黄曲霉毒素生物合成及代谢转换的研究进展
2015-04-19熊江林周华林丁斌鹰刘建新
熊江林,周华林,丁斌鹰,刘建新
(1.武汉轻工大学 动物科学与营养工程学院, 湖北 武汉 430023; 2.浙江大学 奶业科学研究所,浙江 杭州 310058;3.襄阳职业技术学院 生物工程学院,襄阳市动物医学工程技术中心,湖北 襄阳 441100)
黄曲霉毒素生物合成及代谢转换的研究进展
熊江林1, 2,周华林3,丁斌鹰1,刘建新2*
(1.武汉轻工大学 动物科学与营养工程学院, 湖北 武汉 430023; 2.浙江大学 奶业科学研究所,浙江 杭州 310058;3.襄阳职业技术学院 生物工程学院,襄阳市动物医学工程技术中心,湖北 襄阳 441100)
黄曲霉毒素(Aflatoxins,AFs)是真菌产生的有机代谢产物,具有强烈的生物毒性,广泛存在于饲料原料和各类饲料之中。本文综述了AFs的产生条件、合成途径以及AFB1在动物体内的代谢转化,旨为黄曲霉毒素的防控和动物健康养殖提供参考。
黄曲霉毒素;合成途径;代谢转化;研究进展
黄曲霉毒素(Aflatoxins,AFs)名称源于该毒素的主要生产真菌-黄曲霉菌 (Aspergillusflavus)。有关AFs的最早报道是在1960年,英国东南部地区农场给火鸡饲喂巴西和非洲生产的花生粕后,导致10万只火鸡突然死亡。最初因病因不明,该病被命名为火鸡"X"病,后来被证实为黄曲霉菌产生的AFs严重污染饲料所致[1]。50多年来,人们广泛开展了AFs的生成、代谢、毒性和防控技术研究。
1 AFs的种类和产生菌株
AFs的CAS号1402-68-2,是一组结构类似的二呋喃香豆素衍生物,其基本结构为1个二呋喃环和1个氧杂萘邻酮 (香豆素)组成,前者为基本毒性结构而后者与致癌有关。自然界中至少存在14 种不同类型的AFs,主要有AFB1、AFB2、AFG1和AFG2等。其中,AFB1在体内可羟化生成AFM1[2],AFM1可见于动物的肉、蛋、奶、肾脏和肝脏等动物产品及内脏之中。
AFs主要由曲霉属中的黄曲霉 (Aspergillusflavus)和寄生曲霉 (A.parasiticus)污染谷物和谷物产品后所产生的一组次生代谢物[3-4]。其他一些品种,诸如特异曲霉 (A.nomius)、家蚕曲霉 (A.bombycis)、假溜曲霉菌 (A.pseudotamari)和赭曲霉(A.ochraceoroseus)也可产生AFs,但这些霉菌在自然界中出现的频率很低[5-7]。黄曲霉分离菌株在产生AFs能力方面存在重大差异。据报道,上述真菌中的50%菌株可以产生AFs,而一些分离菌株可以产生高达106 μg/kg的AFs[8-9]。
2 AFs生成的外在条件
AFs形成和污染程度除了上述产毒真菌外,还需要适当的产毒外部环境条件包括:温度、湿度、水活力、物理性损伤和其它储存条件[10]以及饲料种类。产生的毒素量将取决于生物学因素 (植物品种、应激、昆虫和霉菌孢子量)和物理因素 (水分、相对湿度、温度和谷物的物理损伤)[11]。比如,黄曲霉和寄生曲霉等在谷物具有一定水分含量 (≥15%)、适当的温度 (20~30 ℃)和相对湿度 (≥80%)条件下即可生长,并生成AFs,其中以AFB1比例最大[12-13]。
产毒真菌在收获前侵染谷物需要的水分含量 (200~250 g/kg)通常比真菌在存储间增殖所需水分含量 (130~180 g/kg)要高些。因此,水分含量在130 g/kg以上的大多数饲料是容易导致霉菌生长和霉菌毒素的形成[14]。另外,谷物在30%~32%水分条件下成熟,水分下降时,谷粒容易发生机械性破损,这些破损的谷粒就会成为有利于产毒真菌生长的基质。大宗谷物在水分不均匀,且不同批次谷物进行混合会有利于AFs产毒真菌的生长,使得谷物受AFs污染情况更加严重,所以维持谷物低且均匀的水分十分重要[12]。
饲料种类不同,营养基质状况差异很大,也会在很大程度上影响产毒真菌的生长和产毒量,比如花生粕、玉米、棉籽粕和青贮饲料等饲料原料非常适合AFs产毒真菌生长和AFs的形成,是导致饲料黄曲霉毒素污染的主要来源[15-17]。
3 AFs的生物合成途径
AFs生物合成共需21个酶促步骤,并且这些酶的基因已经得到克隆。aflR和aflJ基因编码大多数结构基因转录激活的蛋白质,也是基因簇的一部分[18-19]。黄曲霉和寄生曲霉DNA的粘粒和λ噬菌体库的限制性酶图显示所有的基因成簇聚集在真菌基因组70-kb区域[20]。其他曲霉 (如构巢曲霉)生成AFs前体物,诸如杂色曲霉素 (ST)。ST生物合成途径与黄曲霉和寄生曲霉类似,但是染色体上基因顺序不同[21]。AFs的合成是通过一系列高度协调的氧化还原反应实现[22],主要合成途径和相关基因见图1(以黄曲霉合成AFs为例)。AFs的生物合成起始类似于脂肪酸的生物合成,即乙酰CoA作为起始单位,而丙二酸单酰作为延长单位,在聚酮化合物合成酶(PKSA)催化作用下形成AFs的聚酮骨架,该过程由两个脂肪酸合酶基因 (fas-1和fas-2)和聚酮合酶基因 (pksA)编码产物催化[18-19]。无特定的酶催化noranthrone转变为NOR (可以分离的第一个稳定代谢物),但转化可能涉及单加氧酶 (可能cypA编码)和脱氢酶 (可能norB编码)[19-22,24]。
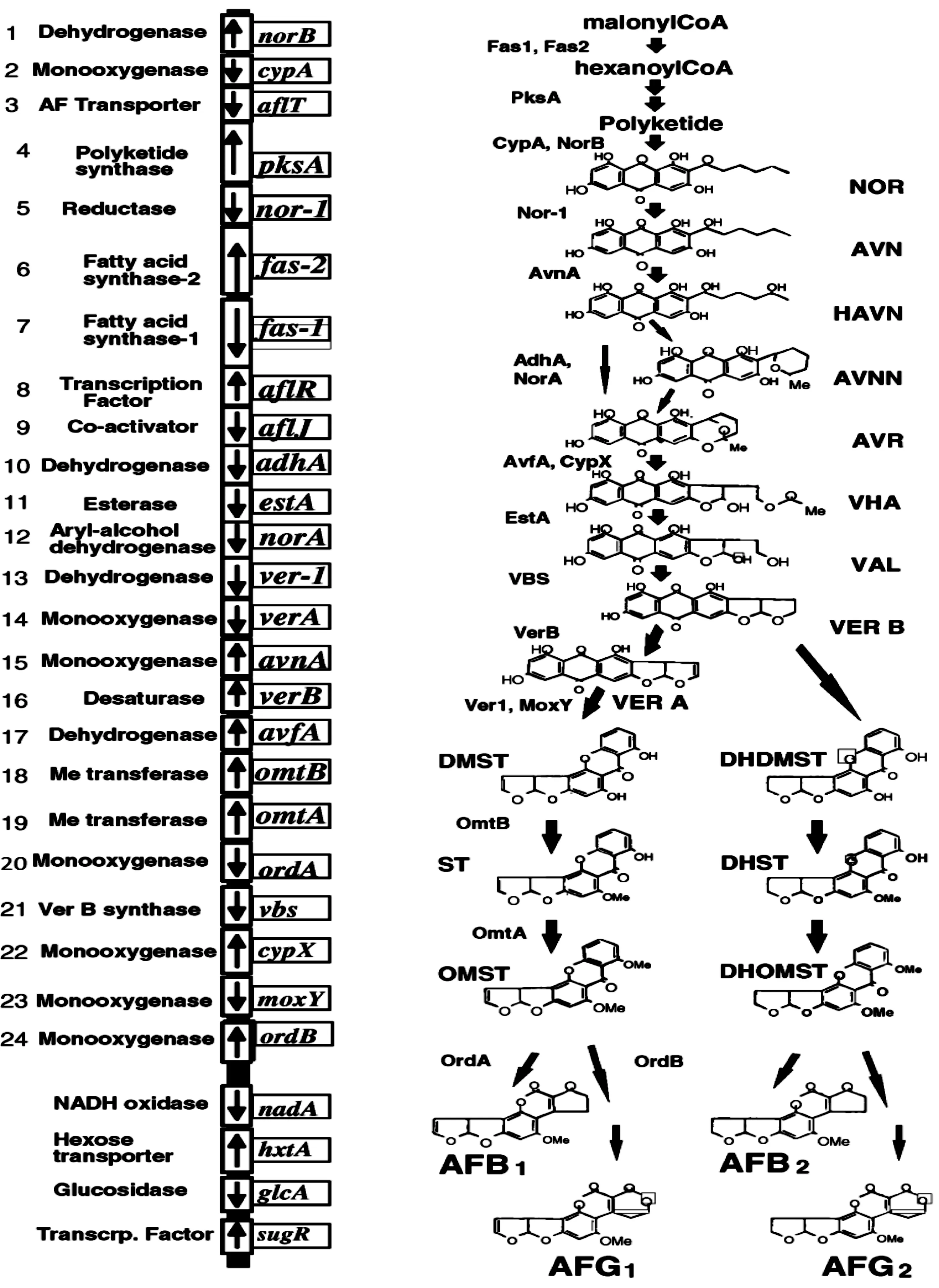
图1 黄曲霉和寄生曲霉合成AFs的途径及相关基因簇和酶[23]
NOR转化为AVN涉及脱氢酶,该酶由nor-1基因编码[25],但也能够由norA编码的脱氢酶催化[26]。奥弗尼红素 (AVR)转化为杂色曲霉B (VERB)的一些催化步骤仍未与基因簇上特定基因建立联系。三个基因 (cypX、moxY和avfA)可能涉及个别步骤;两个基因ver1 (编码酮还原酶)和verA (编码细胞色素P-450单加氧酶)是杂色曲霉A (VERA)转化为脱甲基杂色曲霉素 (DMST)所必需的[27-28]。AFs形成的最后步骤是O-甲基杂色曲霉素(OMST)或二氢-O-甲基杂色曲霉素 (DHOMST)转化为AFB1、B2、G1和G2,该反应需要ordA基因编码的NADPH依赖单加氧酶[29]。AFG毒素形成还可能与ordB编码的酶有关[29]。其他基因,如aflT。编码ABC转运蛋白,该蛋白可能是AFs从细胞转运出来所必需。
4 AFB1的吸收与代谢转化
由于AFB1是脂溶性和低分子量,一旦被摄入后就会很快通过被动扩散机制被瘤胃壁和肠道吸收[30]。实验动物和灵长类动物的有关试验显示AFB1的吸收迅速、完全[31-32]。根据对大鼠的药代动力学研究,AFB1主要在十二指肠段吸收,并且在小肠的2个位点分2个阶段吸收。AFB1在奶牛消化道迅速被吸收,使得AFM1很快迁移到乳中[33]。血液中约90%的AFB1存在于血浆中[34]。AFB1与蛋白质结合的部分主要连接到白蛋白上,白蛋白赖氨酸和AFB1某种衍生物间形成的席夫碱共价键涉及到血蛋白中加合物形成。
肠道上皮组织、肝脏和肾脏是许多化合物发生生物转化的部位,这些生物转化包括两个阶段的反应。第一阶段由还原、氧化和水解反应组成。微粒体细胞色素P-450、含有核黄素的单氧合酶、前列腺素类合成酶、氨基氧化酶和乙醇脱氢酶等是参与氧化反应的主要酶类,然而还原反应受到环氧化物水解酶、醛糖还原酶或酮还原酶控制。另外,哺乳动物组织和体液含有大量能水解异生物质分子的非特异性酯酶和酰胺酶[35-36]。第二阶段由合成反应组成,作用于第一阶段形成的分子。这些反应会减少毒性、增加AFs分子的水溶性以便通过尿 (奶)排除,以保护动物[38]。主要的加成反应酶是微粒体葡糖醛酸基转移酶、S-谷胱甘肽转移酶和N-乙酰基转移酶[35]。AFB1在肝脏中发生的一系列生物转化和和排泄途径见图2,奶牛可将食入的AFB1转化为AFM1,然后分泌到牛奶中[39-40],蛋禽也可将AFM1分泌到蛋中[41-42]。除了肝脏外,氧化系统还会在其他组织中发挥作用,比如骨髓、肥大细胞、皮肤、卵巢、睾丸和白细胞等[47]。

图2 吸收的AFB1在肝脏中的生物转化途径[39]
5 结 语
AFs的产毒真菌、产毒条件和易感谷物等的研究可提供降低饲料和食品中AFs的方法。随着真菌合成AFs的相关基因相继被解析,应用基因修饰方法可得到不产毒的曲霉真菌,再接种谷物等植株上,通过与野生的产毒曲霉真菌的生物生存竞争,达到减少AFs对谷物等的污染。而AFs的代谢转化途径中一些关键酶和代谢中间产物相继被研究楚,将为AFB1解毒药物的开发提供启示。
[1] Forgacs J. Mycotoxicoses-the neglected diseases[J].Feedstuffs,1962(34):124-134.
[2] 李培武, 丁小霞, 白艺珍, 等.农产品黄曲霉毒素风险评估研究进展[J]. 中国农业科学,2013(12):2 534-2 542.
[3] Cheraghali A M, Yazdanpanah H, Dorak N, et al. Incidence of aflatoxins in Iran pistachio nuts[J]. Food and Chemical Toxicology,2007(45):812-816.
[4] Iqbal S Z, Paterson R R M, Bhatti I A, et al. Survey of aflatoxins in chilies from Pakistan produced in rural, semi-rural and urban environments[J]. Food Additives & Contaminants Part B-Surveillance,2010(3):268-274.
[5] Kurtzman C P, Horn B W, Hesseltine C W. Aspergillus nomius,a new flavus-producing species related to aspergillus flavus and aspergillus tamari[J]. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology,1987(5):147-158.
[6] Peterson S W, Ito Y, Horn B W, et al. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius[J]. Mycologia,2001(93):689-703.
[7] Moss M O. Mycotoxin review-1. Aspergillus and Penicillium[J]. Mycologist,2002(16):116-119.
[8] Klich M A, Pitt J I. Differentiation of Aspergillus flavus and A. parasiticus and other closely related species[J].Transactions of the British Mycological Society,1988(9):99-108.
[9] Cotty P J, Bayman P, Egel D S, et al. The genus Aspergillus:From taxonomy and genetics to industrial application.Agriculture, aflatoxins and Aspergillus[M].New York:Plenum Press,1994:1-27.
[10] EFSA (European Food Safety Authority) . Opinion of the Scientiflc Panel on contaminants in the food chain related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds,hazelnuts,pistachios and derived products[J]. European Food Safety Authority Journal,2007(446):1-127.
[11] Frisvad J. C. Stored-Grain Ecosystems[M].New York:Marcel Dekker Inc,1995,251-288.
[12] Prandini A, Tansini G, Sigolo S, et al. On the occurrence of aflatoxin M1 in milk and dairy products[J]. Food Chem Toxicol,2009(47):984-991.
[13] Mostrom M S, Jacobsen B J. Ruminant Mycotoxicosis[J]. Veterinary Clinics of North America-food Animal Practice,2011(2):315-344.
[14] Magan N. Mycotoxin contamination of food in Europe: early detection and prevention strategies[J]. Mycopathologia,2006(162):245-253.
[15] Jaime-Garcia R, Cotty P J. Aflatoxin contamination of commercial cottonseed in South Texas[J]. Phytopathology,2003(93):1 190-1 200.
[16] Ding X X, Li P W, Bai Y Z et al. Aflatoxin B1 in post-harvest peanuts and dietary risk in China[J]. Food Control,2012(23):143-148.
[17] Keller L A M, González Pereyra M L, Keller K M, et al. Fungal and mycotoxins contamination in corn silage:Monitoring risk before and after fermentation[J]. Journal of Stored Products Research,2013(52):42-47.
[18] Cary J W, Linz J E, Bhatnagar D. Microbial foodborne diseases: mechanisms of pathogenesis and toxin synthesis[M].Lancaster: Technomic press,2000:317-361.
[19] Abd-El-Aziz A R M, Mahmoud M A, Al-Othman M R, et al. Genetic Variability of Non-aflatoxigenic Aspergillus flavus Isolates by using Aflatoxin Biosynthesis Genes[J]. Journal of Pure and Applied Microbiology,2013,7(SI): 341-347.
[20] Yu J, Chang P K, Cary J W, et al. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus[J].Applied and Environmental Microbiology,1995(61):2 365-2 371.
[21] Brown D W, Yu J H, Kelkar H S, et al. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans[J].Proceedings of the National Academy of Sciences of the United States of America,1996(93):1 418-1 422.
[22] Minto R E, Townsend C A. Enzymology and molecular biology of aflatoxin biosynthesis[J]. Chemical Reviews,1997(97):2 537-2 555.
[23] Bhatnagar D, Ehrlich K C, Cleveland T E. Molecular genetic analysis and regulation of aflatoxin biosynthesis[J]. Applied Microbiology and Biotechnology,2003(61):83-93.
[24] Bhatnagar D, Ehrlich K C, Cleveland T E. Handbook of applied mycology(vol V):Oxidation-reduction reactions in biosynthesis of secondary metabolites.[M]. New York: Marcel Dekker, Inc,1992:255-285.
[25] Trail F, Chang P K, Cary J, et al. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus[J]. Applied and Environmental Microbiology, 1994(60):4 078-4 085.
[26] Cary J W, Bhatnagar D. Nucleotide sequence of an Aspergillus parasiticus gene strongly repressed by thiamine[J]. Biochimica et Biophysica Acta-gene Structure and Expression,1995(1261):319-320.
[27] Hong S B, Lee M, Kim D H, The proportion of non-aflatoxigenic strains of the Aspergillus flavus/oryzae complex from meju by analyses of the aflatoxin biosynthetic genes[J]. Journal of Microbiology,2013(6):766-772.
[28] Skory C D, Chang P K, Cary J, et al. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis[J]. Applied and Environmental Microbiology,1992(58):3 527-3 537.
[29] Yu J, Chang P K, Ehrlich K C, et al. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P-450 monooxygenase encoded by ordA that is involved in the biosynthesis of aflatoxins B1, G1, B2, and G2[J]. Applied and Environmental Microbiology,1998(64):4 834-4 841.
[30] Moschini M, Mosoero F, Diaz D E. Plasma aflatoxin concentrations over time in bolus fed lactating dairy cows[J]. Journal of Animal Science,2006(84):74.
[31] Dalezios J I, Keriger R I, Hseih D P H. The in vivo metabolism of aflatoxin B1 in rhesus monkey liver[J]. Federation Proceedings,1972(31):733.
[32] Coulombe R A, Sharma R P. Clearance and excretion of intratracheally and orally administered aflatoxin B1 in the rat[J]. Food and Chemical Toxicology,1985(23):827-830.
[33] Polan C E, Hayes J R, Campbell T C. Consumption and fate of aflatoxin B1 by lactating cows[J]. Journal of Agricultural and Food Chemistry,1974(22):635-638.
[34] Wong S A, Hsieh D P H. The comparative metabolism and toxicokinetics of aflatoxin B1 in the monkey rat and mouse[J]. Toxicology and Applied Pharmacology,1980(55):115-125
[35] Galtier P. Biotransformation and fate of mycotoxins[J]. Journal of Toxicology-toxin Reviews,1999(18):295-312.
[36] Karabulut S, Paytakov G, Leszczynski J. Reduction of aflatoxin B1 to aflatoxicol: A comprehensive DFT study provides clues to its toxicity[J]. Journal of the Science of Food and Agriculture,2014(15):3 134-3 140.
[37] Moschini M, Gallo A, Piva G, et al. The effects of rumen fluid on the in vitro aflatoxin binding capacity of different sequestering agents and in vivo release of the sequestered toxin[J]. Animal Feed Science and Technology,2008(147):292-309.
[38] Dominguez-Bello M. G. Detoxification in the rumen[J].Annales de Zootechnie, 1996,45 (Suppl.):323-327.
[39] Kissell L, Davidson S, Hopkins B A,et al. Effect of experimental feed additives on aflatoxin in milk of dairy cows fed aflatoxin-contaminated diets[J]. Journal of Animal Physiology and Animal Nutrition,2012(97):694-700.
[40] Queiroz O C M, Han J H, Staples C R, et al. Effect of adding a mycotoxin-sequestering agent on milk aflatoxin M1 concentration and the performance and immune response of dairy cattle fed an aflatoxin B1-contaminated diet[J]. Journal of Dairy Science,2012(95):5 901-5 908.
[41] Qureshi M A, Brake J, Hamilton P B, et al. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks[J]. Poultry Science,1998,77(6):812-819.
[42] Khalil M M H, Gomaa A M, Sebaei A S. Reliable HPLC Determination of Aflatoxin M1 in Eggs[J]. Journal of Analytical Methods in Chemistry,2013, 817091:1-5.
Review of Biosynthesis and Metabolic Turnover of Aflatoxins
XIONG Jiang-lin1,2, ZHOU Hua-lin3, DING Bin-ying1, LIU Jian-xin2
(1.SchoolofAnimalScience&NutritionalEngineering,WuhanPolytechnicUniversity,Wuhan, 430023,China. 2.InstituteofDairyScience,ZhejiangUniversity,Hangzhou310058,China; 3.XiangyangEngineeringResearchCenterofAnimalMedicine,XiangyangVocationalandTechnicalCollege,Xiangyang441100,China)
Aflatoxins are mebolites produced by fungus and widely exist in feedstuff and all kinds of feed. It has strong biological toxicity, and thus endanger feed production and the healthy development of animal husbandry. In order to assist the prevention and control and animal feeding practice, discussions were made on generating condition and denovo synthesis of aflatoxins and metabolic transformation of aflatoxin B1 in animals.
afltoxins; production; metabolic transformation
2014-12-15,
2015-01-05
现代奶牛产业技术体系建设专项资金资助(CARS-37)
熊江林(1977-),男,湖北鄂州人,讲师,博士,研究方向为动物营养与免疫。E-mail:xiongjianglin@126.com
*[通讯作者] 刘建新(1958-),男,浙江杭州人,博导,教授,主要从事反刍动物营养研究。E-mail:liujx@zju.edu.cn
S811.6
A
1005-5228(2015)04-0085-05
