Hydrochemistry of the Natural Low pH Groundwater in the Coastal Aquifers near Beihai, China
2015-03-31ZHOUXunSHENYeZHANGHuaSONGChaoLIJingweiandLIUYan
ZHOU Xun, SHEN Ye, ZHANG Hua, SONG Chao, LI Jingwei, and LIU Yan
Hydrochemistry of the Natural Low pH Groundwater in the Coastal Aquifers near Beihai, China
ZHOU Xun1), 2), *, SHEN Ye1), 2), ZHANG Hua1), SONG Chao1), LI Jingwei1), and LIU Yan1)
1),(),100083,..2)(()),,100083,..
Natural weak acidic groundwater occurs in the unconfined and confined aquifers consisting of Quaternary and Neogene unconsolidated sediments near Beihai in southern Guangxi, China. Under natural conditions the groundwater has low TDS (less than 200mgL−1) and low concentrations of trace elements (less than 100µgL−1) with a deceasing tend in contents of the Lanthanides (rare earth elements, less than 1µgL−1) towards higher atomic number. The groundwater ranges in pH from 3.33 to 7.0 with an average value of 5.12 (even lower than that of local rainwater, 5.88). pH values in the groundwater are a bit higher in rainy seasons than those in dry seasons and do not show significant increasing or decreasing trend with time. The average pH value in groundwater in the confined aquifers is even a bit lower than that in the unconfined aquifer. Comprehensive analyses of the groundwater environment suggest that H+in the groundwater may be derived from dissociation of H2CO3, release of the absorbed H3O+in clay layers and the acidity of rainwater. The H2CO3in the groundwater may be formed by dissolution of CO2 (g). Minerals in the unconsolidated sediment are predominated by quartz with small amount of clay minerals. The sediments undergoing a long-term weathering contain low levels of soluble constitutes. Lack of alkaline substances in the groundwater system is also helpful in the accumulation of acidity of the groundwater.
acidic groundwater; carbonic equilibrium; coastal aquifer; unconsolidated sediments; trace elements
1 Introduction
Acidic or weak acidic groundwater is found to occur in acidic mining discharge and natural low pH groundwater systems. Acidic discharge occurs in mining areas of metal ore or coal since metal sulphides oxidation may occur in metal ore-bearing or coal-bearing formations in the course of mining (Herbert, 1996; Keith., 2001; Santofimia and López-Pamo, 2013). Natural low pH groundwater exists as a result of a long-term evolution of the natural environment. Natural weak acidic groundwater is found to occur in unconsolidated sediments in the coastal plain near Beihai in southern Guangxi, China. Although hydrogeological studies were carried out for this area (Chen., 1990; Zhou., 1997, 2000, 2006), little is known about the occurrence of the weak acidic groundwater in the coastal unconsolidated sediments. Studies of acidic groundwater by some researchers focused on acidic mining drainage (Stollenwerk, 1994; Herbert, 1996; Keith., 2001; Santofimia and López-Pamo, 2013). Acidification of surface water by acidic rain and effects of acidic rain on the environment were also reported by Lång and Swedberg (1990). Effects of acidic or weak acidic groundwater on surface water, soil and soluble rocks and on transport and transfer of metal elements were examined by some researchers (Herbert, 1996; Choi., 1998; Qian, 2002). Preda and Cox (2000, 2001) suggested that the oxidation and hydrolysis of pyrite are responsible for the low pH water of the Pimpama River in the southeast Queensland, Australia. Many hydrogeochemical studies indicate that a variety of physical and chemical processes may occur and lead to changes in chemical compositions of groundwater and their concentrations in groundwater flow from recharge areas receiving recharge from precipitation to discharge areas in a groundwater system (Bartarya, 1993; Wicks and Herman, 1996; Giménez, 1997; Rosen and Jones, 1998; Petalas and Diamantis, 1999; Lawrence., 2000; Ryzhenko and Cherkasova, 2012). Hydrochemistry of costal aquifers were analyzed by Giménez and Morell (1997), Agoubi. (2013) and Baram. (2014). Low pH groundwater in coastal aquifers in Morocco was examined by Re. (2103). pH in groundwater is controlled by many factors or processes that exist or occur in the unsaturated zone and saturated zone in an aquifer system (Cox, 2000; Isa., 2012). As a preliminary investigation, Zhou. (2007a) suggested the possible mechanism for the formation of the low pH groundwater in Beihai. Based on chemical analyses of groundwater and rain water samples and soil samples, hydrochemical characteristics of the groundwater in coastal aquifers near Beihai are summarized and the mechanism for the occurrence of the natural weak acidic groundwater is also discussed in this paper. Contents of 56 trace elements in the groundwater samples and rain water are examined. The objective of this paper is to provide some insight into the hydrochemical evolution of the coastal groundwater environment near Beihai.
2 Hydrogeology Setting and Sample Collection
The city of Beihai is located in the coastal plain in southern Guangxi, China and faces the Beibuwan Gulf of the South China Sea (Fig.1). The elevation of the flat coastal plain ranges mostly from 8 to 25m. Rivers are sparse and short in the study area. With a subtropical humid climate, the study area receives an annual precipitation varying between 849mm and 2382mm (average value 1677mm). More than 80 % of the total annual precipitation occurs during the summer wet season from May to October. The average annual evaporation in the area is 1756mm and the temperature ranges from 2℃ to 37℃ (average value 22.6℃).
The coastal plain near Beihai is underlain by Quaternary and Neogene unconsolidated sand with gravel and scattered lenses of clay or sandy clay with a total thickness ranging from 5 to 350m. One unconfined aquifer and three confined aquifers can be grouped in the unconsolidated sediments in the area. The unconfined aquifer (2-18m thick) consists mainly of sand with gravel in the lower part and clayey sand in the upper part of the Beihai Group of the Middle Pleistocene (Q2b). The upper confined aquifer, consisting of sand with gravel of the Zhanjiang Group of the Lower Pleistocene (Q1z), occurs at elevation from 2 to −10m with a thickness of 8 to 30m. The middle and lower confined aquifers, which are composed of sand with clayey sand, correspond to the Shangcun Group (N2sh) and Huangniuling Group (N1h) of Neogene age. Impervious basement rocks consisting of mudstone with sandstone and granite underlie the Quaternary and Neogene unconsolidated sediments, and outcrop in the southwestern tip and the northeastern part of the study area. As approaching to the north, the basement rocks uplift, leading to the occurrence of one unconfined aquifer and one confined aquifer. The upper Q1zclay is semi-permeable and acts as an aquitard. Hydraulic connections exist among the aquifers, especially among the confined aquifers, owing to the semiperviousness and termination of clay (Zhou., 2000). Under natural conditions the groundwater in the unconfined aquifer receives recharge from precipitation, and discharges directly into the rivers and the sea and also discharges to the confined aquifers through leakage. Groundwater in the confined aquifers receives recharge from the unconfined aquifer, flows towards northwest and south, and finally discharges into the sea (Zhou., 2000b).
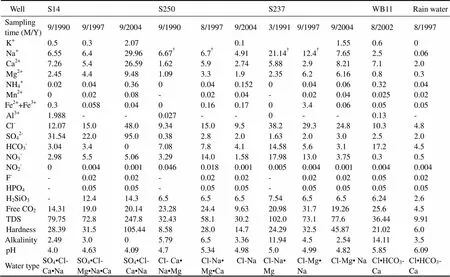
Table 1 Chemical compositions of representative water samples in the unconfined aquifer (in mgL−1, except pH)
Notes:†Na plus K; -not detected.
Monitoring of groundwater quality has been conducted in the Beihai coastal area by the local hydrogeological agency since 1989. Water samples are generally collected twice a year in the dry season (mostly in March) and in the rainy season (mostly in September), respectively. In August, 2002 soil samples (numbered from TB1 to TB6) were collected at depths of 1.5, 3.1, 4.1, 5.1, 6.0 and 8.5 m below the land surface near the northern coast, where both the Q2band Q1zsediments outcrop. Another two soil samples of Q2bsediments (TQ1 and TQ2) were collected at a depth of 1 m below the land surface in the northern part. Soil samples TQ1, TQ2 and TB1 represent the upper Q2bsediments, and TB2, TB3 and TB4 stand for the lower Q2bsediments. Soil samples TB5 and TB6 stand for the upper clay and the lower sand with gravel of the Q1zsediments, respectively. The soil samples were dried and ground for determination of minerals, chemical compositions and soluble ions. Location of the sampling sites is shown in Fig.1. Chemical analyses of water samples at representative wells and at different time points are listed in Table 1 and Table 2. Also shown are chemical compositions of rain water and sea water samples.
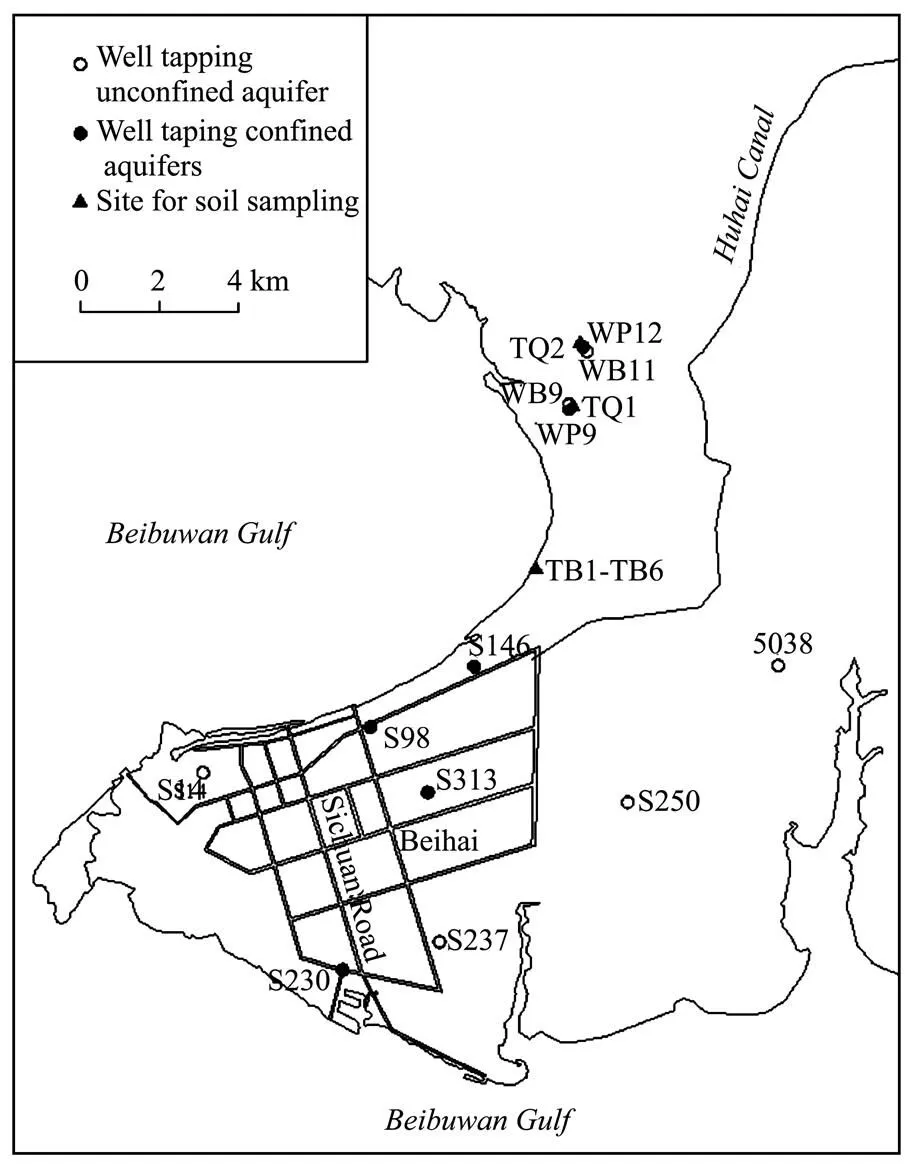
Fig.1 Location of the sampling sites.

Table 2 Chemical compositions of representative water samples in the confined aquifer (in mgL−1, except pH)
Notes:†Na plus K; -not detected.
3 Hydrochemical Characteristics and Changes in pH
The hydrochemical monitoring data show that TDS in groundwater in the coastal aquifers near Beihai is quite low (commonly ranging from 30 to 200mgL−1) and has a slightly increasing trend from inland area to the coast. Hardness varies mostly between 5 and 100mgL−1. Cl−, HCO3−, Na+, Ca2+and Mg2+are the major ions in the groundwater, accounting for 44.24%-79.98%, 7.61%-44.63%, 18.74%-70.41%, 10.84%-61.21%, 0.33%-40.47% equivalent of the anions or cations, respectively. In the representative groundwater samples (Table 1 and Table 2), Cl−contents range from 3.73 to 48.0 mgL−1. At well S14, HCO3−contents are low (0–3.4mgL−1) but SO42−concentrations are relatively high (22.0–95.0mgL−1). NO3−Contents range from 0.3 to 22.0mgL−1. Fe2++Fe3+concentrations vary between 0 to 3.4mgL−1and Mn2+contents, between 0 and 0.04mgL−1. F−levels are lower than 0.05mgL−1. Concentrations of the major ions in S14 sample collected in October, 2004 are occasionally high, leading to the TDS having 3 times the values of the previous samples. Contents of H2SiO3are in the range of 5.8-14.3 mgL−1and free CO2, 6.3-33.5mgL−1. Groundwater in the area is of a variety of water types (Fig.2, Table 1 and Table 2) since spatial and temporal changes in concentrations of the major ions exist even though TDS of the groundwater is low. The groundwater in the confined aquifers is dominated by Cl-Na·Ca (Mg), Cl·HCO3-Na (Ca)and Cl-Na types, whereas Cl-Na, Cl-Na·Mg (Mg·Na) and SO4·Cl-Ca·Natypes prevail in the unconfined aquifer.
The monitoring data also show that the pH value in groundwater in the study area ranges from 3.3 to 7.0 with the average value of 5.12 (lower than that of the local rainwater, 5.88), the minimum value of 3.33 and occasional values close to 7.0. Groundwater in the unconfined aquifers has pH value ranging from 3.68 to 7.0 with an average of 5.17, whereas groundwater in the confined aquifers has pH value in the range of 3.33 to 6.97 with an average value of 5.07, which is a little bit lower than that in the unconfined aquifer (Fig.3). The pH values of the groundwater in both the unconfined and confined aquifers do not show significant increasing and decreasing trends with time and fluctuate slightly by the value less than 2 (Fig.4). Change in pH of groundwater after 1999 is slight- ly larger than that before 1999. On a regional basis of monitoring data, pH value of the groundwater in the Beihai coastal area is slightly higher in the rainy season than that in the dry season. The average pH value of the groundwater in the unconfined aquifer is 5.20 in rainy season and 5.14 in the dry season, whereas the average pH in groundwater in the confined aquifer is 5.09 in the rainy season and 5.05 in the dry season. The representative groundwater samples in Table 1 and Table 2 have pH values in the range 4.0-5.85.
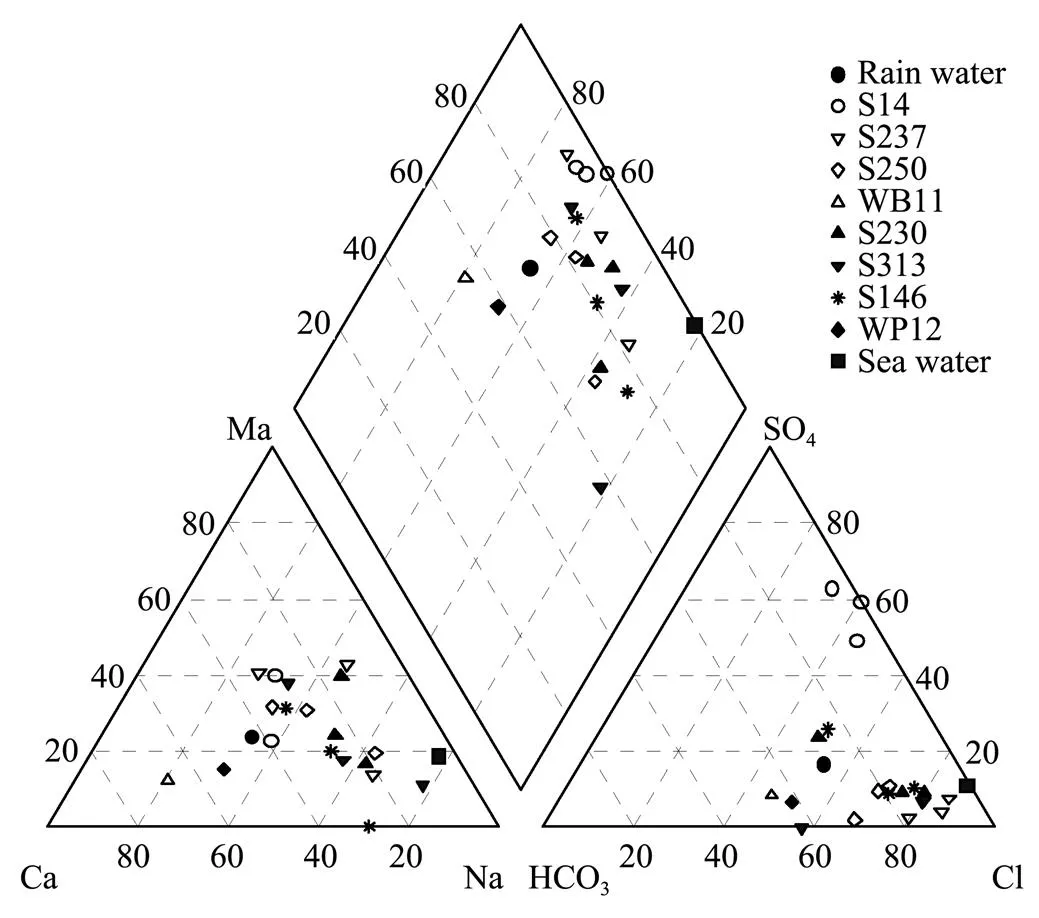
Fig.2 Piper diagram of water samples.

Fig.3 Diagrams showing frequency of pH values of groundwater in (a) the unconfined aquifer and (b) the confined aquifers.
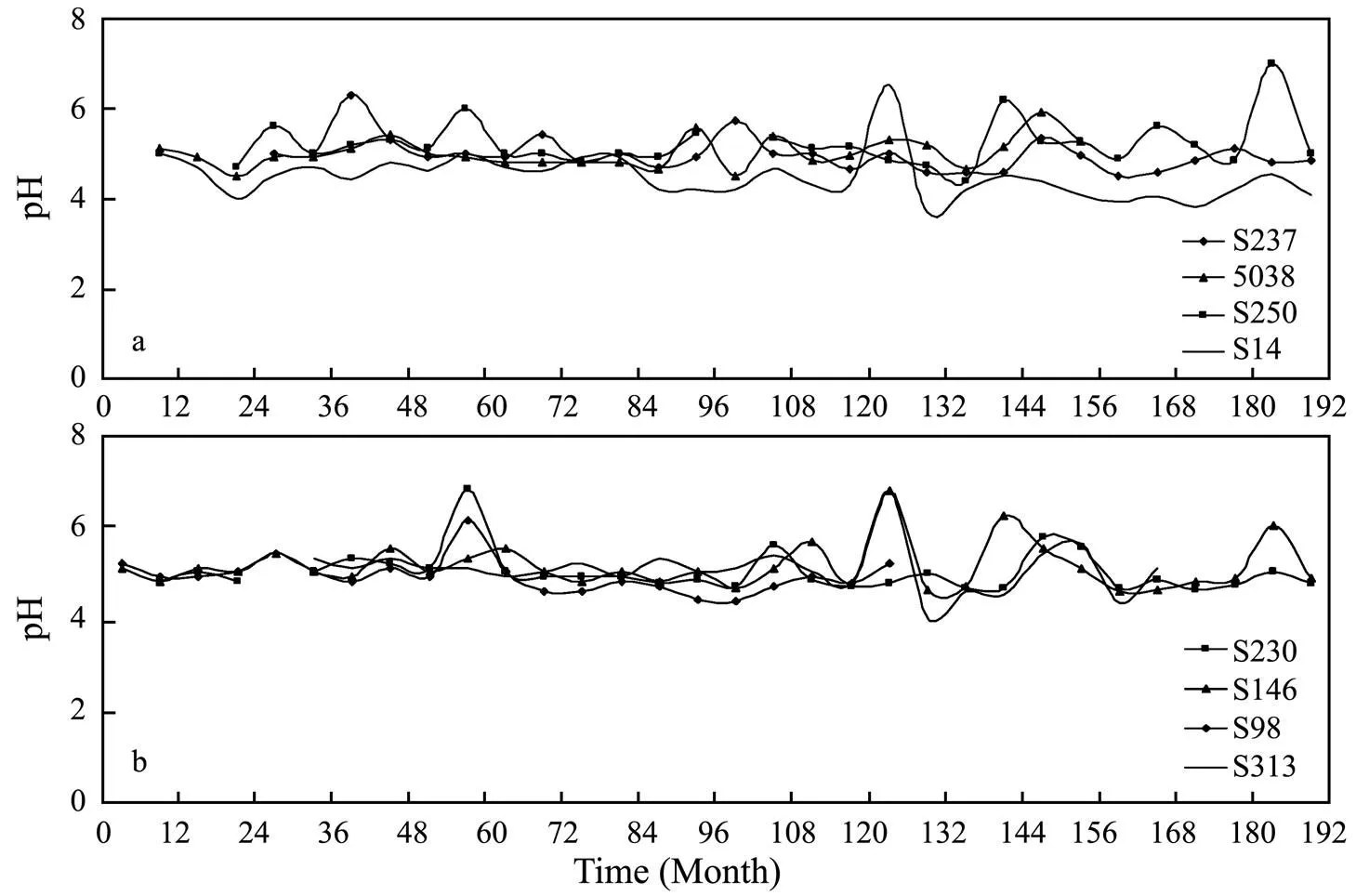
Fig.4 Changes in pH values of groundwater in wells tapping (a) the unconfined aquifer and (b) the confined aquifers from January, 1989 to December, 2004.
Groundwater in the coastal unconsolidated sediments near Beihai is characterized by low TDS, low pH and chloride-dominant water type (Table 1 and Table 2). Hydrochemically, groundwater in the coastal aquifers is thought to be related to rainwater but bears no relation to the sea water under natural conditions (Zhou., 2007b).
4 Discussion of the Occurrence of the Weak Acidic Groundwater
Some reactions occur in the natural groundwater environment which may lead to an increase in H+concentration, resulting in a decrease in pH value and making the groundwater weak acidic or acidic. Factors causing low pH groundwater and affecting the formation of acidic groundwater are summarized as follows. (1) Recharge of infiltration from acidic rainwater may lead to low pH groundwater in some countries, such as those in northern Europe (Lång and Swedberg, 1990). (2) CO2(g)dissolves in groundwater, resulting in the formation of H2CO3(Isa., 2012). Dissociation of H2CO3leads to an increase in concentrations of HCO3-and H+and hence a decrease in pH in groundwater. (3) Pyrite in sediments is oxidized, leading to an increase in H+concentration in groundwater (Preda and Cox, 2000). (4) Silicates decompose in an environment containing organic acid and biogenic CO2in humid areas, leading to acidic groundwater in an unconfined aquifer (Li, 1982). (5) Clay minerals may act as H+buffers and have an influence on pH in groundwater (Sjöström, 1993). (6) Acidic groundwater may be caused by other factors, such as infiltration of precipitation into sediments containing sulfide-bearing volcanic ash.
In general, acids which may occur in groundwater include carbonic acid, boracic acid, silicic acid and other weak acids. Boracic acid, silicic acid and other weak acids play a minor part in the acidity of groundwater owing to their low concentrations in groundwater. Carbonic acid is important in the carbonic equilibrium in groundwater since it has high concentration and occurs extensively in groundwater. Sulfuric acid and hydrochloric acid are strong acids and if they exist in groundwater, pH of the groundwater will decrease. On the contrary, strong alkali may increase the pH value of groundwater.
Acidity in the groundwater in the coastal aquifers near Beihai is possibly associated with the carbonic equilibrium, and changes in the pH values are possibly related to variation in CO2and HCO3−concentrations. CO2(g)keeps on dissolving in the groundwater, leading to the formation of H2CO3. Continual dissociation of H2CO3, HCO3−and H2O results in the production of H+, making the groundwater weak acidic (Zhou., 2007a).
HCO3−in groundwater in the unconfined aquifer partly comes from rainwater and partly derives from dissociation of H2CO3in the carbonic equilibrium. HCO3−in groundwater in the confined aquifers partly comes from groundwater in the unconfined aquifer and partly from dissolution of albite. Kaolinite occurs in the process, which is the major mineral in the soil samples of the confined aquifers (Zhou., 2007b).
The acidity in the groundwater may also come from the acidity of the rainwater. Chemical analyses of seven rainwater samples collected from August, 1997 to August, 2003 show that rainwater had TDS ranging from 8.94 to 21.2mgL−1, pH from 5.29 to 6.48 with an average value of 5.88. The water type of the rainwater is of Cl·HCO3-Ca type and the groundwater in the unconfined and confined aquifers is of a variety of water types but is predominated by the chloride type. Strong infiltration of precipitation in the area occurs due to abundant rainwater and a thin and permeable unsaturated zone.
Minerals in the soil samples are mainly quartz and clay minerals (Zhou., 2007b). Quartz accounts for 50 % of the mineral compositions of the upper clay of the Q1zsediments and more than 70% of the other Q2band Q1zsediments. Clay minerals include kaolinite and chlorite and small amount of illite. The upper Q2bsediments (samples TB1, TQ1, TQ2) contain chlorite with kaolinite (25%-30%) but do not contain illite. Samples TB2 and TB3 contain illite (5%-15%) and chlorite with kaolinite (15%-25%). Samples TB4, TB5, TB6 contain illite (10%-20%) and kaolinite (5%-30%) but do not contain chlorite. The minerals of soil samples are believed to be typical products of long-term weathering. Pyrite was not detected in the soil samples and oxidation of pyrite does not appear to occur.
The chemical compositions (expressed as oxides) of soil samples show that SiO2is the main oxides of the soil, ranging from 73.55 % (TB5) to 95.25 % (TB6). Al2O3varies between 3.94 % (TB2) and 16.59 % (TB5) and Fe2O3is in the range of 0.24 % (TB6)-6.13 % (TB1). In addition, H2O+ranges from 0.71 % (TB6) to 4.52 % (TB3), and H2O−, from 0.0961 % (TB6) to 1.71% (TQ2). Other oxides in soil samples are rare or absent. Insoluble compositions account for most of the chemical compositions of the Q2band Q1zsoils, indicating that the unconsolidated sediments have undergone dissolution in the local water circulation under natural conditions for a long time and extensive leaching of soluble compositions from soils has taken place.
The soil samples have low levels of soluble ions; almost all of the ions have concentrations less than 300µgg−1. SO42−predominates in the anions, ranging from 44.9 µgg−1(TB6) to 306.2µgg−1(TB3). HCO3−varies between 37.0µgg−1(TB1) and 63.1µgg−1(TB6) and Cl−, between 10.1 and 12.2µgg−1. K+, Na+, Ca2+and Mg2+have similar concentrations of several tens of µgg−1, while Mn2+, Fe2+and Fe3+also have concentrations of several tens of µgg−1close to those of the above four cations. Na+is the highest at TB2 (130.5 µgg−1), Fe2+and Fe3+are high at TB3 (76.3 and 74.4 µgg−1, respectively), and SO42−increases from TB1 (204.6µgg−1) to TB3 (306.2µgg−1) and decreases from TB3 to TB6 (44.9µgg−1) in the soil profile. Low levels of soluble ions in the soil samples also indicate extensive leaching of soluble compositions from soils. Thus, alkaline substances in the groundwater system are lacking.
Groundwater in the confined aquifers has hydrochemical characteristics similar to that in the unconfined aquifer, but pH in the former is a bit lower than that in the latter. The semipervious layer of the upper Q1zclay contains H3O+. The absorbed H3O+is possibly released when groundwater flows through this layer from the unconfined aquifer to the confined aquifers, leading to a slight increase in H+concentrations in groundwater in the confined aquifers.
5 Trace Constituents of the Water Sam- ples
Two groundwater samples were taken from wells WB9 (pH 5.5 and Temperature 26.7℃) and WB11 (pH 5.3 and temperature 26.7℃) tapping the unconfined aquifer and another two groundwater samples were taken from wells WP9 (pH 5.2 and temperature 27℃) and WP12 (pH 4.6 and temperature 26.8℃) tapping the confined aquifer in August, 2002. One rainwater sample (pH 5.9 and temperature 25.1℃) was collected in the same period. Well WB9 is close to well WP9 and so is well WB11 to well WP12. The water samples were analyzed for as many as 66 major, minor and trace constituents (Table 3). Concentrations of the major, minor elements are presented in Fig.5 and those of the trace elements are shown in Fig.6, following the order in the Periodic Table of the Elements. In general, concentrations of all the elements in the water samples are relatively low and are coincident with those listed in Tables 1 and 2.
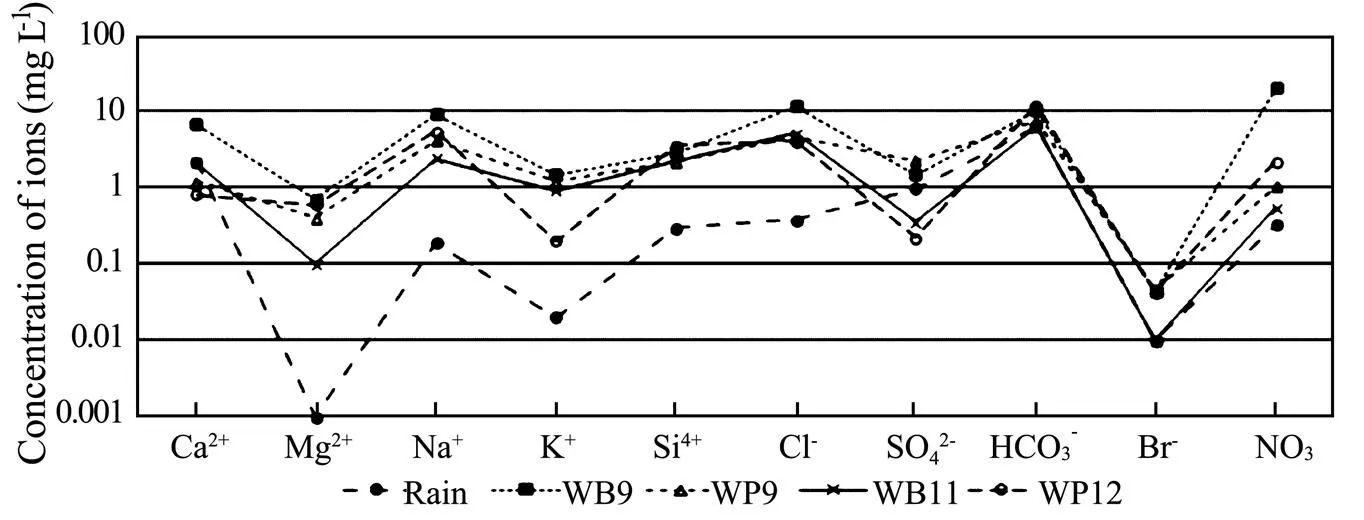
Fig.5 Concentrations of the major and minor constitutes in the groundwater samples and rain water.

Fig.6 Concentrations of the trace constitutes in the groundwater samples and rain water.
Concentrations of the major and minor ions Ca2+(0.8-6.8mgL−1), Mg2+(0.1-0.7mgL−1), Na+(2.4-9.0 mgL−1), K+(0.2-1.5mgL−1), Cl−(4.0-11.5mgL−1), SO42−(0.22-2.28mgL−1), HCO3−(6.1-12.0mgL−1), Si4+(2.18-3.53mgL−1), Br−(<0.02-0.046mgL−1) and NO3−(0.54-20mgL−1) of the four groundwater samples are less than 20mgL−1and are quite close to each other (Table 3), reflecting a good hydraulic connection between the unconfined aquifer and the confined aquifer near the well sites. The rain water has the similar major and minor elements to the groundwater (except SO42−). Concentrations of Mg2+(0mgL−1), Na+(0.19mgL−1), K+(0.02 mgL−1), Cl−(0.38mgL−1) and Si4+(0.3mgL−1) of the rain water are obviously lower than those of the groundwater samples while concentrations of Ca2+(2.2mgL−1), SO42−(0.98mgL−1), HCO3−(6.3mgL−1), Br−(<0.02mgL−1) and NO3−(0.33mgL−1) of the rain water are close to those of the groundwater samples. Since the rain water is the main source of recharge of groundwater in both the unconfined and confined aquifers, the concentrations of Mg2+, Na+, K+, Cl−and Si4+of the groundwater increase in the course of infiltration of recharge from rain water.
Concentrations of the 56 trace elements of the groundwater samples are less than 100μgL−1and those of the rain water are even lower. Concentrations of Mn, Ni, Zn, As, Rb, Sr, I, Cs and Ba among the trace elements in the groundwater samples are relatively high and range from 0.1 to 10μgL−1. The groundwater and rain water samples do not contain Ge, Nb, Ru, Rb, Pd, Ag, In, Ho, Tm, Lu, Hf, Re, Os, Pt, Au, Hg and Th, and have very low concentrations (less than 0.01μgL−1) of V, Cr, Co, Cu, Ga, Se, Y, Mo, Cd, Sn, Te, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Yb, Ta, W, Ir, Tl, Pb, Bi and U. Concentrations of elements from V to Y following the order in the Periodic Table (Fig.6a) are relatively high and concentrations of elements from Nb to Ba (Fig.6b) have an increasing trend. Concentrations of elements from La to U are less than 1 μgL−1. Concentrations of the Lanthanides (rare earth elements) have a decreasing trend (Fig.6c),.., a decreasing slope towards higher atomic number (Edet, 2004). Concentrations of the elements from Hf to U show a rough fluctuation trend (Fig.6d).
6 Summary
Groundwater occurs in the unconfined and confined aquifers consisting of sand and gravel in the coastal area near Beihai, Guangxi, China. It receives recharge from precipitation and discharges into the sea under natural conditions. The groundwater has low TDS of commonly less than 200mgL−1. The concentrations of the major and minor elements are less than 20mgL−1and the concentrations of the trace elements are below 100μgL−1. Although TDS of the groundwater in the Beihai coastal area is quite low, various water types is found in the groundwater due to the spatial and temporal changes in concentrations of the major ions. The pH of the groundwater ranges from 3.3 to 7.0 with an average value of 5.12. H+in the weak acidic groundwater is possibly derived mainly from dissociation of H2CO3and the acidity of rainwater. CO2 (g)dissolves in the groundwater and H2CO3forms in the water. Dissociation of H2CO3leads to an increase in concentrations of H+and a decrease in pH value of the groundwater. Small amount of soluble compositions and lack of alkaline substances in the groundwater system duo to a long term weathering are also helpful in the accumulation of acidity, resulting in a decrease in pH value of the groundwater. The pH value of groundwater in the confined aquifers is a bit lower than that in the unconfined aquifer. The possible reason for this is that H3O+in the upper Q1zclay is released when groundwater flows through this layer, leading to an increase in H+concentrations. Of the 56 trace elements, 39 trace elements are detected in the water samples. The Lanthanides (rare earth elements) have concentrations of less than 1μgL−1and show a decreasing trend towards higher atomic number.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41172227, 40172087) and the Project of Development Field of High Priority of the Specialized Research Fund for the Doctoral Program of Higher Education of China (20110022130002). The authors would like to thank Dr. Warren Wood from the USGS for analyses of trace metals of the water samples. Thanks are given to Mr. Ou Yecheng from the Behai Institute of Hydrogeology, Engineering Geology and Mineral Resources of Guangxi for his help in the fieldwork.
Agoubi, B., Kharroubi, A., and Abida, H., 2013. Hydrochemistry of groundwater and its assessment for irrigation purpose in coastal Jeffara Aquifer, southeastern Tunisia., 6: 1163-1172.
Bartarya, S. K., 1993. Hydrochemistry and rock weathering in a sub-tropical Lesser Himalayan river basin in Kumaun, India., 146: 149-174.
Baram, S., Kurtzman, D., Ronen, Z., Peeters, A., andDahan, O., 2014. Assessing the impact of dairy waste lagoons on groundwater quality using a spatial analysis of vadose zone and groundwater information in coastal phreatic aquifer., 132: 135-144.
Chen, C., Lin, M., Shu, B., Jiang, J., Ye, S., and Wu, N., 1990. The equivalent drainage boundary and its determination of the confined aquifer in a coastal region: For the Hetang area of Beihai city., (4): 2-4 (in Chinese with English abstract).
4.积极争取,落实省级土地确权经费。在咨询部农垦局、部分兄弟垦区、相关测绘公司的基础上,经反复与省财政厅协商,省农业厅在2017年向省政府提交了《关于申请农垦国有土地使用权确权登记发证工作所需经费的请示》,并在2018年年初落实了土地确权登记发证工作省级财政补助经费318.25万元,其补助标准是按照山西省农村土地承包经营权确权登记领证工作每亩15元标准的基础上,考虑到需确权发证的19个农场多为贫困农场且处于贫困县的实际,又增加了工作经费19万元。为确保资金安全、快速使用到土地确权发证工作上,省农业厅与国土厅多次沟通论证,于2018年5月底将资金从省农业厅全部拨付到各市农委(畜牧局)。
Choi, J., Hulseapple, S. M., Conklin, M. H., and Harvey, J. W., 1998. Modeling CO2degassing and pH in a stream-aquifer system., 209: 297-310.
Edet, A. E., 2004. A preliminary assessment of the concentrations of rare earth elements in an acidic fresh groundwater (south-eastern Nigeria)., 113: B100- B109.
Giménez, E., and Morell, L., 1997. Hydrogeochemical analysis of salinization processes in the coastal aquifer of Oropesa (Castellón, Spain)., 29: 118-131.
Herbert, R. B., 1996. Metal retention by iron oxide precipitation from acidic ground water in Dalarna, Sweden., 11: 229-235.
Isa, N. M., Aris, A. Z., and Sulaiman, W., 2012. Extent and severity of groundwater contamination based on hydrochemistry mechanism of sandy tropical coastal aquifer., 438: 414-425.
Keith, D. C., Runnells, D. D., Esposito, K. J., Chermak, J. A., Levy, D. B., Hannula, S. R., Watts, M., and Hall, L., 2001. Geochemical models of the impact of acidic groundwater and evaporative sulfate salts on Boulder Creek at Iron Mountain, California., 16: 947-961
Lång, L. O., and Swedberg, S., 1990. Occurrence of acidic groundwater in Precambrian crystalline bedrock aquifers, southwestern Sweden.,,, 49: 315-328
Li, X., 1982. SiO2in groundwater., (1): 86-92 (in Chinese with English abstract).
Petalas, C. P., and Diamantis, I. B., 1999. Origin and distribution of saline groundwaters in the upper Miocene aquifer system, coastal Rhodope area, northeastern Greece., 7: 305-316.
Preda, M., and Cox, M. E., 2000, Sediment-water interaction acidity and other water quality parameters in a subtropical setting, Pimpama River, southeast Queensland., 39: 319-329.
Preda, M., and Cox, M. E., 2001, Trace metal in acid sediments and waters, Pimpama catchment, southeast Queensland, Australia., 40: 755-768.
Qian, H., 2002.. Xi’an Cartography Publishing House, Xi’an, 24-41 (in Chinese).
Re, V., Sacchi, E., Martin-Bordes, J. L., Aureli, A., Hamouti, N., El, Bouchnan, R., and Zuppi, G. M., 2013. Processes affecting groundwater quality in arid zones: The case of the Bou-Areg coastal aquifer (North Morocco)., 34: 181-198.
Rosen, M., and Jones, S., 1998. Controls on the chemical composition of groundwater from alluvial aquifers in the Wanaka and Wakatipu basins, Central Otago, New Zealand., 6: 264-281.
Ryzhenko, B. M., and Cherkasova, E. V., 2012. Chemical composition of natural waters and brines as a result of hydrogeochemical processes in water-rock-gas systems., 50 (13): 1101-1150.
Santofimia, E., and López-Pamo, E., 2013. The role of surface water and mine groundwater in the chemical stratification of an acidic pit lake (Iberian Pyrite Belt, Spain)., 490: 21-31.
Sjöström, J., 1993. Ionic composition and mineral equilibriums of acidic groundwater on the west coast of Sweden., 21: 219-226.
Stollenwerk, K. G., 1994. Geochemical interactions between constituents in acidic groundwater and alluvium in an aquifer near Globe, Arizona., 9: 353-369.
Wicks, C. M., and Herman, J. S., 1996. Regional hydrogeochemistry of a modern coastal mixing zone., 32: 401-407.
Zhou, X., Chen, M., Ju, X., Wang, J., and Ning, X., 1997. The causes and preliminary ideas of the control countermeasures of sea water intrusion in Beihai City, Guangxi., 8: 77-83 (in Chinese with English abstract).
Zhou, X., Chen, M., Ju, X., Ning, X., and Wang, J., 2000. Numerical simulation of seawater intrusion near Beihai, China., 40: 223-233.
Zhou, X., Ruan, C., Yang, Y., Fang, B., and Ou, Y., 2006. Tidal effects of groundwater levels in the coastal aquifers near Beihai, China., 51: 517-525.
Zhou, X., Zhang, H., Zhao, L., Shen, Y., Yan, X., Ou, Y., and Huang, X., 2007a. A preliminary analysis of the formation of weak acidic groundwater in Beihai, Guangxi., 81 (6): 850-856 (in Chinese with English abstract).
Zhou, X., Zhang, H., Zhao, L., Shen, Y., Yan, X., Li, R., and Zhang, L., 2007b. Some factors affecting TDS and pH values in groundwater of the Beihai coastal area in southern Guangxi, China., 53: 317-323.
(Edited by Ji Dechun)
10.1007/s11802-015-2631-z
(March 26, 2014; revised July 1, 2014; accepted July 23, 2014)
. Tel: 0086-10-82323428E-mail:zhouxun@cugb.edu.cn
ISSN 1672-5182, 2015 14 (3): 475-483
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
猜你喜欢
杂志排行
Journal of Ocean University of China的其它文章
- Monte Carlo Simulation of in situ Gamma-Spectra Recorded by NaI (Tl) Detector in the Marine Environment
- Effects of Waterborne Cu and Cd on Anti-oxidative Response,Lipid Peroxidation and Heavy Metals Accumulation in Abalone Haliotis discus hannai Ino
- A General Overview of the Typical18Frontal-Ventral-Transverse Cirri Oxytrichidae s. l. Genera (Ciliophora, Hypotrichia)
- Application of a Delay-Difference Model for the Stock Assessment of Southern Atlantic Albacore (Thunnus alalunga)
- Invasion and Morphological Variation of the Non- Indigenous Barnacle Chthamalus challengeri (Hoek, 1883) in Yangshan Port and its Surrounding Areas
- The Burial of Biogenic Silica, Organic Carbon and Organic Nitrogen in the Sediments of the East China Sea
