细菌脂蛋白耐受对脓毒症老龄及成年小鼠心脏功能保护作用的对比
2015-04-21周苏明
王 磊,周 静,周苏明
细菌脂蛋白耐受对脓毒症老龄及成年小鼠心脏功能保护作用的对比
王 磊,周 静,周苏明*
(南京医科大学第一附属医院老年重症监护室,南京 210029)
探讨细菌脂蛋白(BLP)耐受对老龄小鼠心肌细胞的保护作用,并比较其对老龄小鼠与成年小鼠心功能影响的差异。选取86只健康雄性C57BL/6老龄小鼠(SPF级,24月龄)和55只成年雄性C57BL/6小鼠(6~8周龄),用小剂量BLP预处理老龄小鼠及成年小鼠诱导BLP耐受,分别比较正常对照组、假手术(sham)组、脓毒症(剖腹手术行盲肠结扎穿孔致脓毒症,CLP)组、BLP耐受+CLP组,其中成年小鼠在盲肠结扎穿孔0,2,6,12h四个时间点做小鼠二维超声心动图,老龄小鼠在0,3,6,12h四个时间点做小鼠二维超声心动图,选择左心室短轴缩短率(FS)、射血分数(EF)、左心室舒张末期内径(LVIDd)等做为观测指标。老龄小鼠CLP组与BLP耐受+CLP组的FS、EF、LVIDd自手术后均呈下降趋势,但是BLP耐受+CLP组的降幅较CLP组小。成年小鼠BLP耐受+CLP组0~6h时段FS、EF、LVIDd的变化趋势与CLP组相同,但各时间点数值均低于CLP组;而6h后BLP耐受+CLP组的3项指标转而上升,至12h时高于CLP组,其中FS、EF差异均有统计学意义(<0.05)。BLP耐受+CLP组老龄小鼠的FS呈现出进行性下降趋势,而在成年小鼠中6h后为进行性增高趋势,12h明显高于假手术组和CLP组(<0.05)。成年小鼠CLP组在术后EF上升,6h后出现下降的趋势,12h低于术前水平;而老龄小鼠CLP组术后EF进行性下降。成年小鼠BLP耐受+CLP组术后出现的EF值呈逐渐上升趋势,相反,老龄组则进行性下降。老龄小鼠BLP耐受+CLP组的LVIDd术后进行性下降,而成年小鼠BLP耐受+CLP组术后2h时低于术前水平(<0.05),6h后逐渐回升。脓毒症对FS、EF、LVIDd有抑制作用,老龄小鼠心脏收缩功能和舒张功能所受抑制更加严重;BLP耐受对心功能有一定的保护作用,此作用在成年小鼠较明显,在老龄小鼠未见明显保护作用。
休克,脓毒性;细菌脂蛋白耐受;心功能;保护
脓毒症是感染引起的全身炎症反应综合征,具有高患病率和死亡率,心脏是易受损伤的靶器官,近50%脓毒症患者出现不同程度心肌抑制。脓毒症的主要病因是细菌感染过程中其胞壁成分,如细菌脂蛋白(bacterial lipoprotein,BLP)、内毒素脂多糖(lipopolysaccharide,LPS)等激活宿主炎症细胞而导致炎症细胞呈高度活化状态,炎症细胞因子被过度合成释放,引起失控的全身性炎症反应。BLP不但可活化宿主炎症免疫细胞,诱导炎症细胞因子释放,导致休克甚至死亡,而且可以诱导自我耐受[1]。目前有文献报道BLP耐受对脓毒症小鼠的心功能有保护作用[2]。本文将通过体外实验探讨老龄小鼠与成年小鼠对BLP耐受的心功能差异。
1 材料与方法
1.1 实验动物和分组
86只健康雄性C57BL/6老龄小鼠,SPF级,24月龄,体质量26~41g;55只成年雄性C57BL/6小鼠,6~8周龄,体质量20~25g(均上海斯莱克实验动物有限公司)。饲养于清洁级小鼠饲养室,环境温度18~25℃,相对湿度45%~55%。适应性喂养1周后,均随机分为正常对照(control)组,假手术(sham)组,剖腹手术行盲肠结扎穿孔致脓毒症(cecal ligation and puncture,CLP)组,BLP耐受+CLP组(BLP+CLP组)。
1.2 动物模型
术前禁食12h,自由饮水,使用10%水合氯醛对实验小鼠进行腹腔麻醉,胸、腹部皮肤去毛,常规消毒腹部皮肤,取下腹正中行长约1.5cm切口,找到盲肠,游离肠系膜。假手术组盲肠不结扎不穿孔,将盲肠还纳腹腔,0号线逐层缝合肌肉和皮肤。CLP组轻轻牵出盲肠,寻找盲肠与回肠和结肠交界处,用3-0号丝线环行结扎盲肠根部,再用18G针头穿刺盲肠2个孔,从孔中挤出粪便少许,然后将盲肠送回腹腔,逐层关腹。BLP+CLP组是按10mg/kg剂量,予小鼠腹腔内注射BLP,24h后制造盲肠结扎穿孔的脓毒症模型。术后均立即给小鼠皮下注射林格液(Ringer’s solution)25ml/kg抗休克,并且让其随意进食进水。以小鼠造模后出现精神萎靡、腹胀,进食、进水及活动减少,开腹后有血性及脓性渗出、盲肠粘连肿胀、肠胀气等表现作为造模成功标志。
1.3 主要试剂与仪器
BLP(Pam3CSK4·3HCl)(美国Alexis公司)。配置方法:加双蒸水,超声振荡混匀,分别制成1g/L和0.1g/L两种稀释液,-40℃保存。小鼠超声心动图仪(美国GE公司)Vivid 7 Dimension。
1.4 观察指标
选用Vivid 7 Dimension彩色超声诊断仪,线阵探头频率为13MHz。小鼠于前述麻醉剂半量腹腔注射,麻醉后取仰卧位,剃须刀剃去胸部毛,再用脱毛剂脱去剩余皮毛。将超声心动图探头置于小鼠左胸前,探头示标向左侧并与胸骨中线保持70°~80°,于左室乳头肌水平短轴切面测量。分别在0h、造模后2,6,12h 4个时间点对成年小鼠做二维超声心动图,在0h、造模后3,6,12h 4个时间点对老龄小鼠做二维超声心动图,选择左心室短轴缩短率(fractional shortening,FS)、射血分数(ejection fraction,EF)、左心室舒张末期内径(left ventricular internal dimension at end-diastole,LVIDd)等做为观测指标,每组原始数据取连续3个心动周期的平均值,保留图像用于脱机分析。
1.5 统计学处理
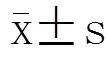
2 结 果
2.1 老龄小鼠与成年小鼠的左心室FS
老龄小鼠对照组与BLP+CLP组在0h相比,差异无统计学意义(>0.05);6h时BLP+CLP组与CLP相比,差异有统计学意义(<0.05),CLP组与假手术组相比,差异亦有统计学意义(<0.05);12h时BLP+CLP组及CLP组与假手术组相比,差异均有统计学意义(<0.05;表1)。
成年小鼠0h BLP+CLP组与相同时间点正常对照组相比,差异有统计学意义(<0.05);6h及12h BLP+CLP组与相同时间点假手术组相比,差异有统计学意义(<0.05);12h BLP+CLP组与相同时间点CLP组相比,差异有统计学意义(<0.05),12h CLP组与假手术组相比,差异有统计学意义(<0.05;表2)。
2.2 老龄小鼠与成年小鼠的EF变化
老龄小鼠0h各组数据相比,差异无统计学意义(>0.05);3h各组数据相比,差异无统计学意义(>0.05);6h时BLP耐受+CLP组与CLP组相比,差异有统计学意义(<0.05),CLP组与假手术组相比,差异有统计学意义(<0.05);12h时BLP耐受+CLP组与假手术组相比,差异有统计学意义(<0.05),CLP组与假手术组相比,差异有统计学意义(<0.05;表3)。
成年小鼠0h时BLP+CLP组与相同时间点正常对照组相比,差异有统计学意义(<0.05);6h及12h时BLP+CLP组与相同时间点假手术组相比,差异有统计学意义(<0.05);12h时BLP+CLP组与相同时间点CLP组相比,差异有统计学意义(<0.05);12h时CLP组与相同时间点假手术组相比,差异有统计学意义(<0.05;表4)。
2.3 老龄小鼠与成年小鼠的LVIDd变化
老龄小鼠6h时CLP组与正常对照组相比,差异有统计学意义(<0.05);12h时BLP+CLP组及CLP组与正常对照组相比,差异均有统计学意义(<0.05),BLP+CLP组及CLP组与假手术组相比,差异均有统计学意义(<0.05;表5)。
成年小鼠0h时BLP+CLP组与相同时间点正常对照组相比,差异无统计学意义(>0.05);2,6及12h时BLP+CLP组与假手术组对比,差异均有统计学意义(<0.05);6,12h时CLP组与假手术组对比,差异有统计学意义(<0.05);另外2,6h时BLP+CLP组与CLP组相比,差异亦有统计学意义(<0.05;表6)。
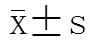
表1 不同处理后老龄小鼠左心室FS的变化
FS: fractional shortening; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with sham group,*<0.05; compared with CLP group,#<0.05
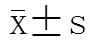
表2 不同处理后成年小鼠左心室FS的变化
FS: fractional shortening; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with control group,*<0.05; compared with sham group,#<0.05; compared with CLP group,△<0.05
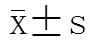
表3 不同处理后老龄小鼠EF的变化
EF: ejection fraction; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with sham group,*<0.05; compared with CLP group,#<0.05
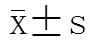
表4 不同处理后成年小鼠EF的变化
EF: ejection fraction; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with control group,*<0.05; compared with sham group,#<0.05; compared with CLP group,△<0.05
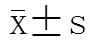
表5 不同处理后老龄小鼠LVIDd
LVIDd: left ventricular internal dimension at end-diastole; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with control group,*<0.05; compared with sham group,#<0.05
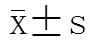
表6 不同处理后成年小鼠LVIDd
LVIDd: left ventricular internal dimension at end-diastole; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with sham group,*<0.05; compared with CLP group,#<0.05
2.4 老龄小鼠和成年小鼠在EF、FS及LVIDd的比较
BLP+CLP组老龄小鼠的FS呈现出进行性下降趋势,其中BLP+CLP组下降幅度较之CLP组小;而在成年小鼠中BLP+CLP组6h后为进行性增高趋势,12h明显高于CLP组,差异有统计学意义(<0.05;图1)。
成年小鼠CLP组在术后EF上升,6h后出现下降的趋势,12h低于术前水平;而老龄小鼠CLP组,术后EF为进行性下降趋势。另外,成年小鼠BLP+CLP组术后出现的EF值呈逐渐上升趋势,相反,老龄组则进行性下降,无代偿性增加(图2)。
老龄小鼠BLP+CLP组的LVIDd在术后出现进行性下降,术后3h时较0h相比,差异有统计学意义(<0.05),而成年小鼠BLP+CLP组术后2h时低于术前水平(<0.05),6h后逐渐回升(图3)。
2.5 小鼠腹腔解剖所见
假手术组小鼠腹腔无明显炎症改变。CLP组小鼠腹腔内可见浓性或血性渗出液,结扎端盲肠肿胀,与周围组织粘连,形成盲肠周围脓肿,肠管扩张。BLP+CLP组小鼠腹腔亦可见脓性或血性渗出液,肠管明显扩张、水肿及充血,结扎端盲肠肿胀明显,与周围组织粘连,肝充血肿大。
3 讨 论
脓毒症是病原菌引起的全身性炎症反应[3],合并感染性休克时病死率>25%,是目前重症监护病房(intensive care unit,ICU)患者死亡的重要原因[4]。而疾病的进展是由于机体自身免疫系统过度激活,导致炎症通路的激活及级联放大的瀑布效应,从而导致全身炎症反应综合征及多器官功能不全综合征[5],其中大约有40%的患者会发生心肌功能障碍[6]。1951年Waisbren最早报道了脓毒症患者出现心功能损害,如心脏扩大、LVEF下降、左室收缩峰压/左室舒张末期压下降、对容量负荷收缩反应差等表现。
BLP是革兰阳性和革兰阴性细菌外膜中最丰富的蛋白,其特征是在蛋白的N端含有独特的脂酰基−氨基酸结构。BLP可由生长或裂解的细菌释放,通过抑制细胞因子等炎症介质的释放来减轻炎症反应,使其在人体免疫中发挥重要作用。机体对细菌的胞壁成分有耐受现象,即机体接触过的某些细菌胞壁成分后,当再次接触大剂量的相同成分或细菌时,会出现暂时性敏感性下降的现象,使致炎因子产生减少,表现为免疫耐受。BLP耐受的重要特征是耐受细胞中的核因子−κB(nuclear factor-κB,NF-κB)活化受到抑制,从而使肿瘤坏死因子−α(tumor necrosis factor-α,TNF-α)、白细胞介素(interleukin,IL)-1、IL-6等NF-κB依赖基因的表达明显减少[7,8]。有实验表明BLP耐受可使小鼠抵抗活细菌或盲肠结扎穿孔造成的脓毒症而免于死亡[2,9]。此外,有临床资料表明,脓毒症患者脂蛋白浓度明显降低与死亡率增加密切相关,以及与入住ICU患者炎症加重相关[10]。
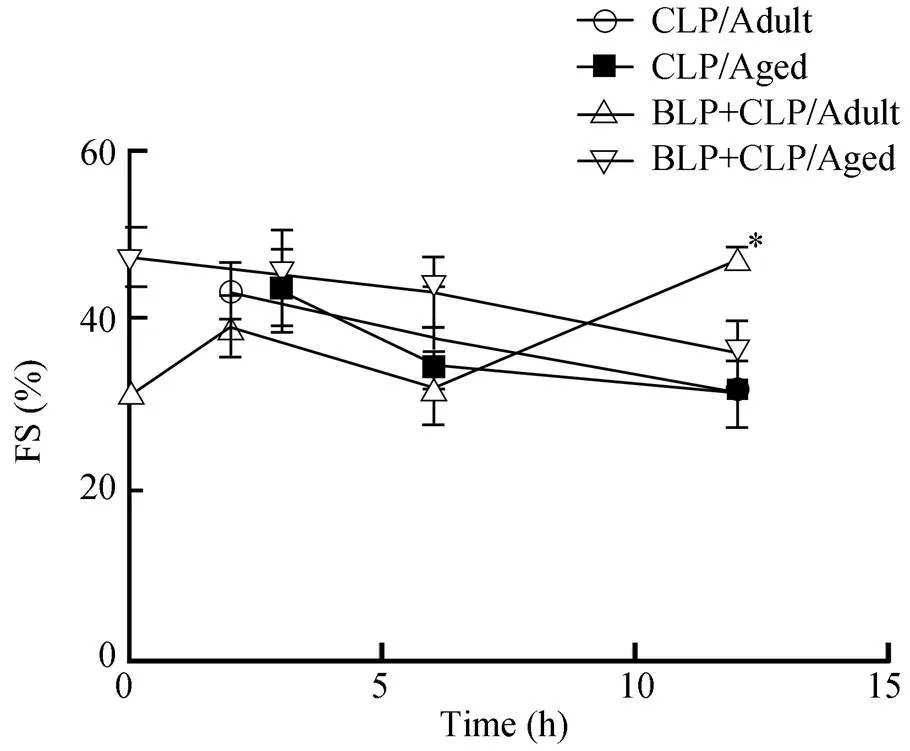
图1 老龄小鼠和成年小鼠FS的比较
Figure 1 The comparison of FS between the aged and adult mice FS: fractional shortening; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with CLP/Adult group,*<0.05

图2 老龄小鼠和成年小鼠EF的比较
Figure 2 The comparison of EF between the aged and adult mice EF: ejection fraction; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein
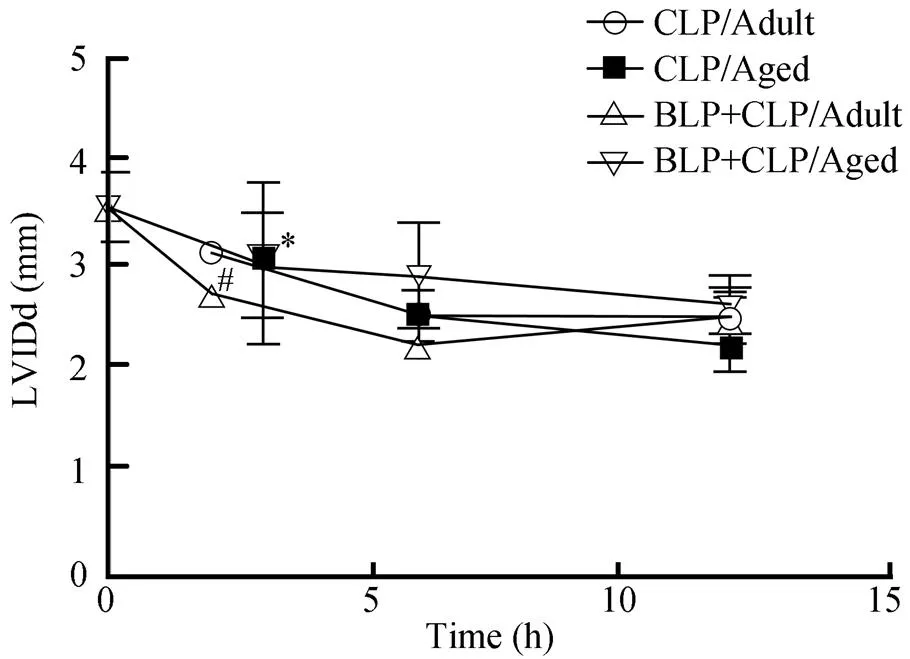
图3 老龄小鼠和成年小鼠LVIDd的比较
Figure 3 The comparison of LVIDd between the aged and adult mice LVIDd: left ventricular internal dimension at end-diastole; CLP: cecal ligation and puncture; BLP: bacterial lipoprotein. Compared with 0h in BLP+CLP/Aged group,*<0.05; compared with 0h in BLP+CLP/Adult group,#<0.05
本研究采用老龄及成年小鼠CLP模型,诱发广泛的全身性炎性反应,表现出与临床相似的早期高动力循环、高代谢和晚期低动力循环状态。心肌功能不全是脓毒症常见的并发症,对脓毒症的预后有着重要影响。本研究就老龄及成年小鼠脓毒症时心功能的变化进行了研究,结果显示,老龄小鼠CLP组与BLP+CLP组的FS、EF、LVIDd自手术后均呈下降趋势,但是BLP+CLP组的降幅较CLP组小。成年小鼠BLP+CLP组0~6h时段FS、EF、LVIDd的变化趋势与CLP组相同,各时间点的数值均低于CLP组。统计学分析显示,0h时BLP+CLP组的FS、EF的值均明显低于正常对照组(<0.05),说明小剂量BLP注射入体内后24h,引起的轻度炎症反应对心脏功能有抑制作用;而6h后BLP+CLP组的3项指标转而上升,至12h时高于CLP组,其中FS、EF均有统计学差异(<0.05),说明BLP耐受对心功能有保护作用。
脓毒症时循环功能的改变是双向性的,初始为高动力循环状态,随着脓毒症的痊愈或进展,高动力循环状态趋向正常或转入低动力循环状态甚至导致死亡。成年小鼠中CLP组及BLP+CLP组术后早期EF值均高于正常,后CLP组出现下降而BLP+CLP组持续升高,而在老龄小鼠则表现为进行性下降。相比于成年小鼠,老龄小鼠的心肌收缩及舒张功能下降,原因可能如下。(1)老龄小鼠心功能低下,随着年龄的增加,心功能及其储备能力下降,老龄小鼠心力衰竭的心室重塑较成年更重,目前机制尚不清;但胶原重建在心室重塑过程中起了重要的作用[11],其中Ⅰ/Ⅲ型胶原比值过度升高,使得心室僵硬度过度增加,从而使得心功能变差。(2)机体调节对外来刺激产生炎症反应的能力出现了随年龄变化,随着年龄的增加,机体对于炎症反应分泌TNF-α、IL-1β、IL-6等炎症因子的能力增强[12]。Vollmar等[13]发现内毒素血症模型的老龄大鼠其外周血TNF-α、IL-1β、IL-6,水平较成年大鼠高2~14倍。Marik等[14]对于NORASEPT Ⅱ临床试验的结果分析发现,高龄脓毒症患者外周血TNF-α明显高于其他年龄组患者。TNF-α、IL-6作为多器官功能衰竭的中心介质,其能够引起心肌延展性和收缩峰值速率的下降,且两者具有协同作用[15]。有报道IL-1β和TNF-α表达一致,说明其可能存在协同作用[16]。
上述结果表明,脓毒症对FS、EF、LVIDd有抑制作用,BLP耐受对心功能有一定的保护作用;而在成年小鼠中此保护作用较老龄小鼠明显。老龄小鼠因其心功能储备低下,及其在脓毒症时所释放的致炎因子增多,其BLP耐受所带来的心功能保护作用有限。
[1] Sato S, Nomura F, Kawai T,. Synergy and cross-tolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways[J]. J Immunol, 2000, 165(12): 7096−7101.
[2] Wang JH, Doyle M, Manning BJ,. Cutting edge: bacterial lipoprotein induces endotoxin-independent tolerance to septic shock[J]. J Immunol, 2003, 170(1): 14−18.
[3] Miyake Y, Yasunaka T, Ikeda F,. SIRS score reflects clinical features of non-acetaminophen-related acute liver failure with hepatic coma[J]. Intern Med, 2012, 51(8): 823−828.
[4] Dellinger RP, Levy MM, Carlet JM,. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2008[J]. Intensive Care Med, 2008, 34(1): 17−60.
[5] Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach[J]. Lancet Infect Dis, 2013, 13(3): 260−268.
[6] Fernandes CJ Jr, de Assuncao MS. Myocardial dysfunction in sepsis: a large, unsolved puzzle[J]. Crit Care Res Pract, 2012, 2012: 896430.
[7] Buckley JM, Wang JH, Redmond HP. Cellular reprogramming by Gram-positive bacterial components: a review[J]. J Leukoc Biol, 2006, 80(4): 731−741.
[8] Coldewey SM, Rogazzo M, Collino M,. Inhibition of IκB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse[J]. Dis Model Mech, 2013, 6(4): 1031−1042.
[9] O’Brien GC, Wang JH, Redmond HP. Bacterial lipoprotein induces resistance to Gram-negative sepsis in TLR4-deficient miceenhanced bacterial clearance[J]. J Immunol, 2005, 174(2): 1020−1026.
[10] Van Leeuwen HJ, Heezius EC, Dallinga GM,. Lipoprotein metabolism in patients with severe sepsis[J]. Crit Care Med, 2003, 31(5): 1359−1366.
[11] Van den Borne SW, Diez J, Blankesteijn WM,. Myocardial remodeling after infarction: the role of myofibroblasts[J]. Nat Rev Cardiol, 2010, 7(1): 30−37.
[12] Inoue S, Sato T, Suzuki-Utsunomiya K,. Sepsis-induced hypercytokinemia and lymphocyte apoptosis in aging-accelerated Klotho knockout mice[J]. Shock, 2013, 39(3): 311−316.
[13] Vollmar B, Pradarutti S, Nickels RM,. Age-associated loss of immunomodulatory protection by granulocyte-colony stimulating factor in endotoxic rats[J]. Shock, 2002, 18(4): 348−354.
[14] Marik PE, Zaloga GP, NORASEPTⅡ Study Investigators,. The effect of aging on circulating levels of proinflammatory cytokines during septic shock. NORASEPTⅡ Study Investigators[J]. J Am Geriatr Soc, 2001, 49(1): 5−9.
[15] Vincent JL. International Sepsis Forum. Hemodynamic support in septic shock[J]. Intensive Care Med, 2001, 27 (Suppl 1): S80−S92.
[16] Wang J, Qian W, Zhu Q,. Martentoxin, a large-conductance Ca(2+)-activated K(+) channel inhibitor, attenuated TNF-α-induced nitric oxide release by human umbilical vein endothelial cells[J]. J Biomed Res, 2013, 27(5): 386−393.
(编辑: 李菁竹)
Comparison of cardiac protection of bacterial lipoprotein tolerance between aged and adult sepsis mice
WANG Lei, ZHOU Jing, ZHOU Su-Ming*
(Geriatric Intensive Care Unit, the First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China)
To determine the protective effect of bacterial lipoprotein (BLP) tolerance on myocardiocytes in aged mice, and compare its effect on cardiac function between aged and adult mice.A total of 86 healthy male aged C57BL/6 mice (SPF grade, 24 months old) and another 55 healthy male adult C57BL/6 mice (SPF grade, 6 to 8 weeks old) were employed in this study. Low-dose BLP was used to pretreat the aged and adult mice to induce BLP tolerance. Then the mice were respectively divided into control, sham operation group (sham), sepsis group (CLP, inflicted by cecal ligation and puncture) and BLP tolerance+CLP group. Echocardiography was carried out in adult mice in 0, 2, 6 and 12h after CLP to measure left ventricle shortening fraction (FS), ejection fraction (EF), left ventricular internal diameter at end-diastole (LVIDd) in adult mice, and in aged mice at time points of 0, 3, 6 and 12h for above-mentioned indices.The FS, EF and LVIDd showed a downward trend after operation in the sepsis group and BLP tolerance+CLP group in aged mice, with the stronger decreases in the former than in the latter group. In the time from 0 to 6h, the BLP tolerance+CLP group in adult mice had similar tendencies in the FS, EF and LVIDd with sepsis group, but lower than the later. Then the 3 indices were increased in the BLP tolerance+CLP group in 6h after CLP, and became higher than those of sepsis group in 12h, with FS and EF having significant differences (<0.05). The FS in the BLP tolerance+CLP aged mice presented a progressive decline tendency, but it was in a progressive increase trend after 6 h in adult mice, and was significantly higher than those of the sham and CLP groups at 12h (<0.05). The EF of CLP group in adult mice increased after operation, then decreased from 6h and became lower than the preoperative level at 12h. While the EF of CLP group in aged mice showed a progressive decline tendency after surgery. The EF of BLP tolerance+CLP group in adult mice was in a progressive increase trend; on the contrary, the corresponding group in aged mice was tending downwards. The LVIDd of the BLP tolerance+CLP group in aged mice was progressively reduced, while that of corresponding group in adult mice was below the preoperative level in 2h after operation (<0.05), then gradually recovered in 6h.Sepsis has inhibitory effects on FS, EF and LVIDd, and the effects on cardiac systolic and diastolic functions are stronger in aged mice. BLP tolerance exerts protective effect on cardiac function. This protection is apparently effective in adult mice than in aged mice.
shock, septic; bacterial lipoprotein tolerance; cardiac function; protection
R631.4
A
10.11915/j.issn.1671-5403.2015.04.064
2015−02−09;
2015−03−30
周苏明, E-mail: zhousmco@yahoo.com.cn
