对LPS诱导的心肌细胞产生TNF-α的影响并分析其作用机制。心肌细胞用0.1 μmol/L B-HT933和/或不同剂量的LPS刺激不同时间,测定心肌细胞培养液中TNF-α的含量、心肌细胞中TLR4 与TNF-α的mRNA表达以及相关信号分子的磷酸化水平。
2.2α2A-肾上腺素能受体免疫荧光染色培养的心肌细胞用4%的多聚甲醛固定15 min,PBS清洗3遍,0.25%的Triton X-100处理10 min。PBS清洗3次后,用1% BSA封闭1 h。随后加入兔抗大鼠α2A-肾上腺素能受体和小鼠抗大鼠心肌肌钙蛋白I的抗体,4℃过夜。洗片后,加入荧光II抗,室温孵育1 h,最后加入DAPI室温孵育10 min,封片后立即用激光共聚焦显微镜观察。
2.3ELISA方法测定TNF-α含量取心肌细胞培养上清液,按照ELISA试剂盒说明书测定TNF-α含量。
2.4Real-time PCR测定心肌细胞TLR4和TNF-α 的mRNA表达LPS处理心肌1.5 h后,从培养箱中取出6孔板,按照加药顺序依次吸弃细胞培养液,用预冷的PBS清洗细胞2次,加入RNAiso Plus 1 mL提取细胞总RNA,逆转录获得cDNA,进行实时荧光定量PCR。PCR条件为95℃10 s,60℃20 s,72℃20 s,所用引物见表1。

表1 Real-time PCR引物Table 1.The sequences of the primers for real-time PCR
2.5Western blotting检测LPS和/或B-HT933处理心肌细胞30 min,冰上裂解细胞,4℃、16 000×g离心15 min,收集蛋白上清液,参照文献报道的方法[12],进行Western blotting分析。
3统计学处理
使用SPSS 13.0统计软件进行统计学处理。计量数据采用均数±标准误(Mean±SEM)表示,多组间比较采用单因素方差分析(one-way ANOVA),组间比较采用Bonferroni法,以P<0.05为差别有统计学意义。
结果
1乳鼠心肌细胞上存在α2A-肾上腺素能受体
如图1所示,cTnI抗体阳性染色呈现红色荧光,显示培养的细胞均为心肌细胞,心肌细胞核被DAPI染色,呈现蓝色荧光。同时用抗α2A-肾上腺素能受体抗体染色,发现多数心肌细胞呈阳性,显示为绿色荧光,其阳性染色主要分布在细胞膜和胞浆中。这些结果表明,乳鼠心肌细胞中存在α2A-肾上腺素能受体。
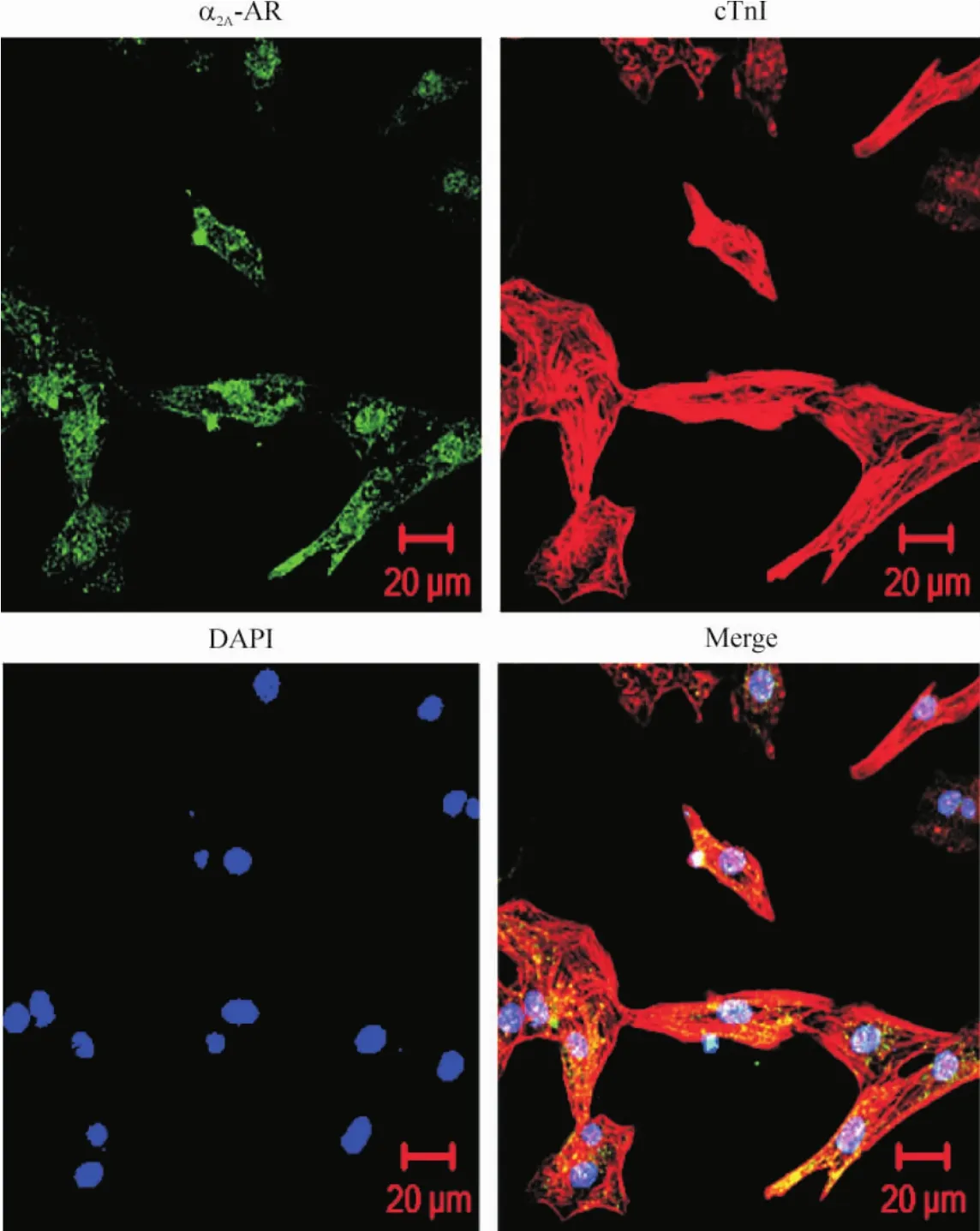
Figure 1.Immunofluorescence staining of α2A-adrenoceptor (α2A-AP) in neonatal rat cardiomyocytes.The cardiomyocytes were cultured for 40 h and then stained with antibodies against α2A-AR (green) and cTnI (red).The nuclei were stained with DAPI (blue).图1乳鼠心肌细胞α2A-肾上腺素能受体免疫荧光染色
2 B-HT933抑制LPS处理的心肌细胞释放TNF-α
LPS以剂量和时间依赖的方式刺激心肌细胞产生TNF-α,0.1 μmol/L的B-HT933处理能显著抑制LPS诱导的TNF-α的产生,见图2。
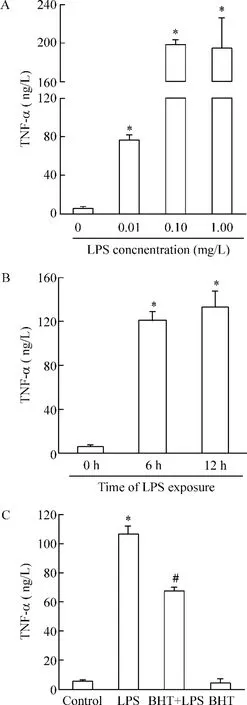
Figure 2.The effects of B-HT933 (BHT),a selective α2-adrenoceptor agonist,on TNF-α production in LPS-challenged cardiomyocytes.A: the cardiomyocytes were cultured for 40 h and then stimulated with 0.01,0.10 and 1.00 μmol/L LPS for 6 h; B: the cardiomyocytes were cultured for 40 h and then stimulated with 1.00 μmol/L LPS for 6 h and 12 h,respectively; C: the cardiomyocytes were cultured for 40 h and stimulated with BHT at concentration of 0.10 μmol/L or vehicle for 30 min,then with 0.10 μmol/L BHT plus 1.00 μmol/L LPS or 1.00 μmol/L LPS for another 6 h.TNF-α levels in the supernatants were examined by ELISA.Mean±SEM.n=3.*P<0.05 vs control;#P<0.05 vs LPS group.图2 α2-肾上腺素能受体激动剂B-HT933对LPS刺激的心肌细胞产生TNF-α的影响
3 B-HT933抑制LPS诱导的TNF-αmRNA的表达,但不影响心肌细胞TLR4的mRNA表达
B-HT933并不影响LPS处理心肌细胞中TLR4 的mRNA表达,然而,LPS显著上调心肌细胞TNF-α 的mRNA表达; B-HT933能显著抑制LPS诱导的TNF-α mRNA表达,见图3。
4 B-HT933处理心肌细胞能显著抑制LPS诱导的IκBα和p38的磷酸化
如图4所示,LPS处理心肌细胞30 min,心肌细胞IκBα和p38的磷酸化明显增加,α2-肾上腺素能受体激动剂B-HT933处理心肌细胞可显著抑制LPS诱导的IκBα磷酸化,但不能影响心肌细胞p38、JNK 和ERK的磷酸化。
讨论
TNF-α作为一种重要的炎症细胞因子参与心肌损伤的发生机制[11]。早期研究认为,胎鼠心肌细胞上存在α2-肾上腺素能受体,该受体在胎鼠心脏发育中发挥重要作用,出生后心肌α2-肾上腺素能受体表达降低,成年鼠心肌细胞上几乎没有α2-肾上腺素能受体[9]。然而,新近的研究发现成年鼠心肌上也存在α2-肾上腺素能受体[10]。本研究发现,乳鼠心肌细胞中存在α2-肾上腺素能受体,B-HT933能显著抑制LPS诱导的心肌细胞表达和释放TNF-α。
目前的研究已证实,心肌细胞上存在TLR4,LPS活化TLR4,启动细胞内的信号转导通路。根据接头分子的不同,LPS/TLR4细胞内信号通路包括MyD88依赖的信号转导通路和MyD88非依赖性信号转导通路,这些通路活化最终激活下游的IκB激酶(inhibitor of nuclear factor-κB kinase,IKK)和MAPK,从而导致IκBα磷酸化和NF-κB的活化,以及p38、JNK和ERK的磷酸化,最终启动TNF-α等靶基因的表达,介导心肌炎症反应[12]。本研究发现,B-HT933并不能影响LPS处理的心肌细胞表达TLR4,说明BHT933显著抑制LPS诱导的心肌细胞表达和释放TNF-α与心肌细胞TLR4的水平无关。研究表明,IκBα磷酸化导致NF-κB的活化以及p38的磷酸化在LPS诱导心肌细胞表达TNF-α的信号通路中发挥重要作用,抑制心肌细胞IκBα磷酸化或阻断p38的磷酸化均能在一定程度上抑制LPS诱导的TNF-α的表达[12-13]。因此,本研究进一步观察了α2-肾上腺素能受体激动剂对LPS诱导IκBα和p38磷酸化的影响。结果发现,LPS显著增加心肌细胞IκBα和p38的磷酸化,而B-HT933明显抑制LPS诱导的IκBα磷酸化。这些结果表明B-HT933可能通过抑制心肌细胞IκBα的磷酸化,从而阻断LPS诱导的TNF-α表达。
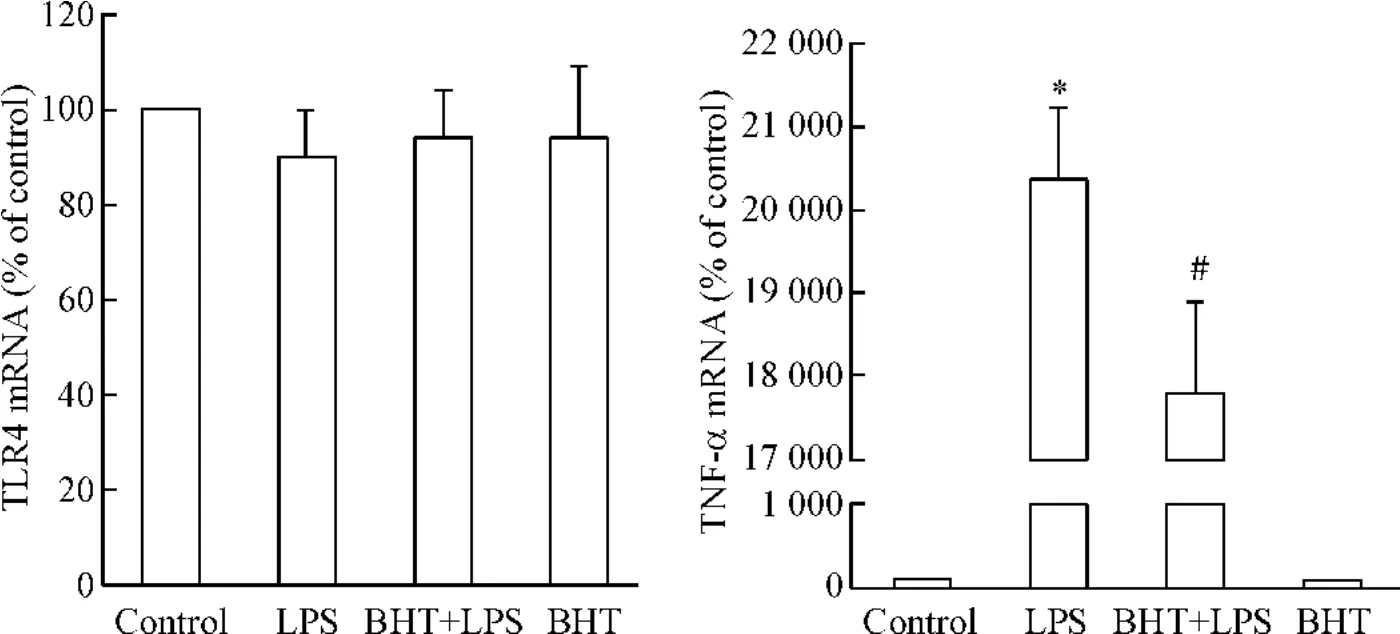
Figure 3.The effects of B-HT933 (BHT),a selective α2-adrenoceptor agonist,on TLR4 and TNF-α mRNA expression in LPS-challenged cardiomyocytes.The cardiomyocytes were cultured for 40 h and stimulated with BHT at concentration of 0.10 μmol/L or vehicle for 30 min,then with 0.10 μmol/L BHT plus 1.00 μmol/L LPS or 1.00 μmol/L LPS for another 1.5 h.The mRNA expression of TLR4 and TNF-α was detected by real-time PCR.Mean±SEM.n=6.*P<0.05 vs control;#P<0.05 vs LPS group.图3 α2-肾上腺素能受体激动剂B-HT933对LPS刺激的心肌细胞TLR4和TNF-α mRNA表达的影响
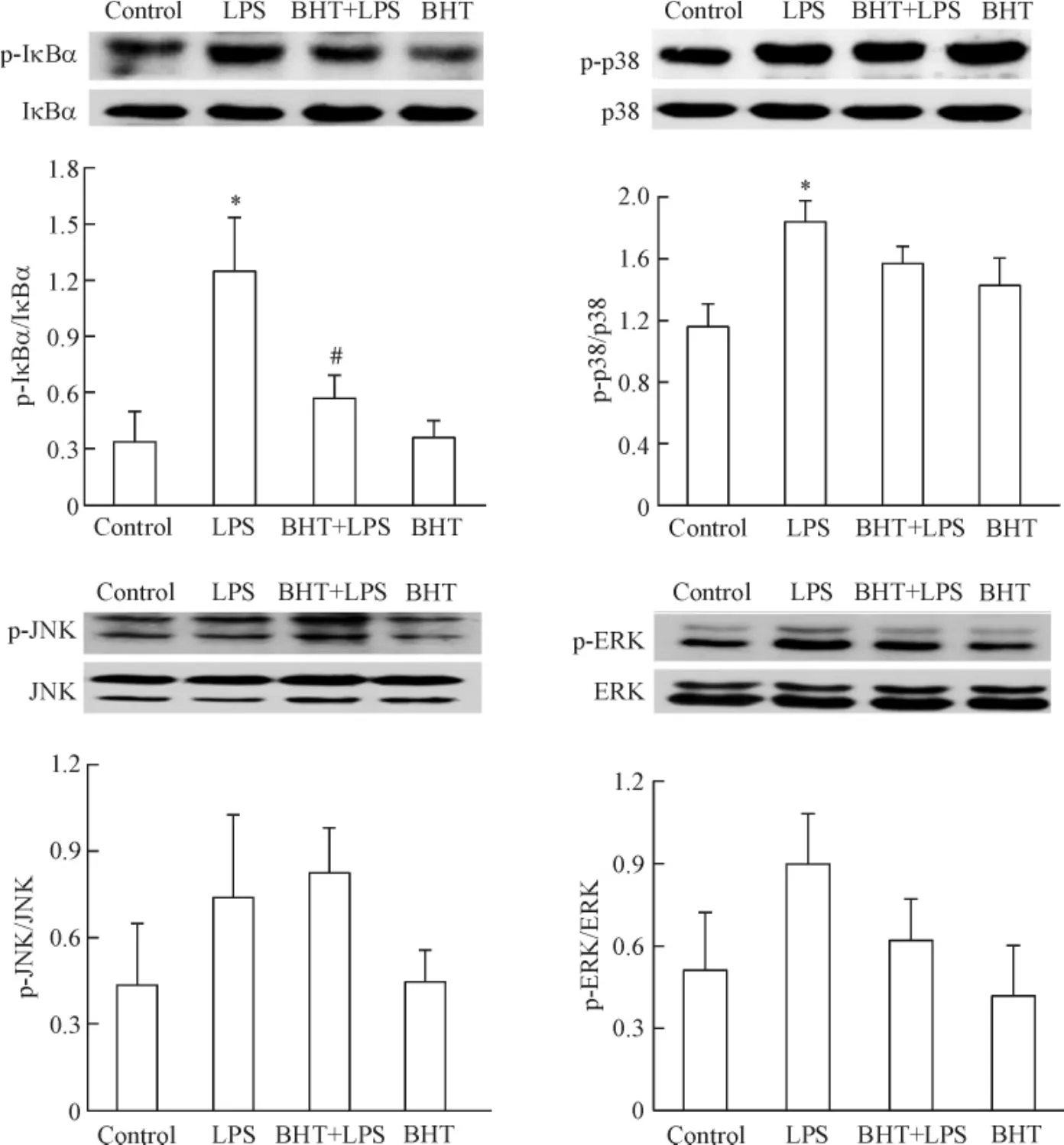
Figure 4.The effects of B-HT933 (BHT),a selective α2-adrenoceptor agonist,on IκBα,p38,JNK and ERK phosphorylation in LPS-challenged cardiomyocytes.The cardiomyocytes were cultured for 40 h and stimulated with BHT at concentration of 0.10 μmol/L or vehicle for 30 min,then with 0.10 μmol/L BHT plus 1.00 μmol/L LPS or 1.00 μmol/L LPS for another 30 min.The phosphorylation levels of IκBα,p38,JNK and ERK were determined by Western blotting.Mean±SEM.n=4~6.*P<0.05 vs control;#P<0.05 vs LPS group.图4 α2-肾上腺素能受体激动剂BHT对LPS刺激的心肌细胞IκBα、p38、JNK和ERK磷酸化的影响
新近,一些研究者发现成体大鼠心肌细胞上存在α2-肾上腺素能受体,激活α2-肾上腺素能受体可活化心肌磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/Akt信号通路[10]。另一方面,激活PI3K/Akt信号通路可抑制LPS诱导的乳鼠心肌细胞产生TNF-α[14]。因此,B-HT933抑制LPS诱导的乳鼠心肌细胞产生TNF-α可能与PI3K/Akt信号通路的活化有关,这一推论是否正确,尚需进一步研究。
综上所述,本研究证实乳鼠心肌细胞上存在α2-肾上腺素能受体,α2-肾上腺素能受体活化可抑制LPS处理的心肌细胞产生TNF-α,这一发现揭示了心肌细胞α2-肾上腺素能受体具有重要的病理生理作用,为探讨心肌细胞α2-肾上腺素能受体在脓毒症性心功能障碍发生机制中的作用奠定了基础。
[参考文献]
[1]Cohen J,Vincent JL,Adhikari NK,et al.Sepsis: a roadmap for future research[J].Lancet Infect Dis,2015,15 (5) : 581-614.
[2]Zaky A,Deem S,Bendjelid K,et al.Characterization of cardiac dysfunction in sepsis: an ongoing challenge[J].Shock,2014,41(1) : 12-24.
[3]Frantz S,Kobzik L,Kim YD,et al.Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium[J].J Clin Invest,1999,104(3) : 271-280.
[4]Comstock KL,Krown KA,Page MT,et al.LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response[J].J Mol Cell Cardiol,1998,30 (12) : 2761-2775.
[5]Cain BS,Meldrum DR,Dinarello CA,et al.Tumor necrosis factor-alpha and interleukin-1β synergistically depress human myocardial function[J].Crit Care Med,1999,27(7) : 1309-1318.
[6]Vincent JL,Bakker J,Marecaux G,et al.Administration of anti-TNF antibody improves left ventricular function in septic shock patients.Results of a pilot study[J].Chest,1992,101(3) : 810-815.
[7]Hahn PY,Wang P,Tait SM,et al.Sustained elevation in circulating catecholamine levels during polymicrobial sepsis[J].Shock,1995,4(4) : 269-273.
[8]Wang Y,Wang Y,Yang D,et al.β1-adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation[J].Crit Care,2015,19: 76.
[9]Porter AC,Svensson SP,Stamer WD,et al.Alpha-2 adrenergic receptors stimulate actin organization in developing fetal rat cardiac myocytes[J].Life Sci,2003,72 (13) : 1455-1466.
[10]Maltsev AV,Kokoz YM,Evdokimovskii EV,et al.Alpha-2 adrenoceptors and imidazoline receptors in cardiomyocytes mediate counterbalancing effect of agmatine on NO synthesis and intracellular calcium handling[J].J Mol Cell Cardiol,2014,68: 66-74.
[11]吴杏,叶任高,汪涛,等.TNF-α、IL-1α、LPS对心肌细胞影响的研究[J].中国病理生理杂志,2004,20 (6) : 923-934.
[12]Yu X,Jia B,Wang F,et al.α1-adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-α production via modulating ERK1/2 and NF-κB pathway[J].J Cell Mol Med,2014,18(2) : 263-273.
[13]Hall G,Singh IS,Hester L,et al.Inhibitor-kappa B kinase-beta regulates LPS-induced TNF-alpha production in cardiac myocytes through modulation of NF-kappa B p65 subunit phosphorylation[J].Am J Physiol Heart Circ Physiol,2005,289(5) : H2103-H2111.
[14]Li XQ,Cao W,Li T,et al.Amlodipine inhibits TNF-alpha production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway[J].Int Immunopharmacol,2009,9(9) : 1032-1041.
(责任编辑:陈妙玲,余小慧)
α2-adrenoceptor agonist B-HT933 suppresses LPS-induced TNF-α production in neonatal rat cardiomyocytes
ZHU Lin-xin1,YANG Duo-meng2,TANG Xiang-xu2,WANG Yuan2,LüXiu-xiu2,LI Hong-mei2,YAN Yu-xia3,QI Ren-bin2,LU Da-xiang2,WANG Hua-dong2
(1Grade 2011,Department of Clinical Medicine,2Department of Pathophysiology,Key Laboratory of State Administration of Traditional Chinese Medicine of People’s Republic of China,3Department of Biochemistry,School of Medicine,Jinan University,Guangzhou 510632,China.E-mail: owanghd@jnu.edu.cn)
[ABSTRACT]AIM: To observe the effect of B-HT933,a selective α2-adrenoceptor agonist,on lipopolysaccharide (LPS) -induced TNF-α production in neonatal rat cardiomyocytes and to explore the underlying mechanisms.METHODS: The neonatal rat cardiomyocytes were cultured.The localization of α(2A)-adrenoceptor in the cardiomyocytes was examined by immunofluorescence staining.The cardiomyocytes were exposed to LPS or/and B-HT933 for different time.The level of TNF-α in the supernatants and the mRNA expression of TNF-α were detected by ELISA and real-time PCR,respectively.In addition,LPS-associated signal molecules in the cardiomyocytes were also examined by Western blotting.RESULTS: Immunofluorescence staining showed that α(2A)-adrenoceptors were localized in the cardiomyocytes.LPS stimulated TNF-α production in the cardiomyocytes in a dose and time-dependent manner.B-HT933 pretreatment significantly inhibited the expression of TNF-α at mRNA and protein levels in LPS-treated cardiomyocytes.Furthermore,LPS exposure induced IκBα and p38 phosphorylation in cardiomyocytes and only IκBα phosphorylation was prevented by BHT933 treatment.CONCLUSION:α(2A)-adrenoceptors are present in neonatal rat cardiomyocytes and its agonist B-HT933 inhibits LPS-induced TNF-α production in cardiomyocytes via suppressing IκBα phosphorylation.
[KEY WORDS]Neonatal rats; Cardiomyocytes; Lipopolysaccharides;α2-adrenoceptor
通讯作者△Tel: 020-85220241; E-mail: owanghd@jnu.edu.cn
*[基金项目]国家自然科学基金资助项目(No.81170222; No.81372028) ;广东省教育厅学科建设科技创新项目(No.2013KJCX0019) ;广州市科技计划(No.12C22071599; No.201508020005)
[收稿日期]2015-06-06
[文章编号]1000-4718(2015)09-1595-06
[中图分类号]R285.5; R363
[文献标志码]A
doi:10.3969/j.issn.1000-4718.2015.09.011
