miRNA153反义核酸上调肺癌细胞对辐射敏感性的体外实验
2015-03-21董国福马德宾马宏达韩雅玲蕾沈阳军区总医院呼吸与重症医学科辽宁沈阳006沈阳军区总医院药剂科辽宁沈阳006解放军总医院肿瘤内科北京0085军事医学科学院北京00850
高 蕊,冯 帆,贾 辉,张 帆,王 涛,董国福,马德宾,马宏达,韩雅玲,刘 蕾沈阳军区总医院 呼吸与重症医学科,辽宁沈阳 006;沈阳军区总医院 药剂科,辽宁沈阳 006;解放军总医院 肿瘤内科,北京 0085;军事医学科学院,北京 00850
GAO Rui1, FENG Fan2, JIA Hui2, ZHANG Fan3, WANG Tao4, DONG Guofu4, MA Debin1, MA Hongda2, HAN Yaling1, LIU Lei11Department of Respiratory Diseases, General Hospital of Shenyang Military Command, Shenyang 110016, Liaoning Province, China;2Department of Pharmacy, General Hospital of Shenyang Military Command, Shenyang 110016, Liaoning Province, China;3Department of Medical Oncology, Chinese PLA General Hospital, Beijing 100853, China;4Military Medical Science Academy of the Chinese PLA, Beijing 100850, China
基础研究论著
miRNA153反义核酸上调肺癌细胞对辐射敏感性的体外实验
高 蕊1,冯 帆2,贾 辉2,张 帆3,王 涛4,董国福4,马德宾1,马宏达2,韩雅玲1,刘 蕾1
1沈阳军区总医院 呼吸与重症医学科,辽宁沈阳 110016;2沈阳军区总医院 药剂科,辽宁沈阳 110016;3解放军总医院 肿瘤内科,北京 100853;4军事医学科学院,北京 100850
目的探讨反义核酸沉默microRNA 153(miRNA153)对电离辐射杀伤肺癌细胞株的影响。方法利用脂质体转染miRNA153的反义核酸;使用60Co-γ射线照射肺癌细胞;使用CCK-8实验、软琼脂成集落实验(锚定非依赖性生长)和Trans-well实验检测miRNA153反义核酸对电离辐射杀伤肺癌细胞的影响;RT-PCR和Western blot实验检测miRNA153的反义核酸对miRNA153、其靶标蛋白PTEN以及细胞存活/凋亡耐受调控蛋白Survivin表达的影响。结果CCK-8实验结果显示,中等剂量射线(4 Gy)照射能够杀伤肺癌细胞A549、H460、H1299和H358,下调miRNA153的表达能够增强辐射对肺癌细胞的杀伤作用;软琼脂成集落和Transwell实验进一步证实miRNA153的反义核酸能够上调辐射对A549细胞锚定非依赖性生长和侵袭的抑制作用。分子机制实验结果表明,miRNA153的反义核酸能够显著降低miRNA153的表达水平,提高肿瘤抑制因子PTEN并降低细胞存活因子Survivin的表达。结论降低miRNA153的表达能够上调辐射对肺癌细胞系的体外杀伤作用。
miRNA153沉默;肺癌细胞;电离辐射
肺癌严重危害人类健康,随着环境污染加剧以及各类环境激素类物质的扩散,其发病率和死亡率都逐年升高,位居人类恶性肿瘤的前列[1]。由于大多数肺癌患者初诊即为进展期,失去了外科手术等根治性治疗的机会,因此放射治疗等在肺癌综合治疗中具有重要意义[2]。Kang等[3]和Bussink等[4]的报道都显示,相当比例的病人对放射治疗并不敏感。尽管目前有临床研究应用分子靶向药物配伍放射治疗,但治疗效果仍然较差[2,4-6]。为此,阐明肺癌放射治疗耐受的分子机制,发现和鉴定新的指示分子并开发新的治疗和干预策略(肺癌辐射增敏)具有重要意义。MicroRNAs(miRNAs)是细胞增殖调控、分化和癌变(Transformation)等生理过程的重要调控因子[7]。其作用广泛,功能多样,能够作为疾病发生的指示分子(Indicator)和治疗靶标(Target)[8-9]。研究表明,miRNA153能够通过下调PTEN基因的表达并诱导多种肿瘤的发生、增殖与进展[10]。Chen等[11]的结果显示,miR-153不仅能够促进肿瘤细胞增殖,还能够诱导肝癌细胞的抗肿瘤药物多药耐药作用(Multi-drug resistance)。miRNA的反义核酸技术能够利用特异性反义序列下调目标miRNA的水平,这使得利用基因治疗相关技术通过miRNA干预疾病的进展成为可能[12]。根据组织学和病理特征,肺癌可分为非小细胞肺癌和小细胞肺癌[13-14]。其中,非小细胞肺癌占肺癌发病总数的80%以上,是肺癌最主要的类型[13-14]。为此,本研究主要使用非小细胞肺癌细胞株A549,观察下调miRNA153对A549辐射敏感性的影响,并在其他类型肺癌细胞H460、H1299和H358等模型上进行检测。
材料和方法
1 药品、试剂和设备 miRNA153反义核酸(Antagomirs/Inhibitor)购自美国Applied Biosystems公司;DMEM高糖培养基和胎牛血清(fetal bovine serum,FBS)购自美国Hyclone公司;CCK-8试剂购自美国Amerresco公司;Lipofectamine-RNAi MAX转染试剂购自美国Invitrogen公司;miRNA153检测使用TaqMan®miRNA Assays试剂盒(美国Applied Biosystems公司);RT-PCR试剂盒购自美国Promega公司;蛋白印迹实验(Western blot)检测试剂盒(包括SDS-蛋白电泳使用Loading Buffer、蛋白Marker以及PVDF膜)购自美国Bio-Rad公司;蛋白印迹实验所用抗体(PTEN、Survivin以及GAPDH)购自美国Santa Cruz公司;化学发光试剂盒(北京,Qiangen公司);其余试剂均为国产分析纯试剂;Trans-well细胞24孔培养板和小室、细胞培养瓶、6 cm直径细胞培养皿以及6孔细胞培养板等购自美国Corning公司。多功能酶标仪(Wallac公司);TS-100倒置相差显微镜购自日本Nikon公司。
2 细胞培养和转染 肺癌细胞株A549、H460、H1299及H358均购自中国医学科学院/中国协和医科大学细胞库,培养于含2 mmol/L的L-谷氨酰胺和10% FBS的高糖DMEM培养液中;置于37℃,5% CO2孵箱中培养。先分别将0.5μl的Lipofectamine-RNAi MAX转染试剂和200 ng核酸(由0.5μl无血清无抗生素的RPMI-1640培养基溶解与稀释)分别加入到2个EP管(含24.5μl无血清无抗生素培养基)中,充分混匀,室温静置15 min后,将两管液体等比例混合,轻轻混匀后室温下静置15 min,备用。
3 细胞抑制率实验 将A549等肺癌细胞培养于75 cm2培养瓶中,待其状态较好时,接种于6孔板中,待每空细胞密度达到80% ~ 90%后换新鲜培养基,每孔1 ml;培养24 h后进行射线照射;60Co-γ射线辐射实验由军事医学科学院放射医学研究所提供实验条件,系列辐射剂量分别为0 Gy、2 Gy、6 Gy、8 Gy和10 Gy;辐射后更换含2% FBS的DMEM培养液;置于37℃,5% CO2孵箱中培养,于0 h、4 h、8 h、12 h、24 h、48 h和72 h时,每孔分别加入100μl CCK-8试剂,置于37℃含5% CO2的细胞培养箱中孵育4 h,多功能酶标仪在450 nm测定吸光度,并计算细胞增殖抑制率[15-16]。辐射对细胞存活的抑制率(Inhibition rate)计算公式:抑制率(%)=(对照组A450nm-射线照射组A450nm)/对照组A450nm×100%。细胞相对存活率(Relative cell survival rate)=100%-抑制率。
4 软琼脂成集落实验 按照Zhu等[17]的方法进行实验:预配置1.2%和0.7%的琼脂溶液,灭菌;预先使用1.2%的琼脂铺6 cm细胞培养皿(每皿约2 ml),待其凝固,4℃保存备用;射线照射后的A549细胞消化和计数后,以500/皿的细胞密度使用预配置的含20% FBS的2倍DMEM重悬细胞,与预配置的0.7%的琼脂溶液等体积混合,加于1.2%琼脂的上层,培养17 ~ 20 d,当克隆肉眼可见时,终止培养并计数、观察和统计。
5 Trans-well实验 参照Zhang等[18]的方法进行实验:利用Transwell实验检测A549细胞的侵袭能力。使用无血清RPMI-1640培养基按1∶5比例稀释ECM(美国Sigma公司)胶(Gel)预铺Transwell小室(每孔30μl),4℃孵育过夜;使用射线照射A549细胞后,消化并使用无血清RPMI-1640培养基重悬至5×105/ml的细胞密度,加入Transwell小室中,每孔0.2 ml;24孔细胞培养板中加入含有20% FBS的RPMI-1640培养基后,将小室放入24孔板;37℃孵育4 ~ 6 h后使用结晶紫染色(无水乙醇配置,0.25%溶液),每孔5个视野拍照,使用冰醋酸洗脱后在590 nm波长处检测吸光度值(O.D.590 nm)。相对侵袭细胞数(Relative invasion cell number)的计算公式:实验组A590nm/对照组A590nm。
6 反转录实验 按照文献[19-20]描述方法,A549细胞转染核酸后,收集细胞,提取总RNA,反转录,DNA电泳检测。
7 蛋白免疫印迹实验 按照文献[15]描述方法进行实验,一抗稀释条件:兔抗人PTEN单克隆抗体(1∶1 000稀释)、兔抗人Survivin单克隆抗体(1∶2 000稀释)、鼠抗人GAPDH多克隆抗体(1∶5 000稀释);二抗稀释条件:HRP-羊抗兔单克隆抗体(1∶5 000稀释)、HRP-羊抗鼠单克隆抗体(1∶5 000稀释)。

图 160Co-γ射线(电离辐射)对A549细胞存活的抑制作用 A:A549 细胞受不同剂量照射后48 h进行CCK-8检测; B:A549受4 Gy强度60Co-γ照射后不同时间点的检测细胞转染miRNA122;aP<0.05图 2 miRNA153的反义核酸对60Co-γ射线抑制A549细胞存活的影响(照射后72 h,进行CCK-8检测) (aP<0.05)Fig. 1 Effect of inhibitory activity of60Co-γ IR (Ionizing radiation) on A549 cell survival a: A549 cells were irradiated by different amount of60Co-γ IR, and then detected by CCK-8 analysis after 48h; b: A549 cells were irradiated by 4 Gy amount of60Co-γ IR, and then detected and analyzed at each time point.aP<0.05Fig. 2 miRNA153 anti-sense nuclear acids enhanced the inhibitory activity of60Co-γ IR on A549 cell survival (A549 cells, which were transfected with control or miRNA513 anti-sense nuclear acid, were irradiated by 4 Gy amount of60Co-γ IR and then were detected by CCK-8 assays.aP<0.05)
8 统计学分析 应用SPSS17.0统计软件,采用单因素方差分析比较多组间的,P<0.05为差异有统计学意义。
结 果
1 电离辐射对A549细胞的杀伤作用 A549细胞的射线照射量-效曲线如图1A所示,照射后48 h后进行CCK-8检测,60Co-γ能够剂量依赖性地杀伤A549细胞,其中间作用剂量约为4 Gy和6 Gy。结合Kang等[3]的方法,我们最终选定4 Gy作为进一步实验剂量。进一步时间-效应实验结果表明(图1B),4 Gy照射后对A549细胞的抑制率在72 h时最为明显。因此,我们选定72 h为检测时间点。
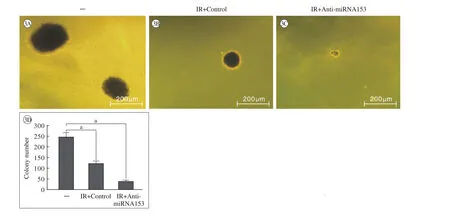
图 3 miRNA153的反义核酸对60Co-γ射线抑制A549细胞锚定非依赖性生长的影响(软琼脂成集落实验); A ~ C:代表性照片; D:克隆计数结果;aP<0.05Fig.3 miRNA153 anti-sense nuclear acids enhanced the inhibitory activity of60Co-γ IR on anchorage-independent growth of A549 (A549 cells were analyzed by soft-agar assays). The Results were shown in photographs (A-C) and colony numbers (D) ().aP<0.05
2 miRNA153的反义核酸能上调射线照射对A549细胞的杀伤作用 4 Gy射线照射能够显著杀伤A549细胞,抑制其存活、锚定非依赖性生长和侵袭作用(图2 ~图4);与对照组相比,miRNA153的反义核酸能够上调射线照射对A549细胞存活(图2)、锚定非依赖性增殖(软琼脂成集落)(图3)以及侵袭(Transwell)(图4)的杀伤和抑制作用。
3 miRNA153的反义核酸对miRNA153及相关蛋白表达的影响 RT-PCR实验结果显示,与对照组相比,miRNA153的反义核酸能够在A549细胞中降低miRNA153的表达水平(图5A);蛋白印迹实验的结果显示,miRNA153的反义核酸能够提高miRNA153作用靶基因PTEN的表达(图5B),下调肿瘤促存活蛋白Survivin的表达(图5B)。这表明,利用反义核酸能够有效下调miRNA153的表达,并调控相关肿瘤增殖、存活调控因子的表达水平。
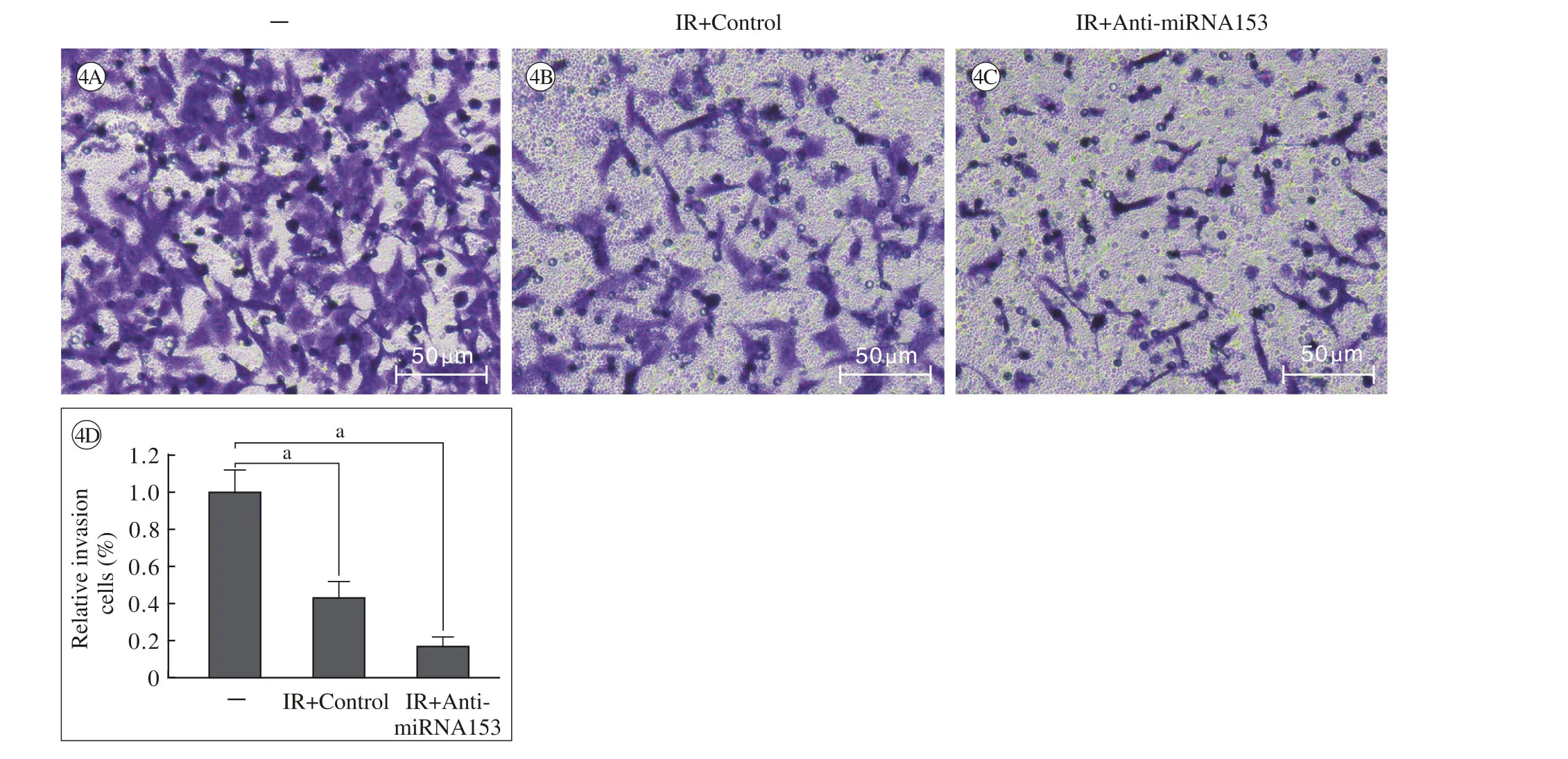
图 4 miRNA153的反义核酸对60Co-γ射线抑制A549细胞侵袭能力的影响(Trans-well实验) A ~ C:代表性照片; D:相对侵袭细胞数;aP<0.05Fig. 4 miRNA153 anti-sense nuclear acids enhanced the inhibitory activity of60Co-γ IR on A549 cell invasion (A549 cells were analyzed by Transwell assays). The Results were shown in photographs (A-C) and relative invasion cell number (D) ().aP<0.05

图 5 miRNA153反义核酸对miRNA153和PTEN、Survivin表达的影响 A:RT-PCR检测miRNA153表达水平;B:蛋白印迹检测miRNA153的作用靶标蛋白PTEN以及细胞存活调控因子Survivin的表达量Fig. 5 Effects of miRNA153 anti-sense nuclear acids on miRNA153, PTEN and Survivin. The expression level of miRNA153 was determined by RT-PCR assays (A). The expression of microRNA153 targeted genes PTEN and cell survival regulator Survivin (B) was detected by western blot
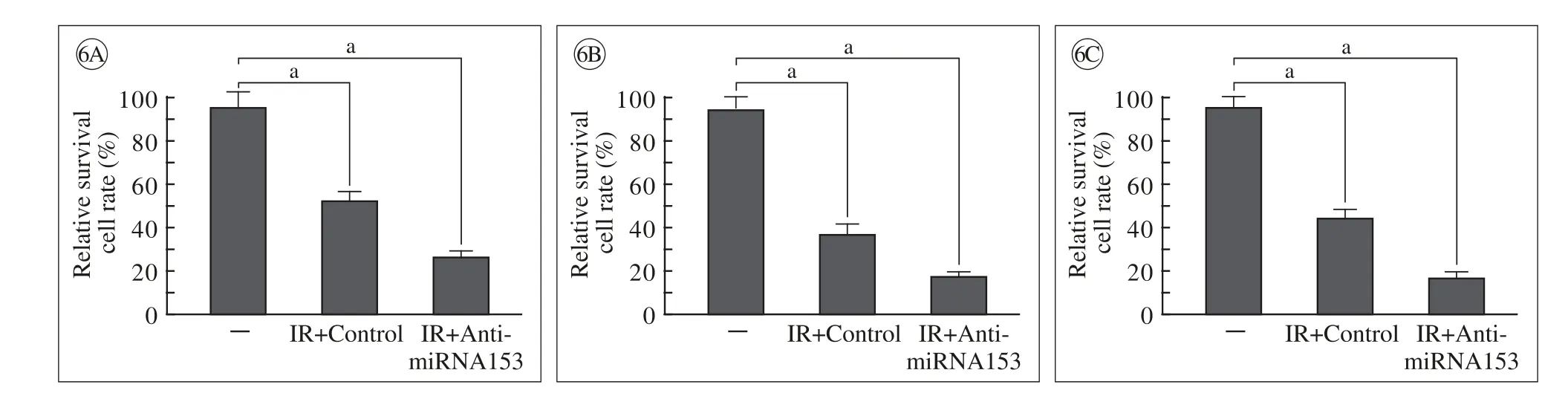
图 6 miRNA153的反义核酸对60Co-γ射线抑制肺癌细胞H460、H1299以及H358存活的影响(CCK-8检测) A: H460; B: H1299; C:H358;aP<0.05Fig. 6 miRNA153 anti-sense nuclear acids enhanced the inhibitory activity of60Co-γ IR on the survival of lung cancer cell H460 (A), H1299 (B) and H358 (C), and H358 cells were detected by CCK-8 assays.aP<0.05
4 miRNA153的反义核酸能上调射线对多种肺癌细胞的杀伤作用 为进一步证实miRNA153反义核酸在肺癌细胞中的作用,H460、H1299和H358细胞转染对照组和miRNA153反义核酸组后,使用4 Gy射线照射。结果显示(图6),与对照组相比,miRNA153的反义核酸能够上调射线照射对H460(图6A)、H1299(图6B)和H358(图6C)细胞的杀伤作用。
讨 论
微小RNA(microRNA)是一类识别靶标基因3′末端18 ~ 25个碱基序列的小RNA分子[7-8]。miRNA为RNA聚合酶Ⅲ转录,通过转录后调节靶标基因的表达,调节真核细胞的增殖、转化和凋亡[7-8]。miRNA是重要的肿瘤调控因子,Qian等[21]系统总结了miRNA的功能和调控机制,包括miR-20a、miR-145、miR-24和miR-25等都有可能参与调控肺癌的发生与进展。miRNA153是新型肿瘤调控因子,Anaya-Ruiz等[12]、Kim等[22]和Wu等[10]均报道,miRNA153能够促进乳腺癌、前列腺癌和神经胶质瘤细胞的增殖,异常表达(Aberrent expression)的miRNA153能够作为乳腺癌进展和患者预后的指示分子(Indicator)。miRNA153不仅能够促进细胞增殖,Chen等[11]的报道也显示,miRNA153还能够调控肝癌细胞的多药耐药作用(Multi-drug resistance)。近年来,随着技术进步,包括“sponges”或“antagomirs”等策略能够通过作用于miRT序列沉默内源miRNA表达,这使得miRNA作为疾病干预靶标成为可能。Anaya-Ruiz等[12]利用miRNA153的Inhibitor(抑制剂)沉默(Silencing)miRNA153的表达,能够诱导乳腺癌细胞MDA-MB-231的细胞凋亡作用。本研究利用类似技术,检测降低miRNA153的表达对肺癌细胞系辐射敏感性的影响,发现转染miRNA153的反义核酸能够上调射线照射对肺癌细胞增殖、锚定非依赖性生长和侵袭的抑制作用。进一步对其作用机制进行分析,miRNA153的反义核酸能够显著下调miRNA153的水平,进而提高miRNA153作用靶标肿瘤抑制蛋白PTEN的表达水平。此外,miRNA153的反义核酸还能降低细胞存活调控蛋白Survivin的表达。PTEN是IGF/PI3K/AKT信号通路的主控负调控因子,能够降低PI3K的磷酸化[23]。作为强势的肿瘤抑制基因,Nagata等[23]证实,PTEN是PI3K信号通路的主要负调控因子,其差异表达可能是HER2阳性乳腺癌患者对曲妥珠单抗(Herceptine)敏感性差异的原因。PI3K信号通路是乳腺癌存活、增殖和侵袭的调控枢纽,能够上调肿瘤细胞存活因子Survivin的表达。结合前述,传统研究主要关注于电离辐射后的DNA损伤修复作用机制和相关信号通路,如P21、P53以及ATM等损伤修复相关调控因子。这些虽然在正常组织/细胞中维持遗传物质的稳定性,但在肿瘤细胞中有可能诱导对放疗的耐受。电离辐射不仅能造成DNA断裂,也能诱导过氧化物或者自由基造成蛋白质等生物大分子的烷基化或相互交联[3]。电离辐射胁迫下,细胞中通过各种感受机制感受DNA和蛋白质等生物大分子损伤,进而活化相关应答机制[3],最终诱导Survivin、BCL-2、IAP和Livin等细胞存活蛋白的表达[24]。在这一过程中,PI3K/ AKT信号通路及Notch-1/NF-κB信号通路起到了重要的作用[3]。进一步研究发现,miRNA126和miRNA34a等也能够调控PI3K/AKT以及Notch-1/ NF-κB信号通路[21]。miRNA126和miRNA34a等的差异表达有可能是肿瘤放疗敏感性差异的主要机制,而本研究首次报道了miRNA153在肿瘤细胞辐射增敏中的作用,表明利用反义核酸技术降低miRNA153等细胞存活及凋亡耐受调控miRNA的表达能增加细胞对辐射等胁迫因素的敏感性。
综上所述,本研究利用miRNA153的反义核酸技术,探讨其在辐射增敏中的意义,对阐明参与电离辐射相关miRNA的作用机制具有重要意义。
1 Niu C, Liang C, Guo J, et al. Downregulation and growth inhibitory role of FHL1 in lung cancer[J]. Int J Cancer, 2012, 130(11):2549-2556.
2 Hillman GG, Singh-Gupta V, Runyan L, et al. Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury[J]. Radiother Oncol, 2011, 101(2): 329-336.
3 Kang J, Kim E, Kim W, et al. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition(EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines[J]. J Biol Chem, 2013, 288(38): 27343-27357.
4 Bussink J, Van der Kogel AJ, Kaanders JH. Activation of the PI3-K/ AKT pathway and implications for radioresistance mechanisms inhead and neck cancer[J]. Lancet Oncol, 2008, 9(3):288-296.
5 Nishimura Y, Nakagawa K, Takeda K, et al. Phase I/II trial of sequential chemoradiotherapy using a novel hypoxic cell radiosensitizer, doranidazole (PR-350), in patients with locally advanced non-small-cell lung Cancer (WJTOG-0002)[J]. Int J Radiat Oncol Biol Phys, 2007, 69(3): 786-792.
6 Toschi L, Cappuzzo F. Impact of biomarkers on non-small cell lung cancer treatment[J]. Target Oncol, 2010, 5(1): 5-17.
7 Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs[J]. Nature, 2009, 457(7228): 421-425.
8 Yang F, Li QJ, Gong ZB, et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment[J]. Technol Cancer Res Treat, 2014, 13(1): 77-86.
9 Maroof H, Salajegheh A, Smith RA, et al. MicroRNA-34 family,mechanisms of action in cancer: a review[J]. Curr Cancer Drug Targets, 2014, 14(8): 737-751.
10 Wu Z, He B, He J, et al. Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer[J]. Prostate, 2013, 73(6): 596-604.
11 Chen Y, Feng F, Gao X, et al. MiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cells[J]. Curr Cancer Drug Targets, 2015, 15(3): 176-187.
12 Anaya-Ruiz M, Cebada J, Delgado-López G, et al. miR-153 silencing induces apoptosis in the MDA-MB-231 breast cancer cell line[J]. Asian Pac J Cancer Prev, 2013, 14(5): 2983-2986.
13 Baumann M, Krause M, Zips D, et al. Molecular targeting in radiotherapy of lung cancer[J]. Lung Cancer, 2004, 45(Suppl 2):S187-S197.
14 Koh PK, Faivre-Finn C, Blackhall FH, et al. Targeted agents in non-small cell lung cancer (NSCLC): clinical developments and rationale for the combination with thoracic radiotherapy[J]. Cancer Treat Rev, 2012, 38(6): 626-640.
15 冯帆, 张琼, 刘洪英, 等. 酪氨酸激酶抑制剂伊马替尼耐药K562细胞系的建立及其耐药特征[J]. 中国药理学与毒理学杂志,2012, 26(4): 563-569.
16 马德宾,冯帆,张帆,等.MicroRNA122促进吉西他滨对体外非小细胞肺癌细胞系A549的杀伤作用[J].解放军医学院学报,2014,35(11):1160-1163.
17 Zhu M, Li M, Zhang F, et al. FBI-1 enhances ETS-1 signaling activity and promotes proliferation of human colorectal carcinoma cells[J]. PLoS One, 2014, 9(5): e98041.
18 Zhang P, Ma X, Song E, et al. Tubulin cofactor A functions as a novel positive regulator of ccRCC progression, invasion and metastasis[J]. Int J Cancer, 2013, 133(12): 2801-2811.
19 Tsai PC, Bake S, Balaraman S, et al. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells[J]. Biol Open, 2014, 3(8):741-758.
20 Yang YP, Qu JH, Chang XJ, et al. High intratumoral metastasisassociated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma[J]. J Transl Med, 2013, 11: 41.
21 Qian B, Nag SA, Su Y, et al. miRNAs in cancer prevention and treatment and as molecular targets for natural product anticancer agents[J]. Curr Cancer Drug Targets, 2013, 13(5): 519-541.
22 Kim TH, Kim YK, Kwon Y, et al. Deregulation of miR-519a, 153,and 485-5p and its clinicopathological relevance in ovarian epithelial tumours[J]. Histopathology, 2010, 57(5): 734-743.
23 Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients[J]. Cancer Cell, 2004, 6(2):117-127.
24 张帆,孙苏华,杨俊兰.Survivin特性与抗肿瘤药物研究[J].军医进修学院学报,2009,30(5):755-757.
Up-regulation of microRNA153 causes radio-sensitization of lung cancer cells in vitro experiments
LIU Lei. Email: doctorliulei@163.com
ObjectiveTo declare whether silencing of miRNA153 via its anti-sense nuclear (inhibitor) induces the radio-sensitization of lung cancer cells.MethodsThe anti-sense nuclear acid (inhibitor) of miRNA153 was transfected into lung cancer cells, which were irradiated by 60Co-γ. The CCK-8 analysis, soft-agar and Transwell assays were performed to identify the effect of miRNA153 silencing on radio-sensitization in lung cancer cells. RT-PCR and Western blot assays were used to detect the effect of anti-sense nuclear acid (inhibitor) of miRNA153 on miRNA153, its target protein PTEN and the expression of Survivin cell proliferation of survival regulators.ResultsCCK-8 analysis revealed that the irradiation of medium dose ray (4 Gy) could kill the lung cancer cells (A549, H460, H1299 and H358), and the down-regulation of expression of miRNA153 could enhance the radio-sensitization in lung cancer cells. The soft-agar and Transwell assays proved that the up-regulation of rays could inhibit the anchorage-independent growth and invasion of A549 cells. And the molecular mechanism experiment indicated that the anti-sense nuclear acid of miRNA153 significantly disrupted the endogenous expression of miRNA153 and Survivin, and in turn upregulated the expression of PTEN.ConclusionSilencing of miR-153 significantly enhances the sensitivity of lung cancers.
miRNA153 silencing; lung cancer cells; ionizing radiation
R 73-3
A
2095-5227(2015)10-1033-06 DOI:10.3969/j.issn.2095-5227.2015.10.019
时间:2015-07-10 10:42
http://www.cnki.net/kcms/detail/11.3275.R.20150710.1042.001.html
GAO Rui1, FENG Fan2, JIA Hui2, ZHANG Fan3, WANG Tao4, DONG Guofu4, MA Debin1, MA Hongda2, HAN Yaling1, LIU Lei1
1Department of Respiratory Diseases, General Hospital of Shenyang Military Command, Shenyang 110016, Liaoning Province, China;2Department of Pharmacy, General Hospital of Shenyang Military Command, Shenyang 110016, Liaoning Province, China;3Department of Medical Oncology, Chinese PLA General Hospital, Beijing 100853, China;4Military Medical Science Academy of the Chinese PLA, Beijing 100850, China
2015-04-22
“十二五”全军医学科研项目(BWS12J007)
Supported by the "12th Five-Year" Medical Science Research Foundation of PLA(BWS12J007)
高蕊,女,硕士,主治医师。研究方向:呼吸系统疾病和肺部肿瘤。Email: zgydgaorui@163.com
刘蕾,女,博士,副主任医师。Email: doctorliulei@163. com
