乳酸杆菌与双歧杆菌对高脂血症治疗的相关性研究
2015-03-21闫志辉崔立红王晓辉弓三东季梦辰
闫志辉,崔立红,王晓辉,贺 星,李 超,弓三东,罗 哲,季梦辰
海军总医院 消化内科,北京 100048
乳酸杆菌与双歧杆菌对高脂血症治疗的相关性研究
闫志辉,崔立红,王晓辉,贺 星,李 超,弓三东,罗 哲,季梦辰
海军总医院 消化内科,北京 100048
目的分析高脂血症患者临床治疗效果与补充肠道乳酸杆菌、双歧杆菌的相关性。方法对2012年3月- 2014年6月于我院就诊的120例高脂血症患者[男性85例,女性35例,年龄(48.1±11.6)岁]随机分为4组:A组低脂饮食+运动,B组给予降血脂药物,C组给予肠道菌群调节剂,D组给予降血脂药物+肠道菌群调节剂治疗,疗程为1个月。所有患者于入院前、治疗1个月、3个月时进行血脂、肠道菌群检测。结果治疗前、治疗1个月、3个月血脂水平:C组总胆固醇(total cholesterol,TC)(mmol/L)6.69±1.29、6.18±1.04、6.78±1.14;三酰甘油(triglyceride,TG)(mmol/L):1.91±0.32、1.87±0.46、1.93±0.28;高密度脂蛋白胆固醇(high density lipoprotein cholesterol,HDL-C)(mmol/L):0.91±0.35、0.97±0.33、0.97±0.36;D组TC:6.79±1.39、4.11±1.07、4.27±1.03;TG:1.90±0.55、1.36±0.41、1.39±0.29;HDL-C:0.88±0.30、1.32±0.39、1.24±0.35。C组降血脂效果最差(1个月:30%;3个月:16.7%),D组降血脂效果最好(1个月:76.6%;3个月:70%)。随时间变化HDL-C水平在C组存在回升和反弹现象(治疗前:0.88±0.30;1个月:1.32±0.39;3个月:0.97±0.36)。C组治疗1个月时乳酸杆菌和双歧杆菌(logn/g)(7.49±0.34,9.50±0.15)显著高于治疗前(6.85±0.26,9.32±0.12)、治疗3个月(6.73±0.28,9.22±0.14)后;而D组治疗1个月(7.63±0.62,9.55±0.14)、3个月(7.42±0.59,9.51±0.11)时乳酸杆菌和双歧杆菌均显著高于治疗前(6.88±0.23,9.27±0.13)。结论乳酸杆菌、双歧杆菌干预可能对高脂血症患者临床疗效起到积极作用。
肠道菌群;高脂血症;降血脂治疗
正常人体肠道内定植500 ~ 1 000种不同菌群,其细胞数量为1014,是人体细胞总数1013的10倍,所携带的遗传基因数量是人体遗传基因总数100倍[1-3](又称为人类第二基因组),也被一些学者视为机体中一个代谢重要器官,具有不可替代的生理功能[4]。随着基础研究的不断深入,肠道菌群在高脂血症、肥胖及相关代谢性疾病发生、发展中的作用被广泛认识和研究[5-10]。但其具体机制尚未完全阐明,同时由于临床高脂血症并发代谢疾病的多样性和治疗方案个体化差异,导致临床干预和治疗效果亦未达成共识,本研究对我院120例高脂血症患者进行降血脂药物及肠道菌群调节剂的临床治疗观察,以探讨肠道菌群对降脂治疗是否产生影响。
资料和方法
1 一般资料 选择我院2012年3月- 2014年6月120例高脂血症患者为观察对象,根据住院号及门诊就诊尾号随机平均分为4组(尾号1 ~ 2 A组;3 ~ 4 B组;5 ~ 6 C组;7 ~ 8 D组,0,9不选入,直至4组样本量均到达30例)。各组在年龄、性别、体质量指数(body mass index,BMI)、血脂水平分布差异无统计学意义(P>0.05),具体见表1。入组患者临床治疗前1个月及整个临床观察期内均未服用任何抗生素及其他微生态制剂,同时排除糖尿病、高尿酸血症等代谢性疾病。高血脂诊断依据《中国成人血脂异常防治指南》[10],入组患者均签署临床观察知情同意书。
2 治疗方法 A组采用非药物的饮食、运动治疗,要求每日严格控制脂肪摄入量,保证每日活动量为步行≥1 km;B组根据血脂检测结果分别采用他汀类或贝特类降血脂药物,胆固醇(total cholesterol,TC)升高为主患者,采用阿托伐他汀(辉瑞制药有限公司J20070061),10 mg/d,每天8:00 pm时口服,疗程1个月;单纯三酰甘油(triglyceride,TG)升高者,采用非诺贝特胶囊(宜昌人福药业有限责任公司H42022051),0.2 g/d,1次/d,口服,疗程1个月[10]。C组采用肠道菌群调节剂,口服丽珠肠乐胶囊(康田制药(中山)有限公司S10960040),2粒/次,3次/d,同时复合乳酸菌胶囊(江苏美通制药有限公司H19980184)2粒/次,3次/d,疗程1个月。D组给予上述降血脂药物联合肠道菌群调节剂治疗,疗程1个月。所有患者定期复查肝、肾功能,若出现肝、肾功能异常或腹泻、腹痛等药物不良反应,立即停用相关药物。各组分别于入院前、治疗1个月、3个月时进行血脂、肠道菌群检测。
3 样本采集及检测 粪便样本:留取晨起新鲜粪便于无菌干燥采便盒内,采集完成后立即送检,进行稀释、接种和培养,整个检测过程不超过2 h。血液样本:留取晨起空腹肘前静脉血液5 ml,采集完毕后立即送检,整个检测过程不超过2 h。肠道菌群数量检测:无菌称取均匀混合后的粪便样本10 g于90 ml厌氧稀释液中,充分混匀后进行10倍浓度梯度稀释至108,选择合适稀释度的样本20μl,分别接种在各培养基上,不同稀释度均完成3个平行样。按相应要求和条件进行培养后,以菌落形态、革兰染色镜检、生化反应等鉴定计数菌落,求平均值,并计算出每克湿便中的菌数。选择性培养基主要采用:双歧杆菌改良BBL培养基,37℃,48 h厌氧培养;乳酸杆菌LBS培养基,37℃,48 h烛缸培养。上述操作均参照《保健食品检验与评价技术规范》相关要求进行[11]。
4 疗效评价 血脂降低效果主要依据血清TG、
表1 4组高脂血症患者一般资料比较Tab. 1 Comparison of general information of patients in four groups ()

表1 4组高脂血症患者一般资料比较Tab. 1 Comparison of general information of patients in four groups ()
Group A (n=30)Group B (n=30)Group C (n=30)Group D (n=30) Sex (n, %) Male21(70)23(76.67)21(70)20(66.67) Female9(30)7(23.33)9(30)10(33.33) Age (yrs)47.70±8.1049.10±9.1046.80±10.3048.50±7.90 BMI29.40±4.4030.40±3.7029.10±4.3031.10±4.20 TC level (mmol/L)6.54±1.616.72±1.516.69±1.296.79±1.39 TG level (mmol/L)1.88±0.301.85±0.281.91±0.321.90±0.55 HDL-C level (mmol/L)0.93±0.270.90±0.310.91±0.350.88±0.30
TC、高密度脂蛋白胆固醇(high density lipoprotein cholesterol,HDL-C)水平[12],于治疗3个月时进行临床疗效评价:显效:TC下降≥20%,TG下降≥40%,HDL-C上升≥0.26 mmol/L;有效:TC下降10% ~ 20%,TG下降20% ~ 40%,HDL-C上升≥0.18 mmol/L;无效:TG、TC降低未达到有效标准;恶化:TC上升≥10%,TG上升≥10%,HDL-C下降≥0.18 mmol/L。相关评价标准参照心血管系统药物临床研究指导原则(1993年版)。
5 统计学分析 资料采用SPSS13.0软件进行统计学分析,血脂水平比较使用析因实验方差分析,肠道菌群计数比较采用单因素方差LSD法分析,临床疗效计量资料采用χ2检验,P<0.05为差异有统计学意义。
结 果
1 4组疗效比较 治疗1个月时,A组有效率为40%,与B组73.3%(χ2=6.787,P=0.009)及D组76.6%差异均有统计学意义(χ2=8.297,P=0.004),与C组30.0%差异无统计学意义;B组与C组差异有统计学意义(χ2=11.279,P=0.001),与D组差异无统计学意义;C组与D组差异有统计学意义(χ2=13.125,P=0.000)。治疗3个月时,A组有效率为26.7%,与D组70%差异有统计学意义(χ2=11.279,P=0.001),与B组40%和C组16.7%差异无统计学意义;B组与C组差异有统计学意义(χ2=4.022,P=0.045),与D组差异无统计学意义,C组与D组差异有统计学意义(χ2=17.376,P=0.000)。其中D组降血脂效果最好,C组降血脂效果最差。见表2。
2 4组治疗前后血脂水平比较 在TC、TG水平变化中,A、C组在不同治疗方法和不同时间段内均存在统计学差异(FTC=28.982、25.048,PTC<0.05,FTG=20.933、10.812,PTG<0.05),HDL-C水平变化在C组均存在统计学差异(FHDL-C=6.565、16.045,PHDL-C<0.05)。TC、TG水平在治疗1个月时出现下降,治疗3个月时较治疗前升高,HDL-C水平在治疗1个月及3个月时均出现下降,随时间变化TC、TG水平在A、C组存在回升和反弹现象,HDL-C水平在C组存在回升和反弹现象。见表3。
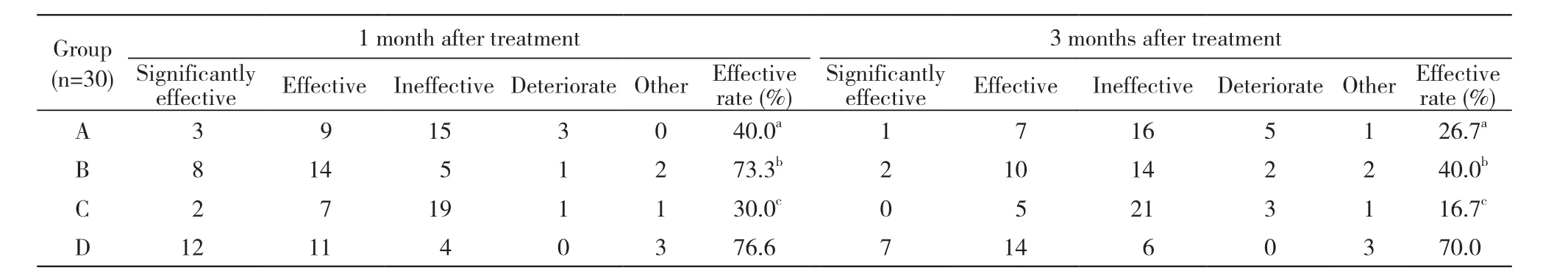
表2 4组高脂血症患者临床观察期内降血脂效果比较Tab. 2 Comparison of treatment effect of patients with hyperlipidemia in four groups during clinical observation period (n)

表3 4组高脂血症患者临床观察期内血脂水平比较Tab. 3 Comparison of blood lipid level of patients with hyperlipidemia in four groups during clinical observation period (mmol/L)
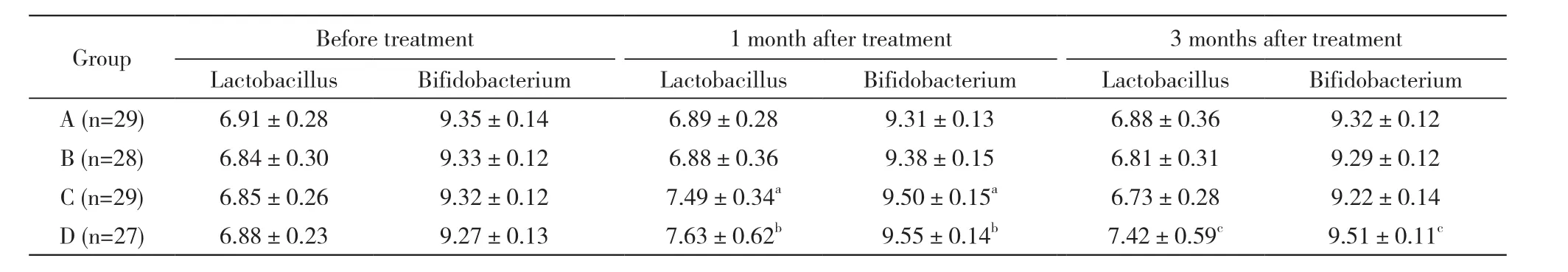
表4 4组高脂血症患者临床观察期内肠道菌群变化Tab. 4 Changes of intestinal flora of patients with hyperlipidemia in four groups during clinical observation period (logn/g)
3 4组治疗前后肠道乳酸杆菌、双歧杆菌变化情况 A、B组治疗前、治疗1个月、3个月时,乳酸杆菌和双歧杆菌肠道分布差异无统计学意义。C、D组治疗前、治疗1个月时、3个月时,乳酸杆菌和双歧杆菌分布差异有统计学意义(Fc=4.127、2.809,P=0.000、0.000;Fd=1.801、2.344,P=0.033、0.004),其中C组,治疗1个月时乳酸杆菌和双歧杆菌显著高于治疗前、治疗3个月时;而D组,治疗1个月、3个月时乳酸杆菌和双歧杆菌均显著高于治疗前。见表4。
讨 论
肠道微生态菌群对人体能量摄入、脂肪吸收、胰岛素抵抗、炎症反应、肝纤维化等均密切相关[13-15]。被视为人体肥胖、高脂血症的重要相关因素。具体机制包括:1)多形拟杆菌等特定肠道菌群可增加食物能量摄入率[16],且同时促进肠道内大量游离脂肪酸进入肝;2)正常优势菌群失调,导致定植菌群异型性和多态性增加,从而引起宿主葡萄糖吸收增多和血清胰岛素含量和分泌节律异常,多种肥胖相关转录因子如ChREBP和SREBP-1异常增高,最终诱导肝脂肪合成增加[17];3)正常肠道菌群和肠道黏膜屏障的破坏,导致脂多糖吸收增加,而脂多糖作用于Toll样受体4,可明显引起胰岛素抵抗和慢性炎症反应,均可引起非高脂饮食性的血脂代谢紊乱[18-19]。国内外大量的生理基础研究和临床试验已基本证实肠道菌群在人体脂肪代谢及高脂血症发展中起到关键作用。
介于临床高脂血症发病原因的复杂性和多样性,除常规明确病因治疗及对症降血脂药物使用外,肠道菌群干预是否能作为一线治疗措施尚未达成共识。因此,我院对120例高脂血症患者进行临床治疗观察,研究发现常规降血脂药物阿托伐他汀及非诺贝特胶囊临床治疗效果明显优于非药物性的运动、饮食治疗及单纯肠道菌群调节剂治疗。在临床因素的交互效应检验中,更可清楚发现非药物性的运动、饮食治疗(A组)、单纯肠道菌群调节剂治疗(C组)在结束为期1个月疗程后,存在显著性血脂水平波动及回升趋势,且部分患者可回到治疗前的血脂水平。降血脂药物联合肠道菌群调节剂(D组)虽然与单纯降血脂药物(B组)在治疗1个月时,临床有效率并未见显著统计学差异,但随着停药时间延长,联合肠道菌群调节剂组患者能较好维持临床有效率,治疗3个月时血脂降低有效率仍为70%,且个别血脂水平甚高患者也能到达有效降低血脂目的,与早期Kawai[20]和张磊艺等[21]的临床研究结论基本一致。在肠道菌群变化方面,大量临床研究已经证实[22-25],脂质代谢异常导致的肠道菌群分布变化主要涉及大肠埃希菌、乳酸杆菌及双歧杆菌等优势菌群呈下降趋势,而拟杆菌,肠球菌等非优势菌群丰度增高,菌群数量呈增高及多态性趋势,且熊静芳和傅国胜[26]研究报道肠道双歧杆菌和乳杆菌可促进肝利用胆固醇增加胆汁酸合成,使更多的胆固醇被转化为无法吸收的粪甾醇,达到降脂作用。因此,在本临床研究中主要选择的肠道菌群调节剂为乳酸杆菌和双歧杆菌微生物制剂,通过外源性提高相关肠道菌群比例,观察对血脂水平的影响。其中单纯使用降血脂药物治疗患者(B组),肠道菌群分布并未见显著性变化。根据肠道菌群对脂质代谢的作用,笔者有理由相信将肠道菌群调节剂作为高脂血症治疗的常规方法是具有一定临床价值,尤其针对血脂水平过高或经饮食、运动治疗后血脂水平下降不理想者,但疗效是否确切还有待进一步的临床观察。
综上所述,肠道菌群外源性干预可能对高脂血症患者临床疗效起到积极作用,值得临床广泛关注和研究。
1 Ventura M, Turroni F, Canchaya C, et al. Microbial diversity in the human intestine and novel insights from metagenomics[J]. Front Biosci (Landmark Ed), 2009, 14(1): 3214-3221.
2 Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing[J]. Nature, 2010, 464(7285): 59-65.
3 Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes[J]. DNA Res, 2007, 14(4): 169-181.
4 Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage[J]. Proc Natl Acad Sci U S A, 2004, 101(44): 15718-15723.
5 Martin FP, Dumas ME, Wang Y, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model[J]. Mol Syst Biol, 2007, 3: 112.
6 Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy[J]. Cell,2012, 150(3): 470-480.
7 Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulinresistant mice[J]. Proc Natl Acad Sci U S A, 2006, 103(33):12511-12516.
8 张晨虹,赵立平.肠道菌群在肥胖及相关的代谢性疾病发生发展中的地位和作用[J].前沿科学,2007(3):75-80.9 Yeretssian G. Effector functions of NLRs in the intestine: innate sensing, cell death, and disease[J]. Immunol Res, 2012, 54(1/3):25-36.
10 中国成人血脂异常防治指南制订联合委员会.中国成人血脂异常防治指南[J].中华心血管病杂志,2007,35(5):390-419.
11 中华人民共和国卫生部. 保健食品检验与评价技术规范(2003版)[S]. 卫法监发[2003]42号.
12 刘红军,高亚丽,王伟.阿托伐他汀治疗原发性高脂血症的疗效评价[J].中西医结合心脑血管病杂志,2010,8(9):1135-1136.
13 Vidhyasagar V, Jeevaratnam K. Evaluation of pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro[J]. J Funct Foods, 2013, 5(1): 235-243.
14 Wang Y, Kirpich I, Liu Y, et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury[J]. Am J Pathol, 2011, 179(6): 2866-2875.
15 Kirpich IA, Feng W, Wang Y, et al. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol[J]. Alcohol, 2013, 47(3): 257-264.
16 Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature, 2006, 444(7122): 1027-1031.
17 Dentin R, Pégorier JP, Benhamed F, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression[J]. J Biol Chem, 2004,279(19): 20314-20326.
18 Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice[J]. Diabetes, 2008, 57(6):1470-1481.
19 Poggi M, Bastelica D, Gual P, et al. C3H/HeJ mice carrying a tolllike receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet[J]. Diabetologia, 2007, 50(6): 1267-1276.
20 Kawai Y. Effect Of cellular extrancts of streptococci on Hyperlipidemia in rats, rabbits, and humans[J]. Microecology and Therapy,1984,14:109-126.
21 张磊艺,丁淑兰,王学翔.双歧杆菌复合制剂对脂质代谢的影响[J].中国微生态学杂志,1997,9(3):35-36.
22 Denipote FG, Trindade EB, Burini RC. Probiotics and prebiotics in primary care for colon cancer[J]. Arq Gastroenterol, 2010, 47(1):93-98.
23 Neyrinck AM, Possemiers S, Verstraete W, et al. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitinglucan fiber improves host metabolic alterations induced by high-fat diet in mice[J]. J Nutr Biochem, 2012, 23(1): 51-59.
24 Turnbaugh PJ, Ridaura VK, Faith JJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice[J]. Sci Transl Med, 2009, 1(6): 6ra14.
25 De Lartigue G, De La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin[J]. Physiol Behav, 2011, 105(1):100-105.
26 熊静芳,傅国胜.高脂血症患者肠道优势菌群与血清脂质水平相关性研究[J].中国微生态学杂志,2013,25(11):1282-1285.
Correlation of Lactobacillus and Bifidobacterium treatment for hyperlipidemia
YAN Zhihui, CUI Lihong, WANG Xiaohui, He Xing, LI Chao, GONG Sandong, LUO Zhe, JI Mengchen
Department of Gastroenterology, Navy General Hospital, Beijing 100048, China
CUI Lihong. Email: luckycui861@sina.com
ObjectiveTo investigate the correlation between clinical outcomes in patients with hyperlipidemia and the distribution of Lactobacillus and Bifidobacterium.MethodsFrom March 2012 to June 2014, 120 cases with hyperlipidemia (85 males, 35 females, average age of (48.1±11.6) years) in our hospital were randomly divided into four groups, and they were provided with one-month treatment. Group A were treated with low fat diet + exercise; group B were treated with lipid-lowering drug therapy; group C were treated with intestinal flora regulator treatment; group D were treated with lipid-lowering drugs + modulators. The serum lipids and intestinal flora detections were given in all patients separately before admission, 1 month after treatment, and 3 months after treatment. The lipid levels of patients in four groups, the changes of intestinal microflora and the blood lipid sustainment after treatment in different periods were analyzed and compared.ResultsThe total cholesterol (TC) of group C before treatment, 1 month and 3 months after treatment was (6.69±1.29) mmol/L, (6.18±1.04) mmol/L and (6.78±1.14) mmol/L, triglyceride (TG) was (1.91±0.32) mmol/L, (1.87±0.46) mmol/L, (1.93±0.28) mmol/L; high density lipoprotein cholesterol (HDL-C) was (0.91±0.35) mmol/L, (0.97±0.33) mmol/L, (0.97±0.36) mmol/L; while TC in group D was (6.79±1.39) mmol/L, (4.11±1.07) mmol/L, (4.27±1.03) mmol/L, TG: (1.90±0.55) mmol/L, (1.36±0.41) mmol/L, (1.39±0.29) mmol/L, HDL-C: (0.88±0.30) mmol/L, (1.32±0.39) mmol/L, (1.24±0.35) mmol/L. The effect of lowering blood lipid in group C was the worst (1 month 30%; 3 months 16.7%), in group D was the best (1 month 76.6%; 3 months 70%). There was rebound phenomenon of HDL-C level in group D as time changed [HDL-C: before treatment (0.88±0.30) mmol/L, 1 month (1.32±0.39) mmol/L, 3 months (0.97±0.36) mmol/L]. The Lactobacillus and Bifidobacteria in group C at 1 month after treatment was significantly higher than before treatment and three months after treatment [(7.49±0.34) vs (6.85±0.26), (6.73±0.28); (9.50±0.15) vs (9.32±0.12), (9.22±0.14)], and Lactobacillus and Bifidobacteria in group D at 1 month and 3 months after treatment was significantly higher than before treatment [(7.63±0.62), (7.42±0.59) vs (6.88±0.23); (9.55±0.14), (9.51±0.11) vs (9.27±0.13)].ConclusionLactobacillus and Bifidobacteriumintervention treatment has positive therapeutic effect in patients with hyperlipidemia.
intestinal microflora; hyperlipidemia; lipid-lowering therapy
R 575
A
2095-5227(2015)10-0983-05 DOI:10.3969/j.issn.2095-5227.2015.10.006
时间:2015-08-25 10:02
http://www.cnki.net/kcms/detail/11.3275.R.20150825.1002.002.html
2015-06-15
海军后勤科研计划课题(CHJ12J027)
Supported by the Foundation of Logistics of Chinese Navy(CHJ12J027)
闫志辉,男,硕士,主治医师。研究方向:消化道肿瘤、肠道疾病。Email: yisheng1018@163com
崔立红,女,博士,主任医师,教授,主任,博士生导师。Email: luckycui861@sina.com
