水通道蛋白4基因表达与阿尔茨海默病相关性的动物实验
2015-04-01周明月王可伊张延明温宏峰孟凡磊方伯言
周明月,王可伊,张延明,赵 云,温宏峰,孟凡磊,方伯言
1辽宁医学院北京航天中心医院研究生培养基地,北京 100049;2北京航天中心医院,北京 100049;
3北京航天中心医院 神经内科,北京 100049;4辽宁医学院,辽宁锦州 121000
水通道蛋白4基因表达与阿尔茨海默病相关性的动物实验
周明月1,王可伊2,张延明1,赵 云1,温宏峰3,孟凡磊4,方伯言3
1辽宁医学院北京航天中心医院研究生培养基地,北京 100049;2北京航天中心医院,北京 100049;
3北京航天中心医院 神经内科,北京 100049;4辽宁医学院,辽宁锦州 121000
目的探讨水通道蛋白-4 (aquaporin 4,AQP4)基因表达与阿尔茨海默病的相关性。方法应用Agilent表达谱芯片,选用快速老化痴呆模型小鼠(SAMP8),通过与同月龄正常对照小鼠(SAMR1)比较,提取脑组织总RNA,筛选出差异基因并应用Real time-PCR技术和免疫荧光方法进行鉴定。结果筛选差异表达基因共804个,其中表达上调521个,表达下调283个,在GeneBank查找相关基因的生物学功能及通路,找到与AD密切相关基因有6条:Herc6,Lyst,Nkain1,Aqp4,Uch13,Slc9a8,其中Aqp4基因的差异倍数为6.26 (P<0.001)。Real time-PCR检测结果显示,SAMP8组Aqp4基因表达量较SAMR1组增高(P=0.037 6),免疫荧光提示SAMP8组中Aqp4表达增加。结论Aqp4在SAMP8小鼠中表达量增多,可能与清除β淀粉样蛋白沉积、代偿性星形胶质细胞增生有关。
阿尔茨海默病;基因芯片;水通道蛋白-4
阿尔茨海默病(Alzheimer′s disease,AD)是一种严重危害人类健康的神经系统退行性疾病,占进行性认知功能障碍的老年人群的50% ~ 60%[1]。目前公认的病理特征为脑组织细胞外以β淀粉样蛋白(β-amyloid,Aβ)沉积为中心的神经炎性斑(neuritic plaques,NP),细胞内以Tau蛋白过度磷酸化形成的神经纤维缠结(neurofibrillary tangles,NFT)和神经细胞凋亡。普遍认为Aβ生成增多和Aβ清除障碍是Aβ沉积的主要原因。参与Aβ生成和清除的众多因子中,类淋巴途径及水通道蛋白4 (aquaporin 4,AQP4)近来备受关注[2-3]。AQP4蛋白存在于星形胶质细胞足突,围绕在血管周围,在类淋巴途径的循环中起到重要的作用[4-5]。本研究利用Agilent表达谱芯片技术同时观察SAMP8和SAMR1小鼠海马组织基因表达的变化趋势,筛选并通过进一步的验证发现,AQP4在SAMP8痴呆小鼠表达增加,为进一步探究AQP4在类淋巴途径循环中清除Aβ的研究提供依据。
材料和方法
1实验动物及分组 采用10月龄SAMP8雄性小鼠20只,体质量26 ~ 30 g;10月龄SAMR1雄性小鼠10只,体质量30 ~ 35 g。小鼠由天津中医药大学第一附属医院老年动物中心提供(动物合格证号:SCXK津-2008-0001)。饲养于军事医学科学院SPF级动物房,饲养环境温度(24±2)℃,湿度50% ~ 80%,12 h光照,自由饮水和摄食,单笼饲养。实验分为正常老化SAMR1小鼠组(A组,n=10)、快速衰老SAMP8小鼠组(B组,n=10)。
2基因表达谱检测 每组随机选取3只小鼠,断头处死后,迅速在冰盒表面分离左侧海马,提取纯化总RNA,利用NanoDrop ND-2000(Thermo Scientific)定量并经Agilent Bioanalyzer 2100(Agilent Technologies)检测RNA完整性。质检合格后,样本标记、芯片杂交及洗脱参照芯片标准流程。首先,总RNA反转录成双链cDNA,再进一步合成用Cyanine-3-CTP(Cy3)标记的cRNA。标记好的cRNA和芯片杂交,洗脱后利用Agilent Scanner G2505C(Agilent Technologies)扫描得到原始图像提取原始数据。利用Genesrping软件(Version 12.5;Agilent Technologies)进行Quantile标准化和后续处理。标准化后的数据进行过滤,在用于比较的每组样本中至少有1组100%标记为Detected的探针留下进行后续分析。应用t检验的P值和倍数变化值进行差异基因筛选,筛选的标准为上调或者下调倍数变化值≥2.0且P≤0.05。接着,对差异基因进行GO和KEGG富集分析,以判定差异基因主要影响的生物学功能或者通路。
3差异表达基因的实时荧光定量PCR验证 选取A、B两组共6个样本并挑选共同表达差异的基因进行验证,以GAPDH为内参照,引物参照GenBank数据库基因序列进行设计。Aqp4引物序列,上游引物:TTAATGAAGTGCATAGTGCCG;下游引物:GCATTGTTTCATGGCTCG,扩增产物约为117 bp。内参GAPDH引物序列,上游引物:CTTTGGCATTGTGGAAGGGC;下游引物:CAGGG ATGATGTTCTGGGCA,扩增产物约为125 bp。提取A组和B组总RNA,利用NanoDrop 2000分光光度计(Thermo Scientific,USA)测定浓度及OD260/ OD280,琼脂糖凝胶电泳检测RNA完整性,利用PrimerScript RT reagent Kit(TaKaRa,Japan)将待测RNA逆转录成cDNA。利用LightCycler 480 SYBR Green I Master试剂盒(Roche,Swiss),采用10 μl体系在LightCycler 480Ⅱ型荧光定量PCR仪(Roche,Swiss)上进行反应。反应条件:95℃变性10 min,退火10 s,60℃延伸30 s。40个循环结束后利用熔解曲线检测产物特异性:从60℃缓慢升温至97℃,每摄氏度采集5次荧光信号。分析扩增曲线,采用2-ΔΔCt法计算靶基因mRNA经内参GAPDH标准化后的相对水平。
4间接免疫荧光法检测两组AQP4蛋白的表达量将鼠海马组织在液氮中快速冷冻并制成4 μm的切片。应用0.5% Trion-100处理4 min,浸入在10%的甲醛中固定4 min,PBS冲洗。并在10%的常规血清中封闭1 h。加入一抗(AQP-4,sc-32739,Santa Cruz Biotechnology)室温孵育1 h,PBS冲洗3次后,加入FITC抗兔IgG (Southern Biotechnology Associates,Inc)(用PBS和1% BSA稀释为1∶200)避光室温孵育30 min,PBS冲洗后在荧光显微镜下应用Zeiss Axiovision软件(Zeiss,Thornwood,NY,USA)观察并拍照。
5统计学分析 应用SPSS16.0软件进行数据处理,数据以表示,两组均数之间比较应用t检验,检验水准a=0.05。
结 果
1基因表达谱概况 经Agilent Bioanalyzer 2100 (Agilent Technologies)检测RNA完整性符合标准(图1)。利用t检验分析出两组样本间差异表达的基因后,以Log2(fold change)为横坐标,以t检验显著性检验P值的负对数-Log10(P-value)为纵坐标,得火山图(图2)。B组与A组两组筛选出基因表达差异在2倍以上的有804个,其中表达上调的有521个,表达下调的有283个。
2与AD密切相关的差异基因筛选 在GeneBank上查找差异基因中相关的生物学功能或通路,找到与AD密切相关基因有6条:Herc6、Lyst、Nkain1、Aqp4、Uch13、Slc9a8。见表1。
3差异表达基因的实时荧光定量PCR验证 筛选出与AD密切相关的6条差异表达基因中差异倍数最高的Aqp4基因进行定量PCR检测。结果发现,SAMP8小鼠组Aqp4基因表达量较SAMR1小鼠组上升(P=0.037 6)。与基因表达谱结果相比,具有相同的方向性,并且数据结果十分接近。见图3。
4免疫荧光检测 结果显示,AQP4蛋白被FITC标记,表达为绿色荧光。图4可见,实验组即SAMP8组鼠内囊组织较对照组绿色荧光的表达增多,同时在SAMP8组鼠的海马组织内也可见大量绿色荧光的表达。
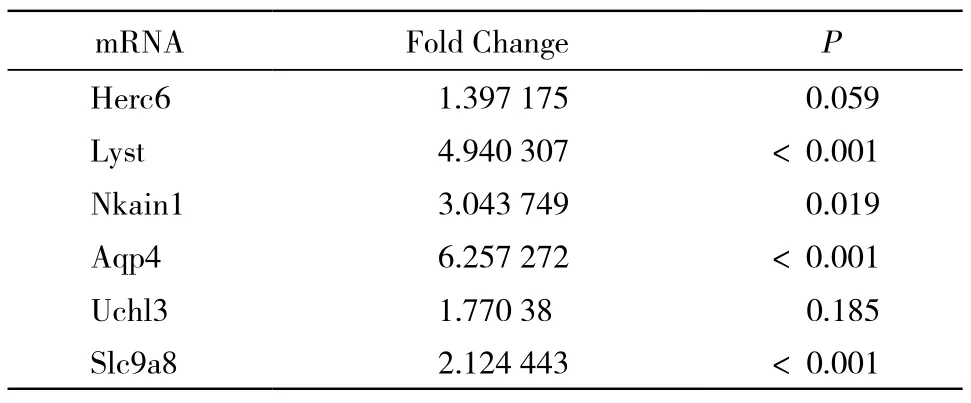
表1 SAMP8与SAMR1组差异基因Tab. 1 Differential genes of SAMP8 and SAMR1
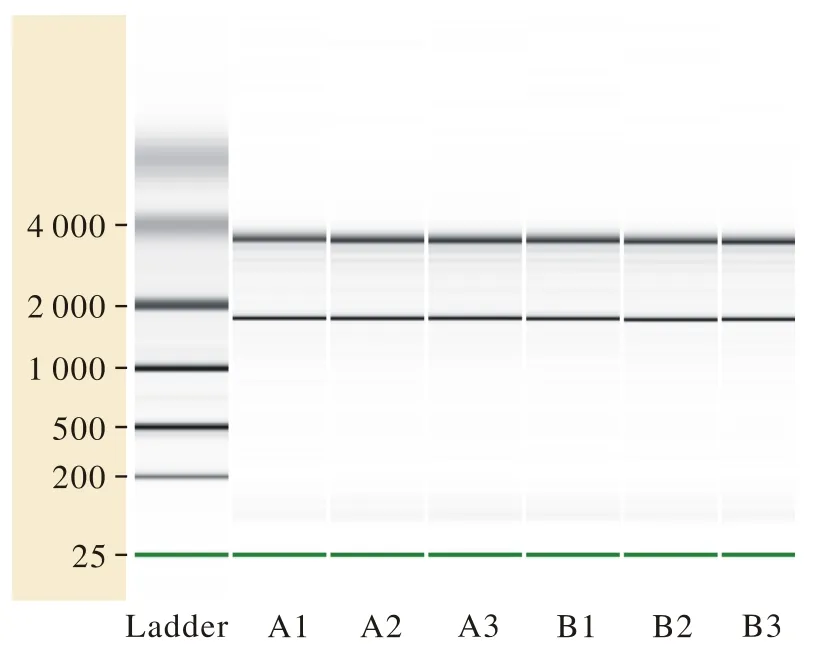
图 1 SAMP8与SAMR1总RNA样品电泳图。 A1、A2和A3为SAMR1小鼠组随机挑选出的3组总RNA样品,B1、B2和B3为SAMP8小鼠组随机挑选出的3组样品Fig. 1 Electrophoregram of total RNA samples of SAMP8 and SAMR1. A1, A2 and A3 were three groups of total RNA samples that were randomly selected from SAMR1 rats. B1, B2 and B3 were three groups of total RNA samples that were randomly selected from SAMP8 rats
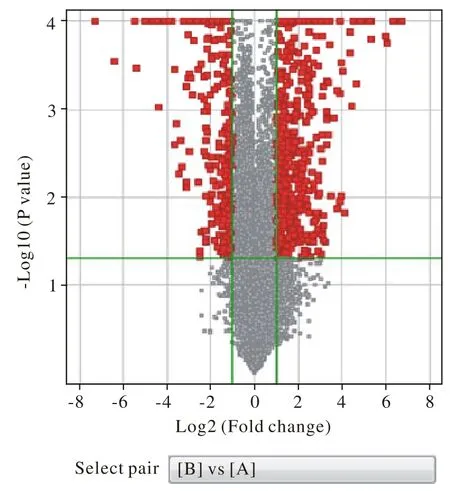
图 2 SAMP8与SAMR1差异基因筛选火山图(黑色部分即为差异基因即≥2倍变化且P<0.05)Fig. 2 Volcano plot of differential gene screening between SAMP8 and SAMR1 (The black part is the differential genes whose fold change≥2 times with P< 0.05)
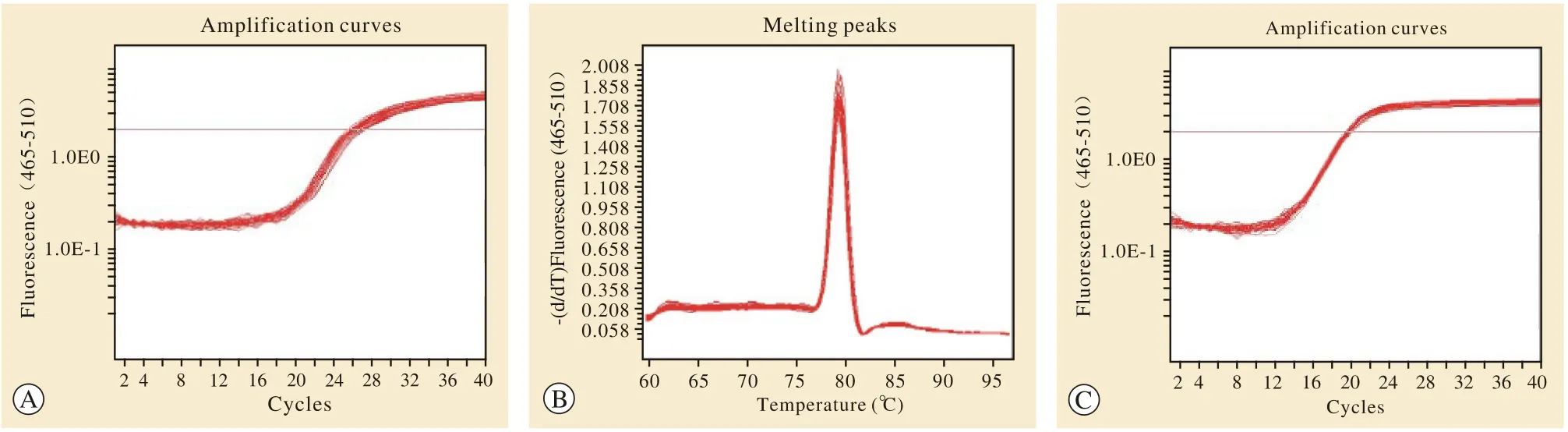
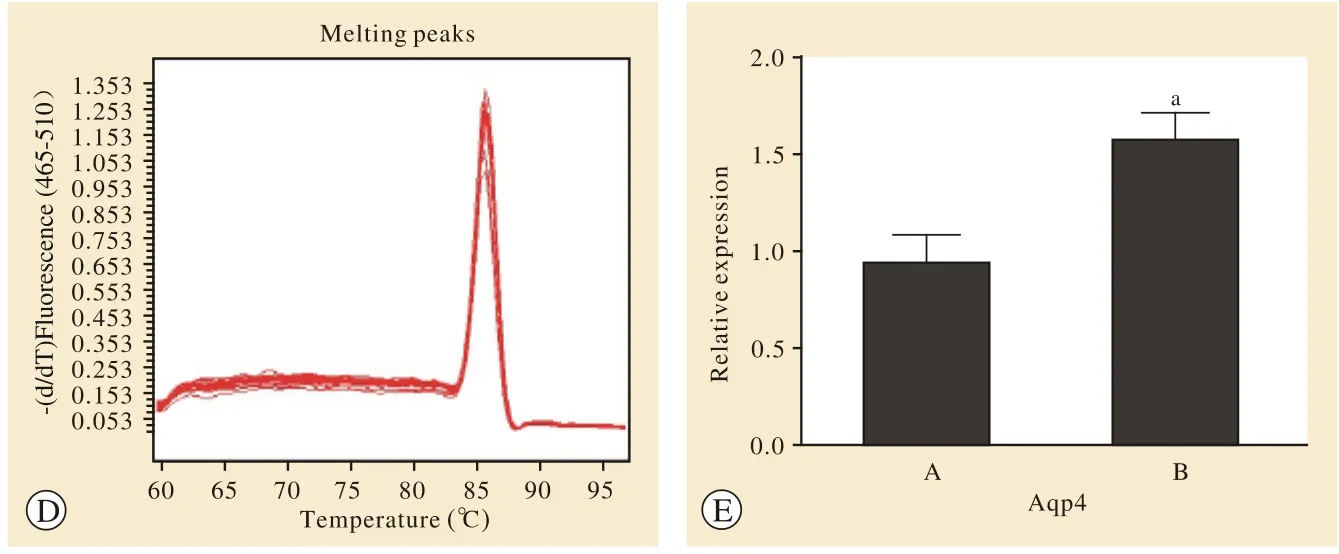
图 3 RT-PCR检测AQP4 mRNA的相对表达量结果。 RT-PCR扩增Aqp4的扩增曲线(a)、溶解曲线(b);扩增GAPDH的扩增曲线(c),其溶解曲线(d);统计图(e) (A: SAMR1组; B: SAMP8组; aP<0.05) Fig. 3 RT-PCR assay showing the relative expression of AQP4 mRNA. The amplification curve (a) and dissolve curve (b) of Aqp4 by RT-PCR; The amplification curve (c) and dissolution curve (d) of GAPDH; Statistical chart (e) [SAMR1 group (A) and SAMP8 group (B); aP<0.05]
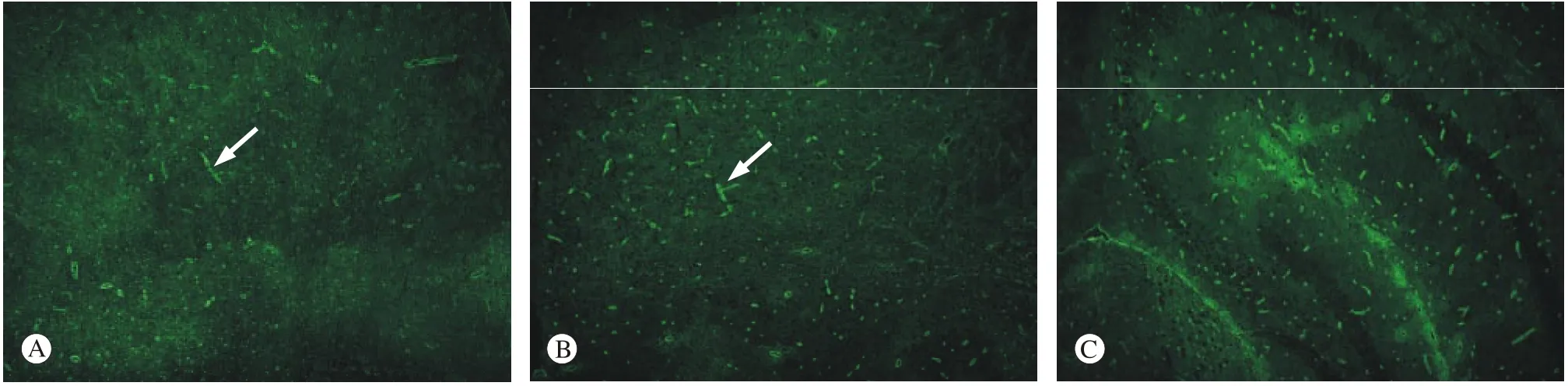
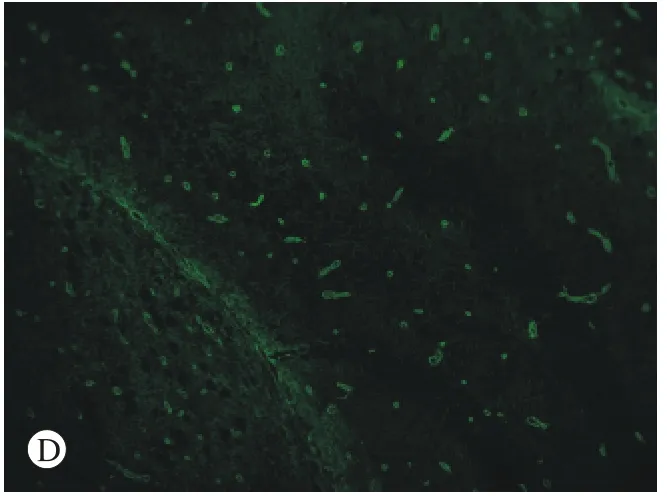
图 4 免疫荧光检测AQP4蛋白的表达 A:对照组SAMR1小鼠内囊(×10); B:SAMP8小鼠内囊(×10); C:SAMP8组鼠海马(×10); D:C图的部分放大(×20)Fig. 4 Immunofluorescence assay showing expression level of AQP4 in the internal capsule of SAMR1 group (A), the internal capsule of SAMP8 group (B), the hippocampus of SAMP8 group (C) and the amplification results of figure C (D) (×20)
讨 论
基因芯片技术又称为DNA微阵列,其高效率、高精度并且能同时分析大量的DNA片段等优势在生命科学领域的研究中得到了广泛的应用,其原理就是将大量探针固定于支持物上并与标记后的样品分子杂交,通过杂交信号强度获得样品分子的序列信息。阿尔茨海默病是一种复杂的神经退行性疾病,我们利用阿尔茨海默病替代研究模型SAMP8[6],应用基因芯片技术寻找阿尔茨海默病小鼠海马中差异表达的基因并挑选差异倍数较高的Aqp4基因进行荧光定量PCR检测。由结果我们看出,PCR检测结果与基因芯片检测结果具有相同的方向性,所以我们认为筛选的结果是可信的。
Aqp4基因编码水通道蛋白4,AQP4是水通道蛋白的一种并首先由Jung等学者在大鼠的肺部分离出来[7],广泛存在于哺乳动物的脑、脊髓、视网膜和骨骼肌等容易兴奋的组织中。AQP4在中枢神经系统中主要存在于星形胶质细胞,并在脑室脉络丛上皮细胞和室管膜细胞也有分布,其在脑中表现的功能较为复杂[8-10]。最新研究认为,AQP4与阿尔茨海默病、帕金森病的发生与进展密切相关[11-12]。据此,我们利用荧光免疫技术检测快速衰老痴呆模型小鼠脑切片,发现AQP4在内囊组织和海马组织的表达较对照组增多,AQP4的高表达在神经元变性疾病的过程中扮演重要角色。有学者认为,脑中的β-淀粉样蛋白和Tau缠结等“垃圾蛋白”的清除依赖类淋巴途径,即类似于身体的淋巴循环系统,脑脊液和组织间液中流动的类淋巴液推动神经元代谢所产生的废物进入静脉旁间隙,使类淋巴液直接汇入淋巴管进入体循环,最终在肾和肝内清除[13-14]。AQP4蛋白则存在于由星形胶质细胞围绕构成的脑血管外壁上,促进CSF和ISF的流动,在类淋巴途径的循环中起到重要的作用[4,15]。人为地减少实验动物脑中星形胶质细胞上的AQP4表达能够使类淋巴循环速率延缓70%左右,同时,荧光示踪显示Aβ的清除率也随之降低[16-17]。
国外研究表明,AQP4蛋白通过增加水的流量来增大细胞的体积和容积,进而增加胶质细胞的迁移速率[18-19]。研究发现,与抗快速老化模型SAMR1小鼠组相比,SAMP8痴呆小鼠组中Aqp4基因的表达量增高。同时,免疫荧光的结果与基因筛选和RT-PCR结果具有一致性。因此,我们推测快速老化模型小鼠AQP4的高表达为代偿性表达增高,试图通过改变血管外壁星形胶质细胞体积和形态的同时增加细胞对水的通透作用来增加类淋巴循环,进而清除脑内Aβ蛋白和Tau缠结等“垃圾蛋白”,但是,其表达是否在病程的不同发展阶段发生动态变化及其相关的具体机制还需要进一步的探究。
1 Li J, Jin Y, Shi Y, et al. Voxelwise spectral diffusional connectivity and its applications to Alzheimer’s disease and intelligence prediction[J]. Med Image Comput Comput Assist Interv, 2013, 16(Pt 1): 655-662.
2 Brundel M, De Bresser J, Van Dillen JJ, et al. Cerebral microinfarcts: a systematic review of neuropathological studies[J]. J Cereb Blood Flow Metab, 2012, 32(3):425-436.
3 Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus[J]. Cerebrospinal Fluid Res, 2010, 7:15.
4 Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β[J]. Sci Transl Med,2012, 4(147):147ra111.
5 Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport[J]. Neuroscience, 2004, 129(4): 999-1010.
6 Fang B, Jia L, Jia J. Chinese Presenilin-1 V97L mutation enhanced Abeta42 levels in SH-SY5Y neuroblastoma cells[J]. Neurosci Lett, 2006, 406(1-2):33-37.
7 Mathew LG, Campbell EM, Yool AJ, et al. Identification and characterization of functional aquaporin water channel protein from alimentary tract of whitefly, Bemisia tabaci[J]. Insect Biochem Mol Biol, 2011, 41(3):178-190.
8 Benfenati V, Ferroni S. Water transport between CNS compartments:functional and molecular interactions between aquaporins and ion channels[J]. Neuroscience, 2010, 168(4):926-940.
9 Jin BJ, Zhang H, Binder DK, et al. Aquaporin-4-dependent K(+)and water transport modeled in brain extracellular space following neuroexcitation[J]. J Gen Physiol, 2013, 141(1):119-132.
10 Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, et al. Glialconditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet[J]. Proc Natl Acad Sci U S A, 2011, 108(43):17815-17820.
11 Fang B, Wang D, Huang M, et al. Hypothesis on the relationship between the change in intracellular pH and incidence of sporadic Alzheimer’s disease or vascular dementia[J]. Int J Neurosci,2010, 120(9):591-595.
12 Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias[J]. Brain, 2012, 135(Pt 12):3749-3756.
13 Eberhardt C, Amann B, Feuchtinger A, et al. Differential expression of inwardly rectifying K+ channels and aquaporins 4 and 5 in autoimmune uveitis indicates misbalance in Müller glial celldependent ion and water homeostasis[J]. Glia, 2011, 59(5):697-707.
14 Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain[J]. J Neurosci, 2013, 33(46): 18190-18199.
15 Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain[J]. Science, 2013, 342(6156):373-377.
16 Nedergaard M. Garbage truck of the brain[J]. Science, 2013, 340(6140): 1529-1530.
17 Yang L, Kress BT, Weber HJ, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer[J]. J Transl Med, 2013, 11:107.
18 Nagae T, Miyake S, Kosaki S, et al. Identification and characterisation of a functional aquaporin water channel (Anomala cuprea DRIP) in a coleopteran insect[J]. J Exp Biol, 2013, 216(Pt 14):2564-2572.
19 Auguste KI, Jin S, Uchida K, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury[J]. FASEB J, 2007, 21(1):108-116.
Correlation between gene expression of aquaporin 4 and Alzheimer's disease in animal experiment
ZHOU Mingyue1, WANG Keyi2, ZHANG Yanming1, ZHAO Yun1, WEN Hongfeng3, MENG Fanlei4, FANG Boyan3
1The Graduate Student Training Base-Aerospace Center Hospital of Liaoning Medical College, Beijing 100049, China;2Beijing Aerospace Center Hospital, Beijing 100049, China;3Department of Neurology, Beijing Aerospace Center Hospital, Beijing 100049, China;4Liaoning Medical University, Jinzhou 121000, Liaoning Province, China
FANG Boyan. Email: fangboyanv@163.com
ObjectiveTo explore the correlation between aquaporin 4 (AQP4) and Alzheimer's disease (AD).MethodsAgilent expression profile chip was used to screen the gene expression differences through the comparison between SAMP8 and SAMR1 after total RNA of brain tissue being extracted out, then Real-Time PCR technology and indirect immunofluorescence assay were applied for identification.ResultsTotally 804 genes related to dementia were selected, among which the number of up-regulated genes was 521 and down-regulated genes was 283. The biological function and pathways of the related genes were searched in GeneBank, then six genes were found to be closely related to AD: Herc6, Lyst, Nkain1, Aqp4, Uch13, Slc9a8. The fold change of Aqp4 was 6.26 with P<0.001. The Real-time PCR of the Aqp4 gene showed that the expression quantity in SAMP8 group was higher than that in SAMR1 group (P= 0.037 6), and the immunofluorescence assay showed that the expression of Aqp4 in SAMP8 also increased.ConclusionIncreased Aqp4 expression is related to the increased need for Aβ clearance in SAMP8 rats which has astrocytes compensatory proliferation.
Alzheimer's disease; gene microarray; aquaporin 4
R 749.16
A
2095-5227(2015)05-0482-05
10.3969/j.issn.2095-5227.2015.05.020
时间:2015-03-03 09:58
http://www.cnki.net/kcms/detail/11.3275.R.20150303.0958.002.html
2014-12-04
国家自然科学基金项目(81100962)
Supported by the National Natural Science Foundation of China(81100962)
周明月,女,在读硕士。研究方向:神经变性病的基础与临床。Email: 1135957366@qq.com
方伯言,博士,硕士生导师。Email: fangboyanv@163.com
