稻壳基介孔炭的制备及其在超级电容器中的应用
2015-03-15吴明铂李玲燕艾培培吴文婷郑经堂
吴明铂, 李玲燕,2, 刘 军, 李 杨, 艾培培, 吴文婷, 郑经堂
(1.中国石油大学重质油国家重点实验室,山东青岛 266580;2.中建安装工程有限公司,江苏南京 210046)
稻壳基介孔炭的制备及其在超级电容器中的应用
吴明铂1, 李玲燕1,2, 刘 军1, 李 杨1, 艾培培1, 吴文婷1, 郑经堂1
(1.中国石油大学重质油国家重点实验室,山东青岛 266580;2.中建安装工程有限公司,江苏南京 210046)
以量大价廉的稻壳为原料,先后经NaOH脱硅处理、预氧化、磷酸活化,在无模板的情况下制备出介孔炭材料。NaOH脱硅处理可有效除去稻壳中的硅并破坏纤维素的晶体结构,脱硅过程中形成的孔隙亦有利于高比表面积和高中孔率介孔炭的制备。所制介孔炭比表面积和中孔率分别高达2 009 m2·g-1和90.8%。其在50 mA·g-1电流密度下的比电容达176 F·g-1,即使在1 000 mA·g-1的大电流下,其比电容仍保持在126 F·g-1,表现出优异的倍率能力。所制介孔炭具有良好的循环稳定性,在200·mA g-1电流密度下的比电容高达150 F·g-1,1 000次循环无容量衰减。稻壳基介孔炭在超级电容器领域具有良好的应用前景。
稻壳;介孔炭;碱预处理;超级电容器
Received date:2015-04-05; Revised date:2015-09-28
Foundation item:National Natural Science Foundation of China(51172285,51372277,21302224);Fundamental Research Funds for the Central Universities(CX-1217,14CX06045A,14CX02060A).
Author introduction:WU Ming-bo,Professor,Ph D.E-mail:wumb@upc.edu.cn.
English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).
1 Introduction
Supercapacitors are attracting much attention as a kind of promising energy storage devices owing to their high power density,high energy efficiency, long cycle life and environmental-friendly nature[1-4]. Porous carbons have been widely used as the electrode materials for commercial supercapacitors owing to their high surface area and low cost.It is noted that only the pores that electrolyte ions can access contribute to the double layer capacitance,and only the mesopores can facilitate the transport of electrolyte ions and exhibit high ion-accessible surface area of the spectrum of pores in porous carbon[5-9].Therefore, mesoporous carbons(MCs)always show better rate performance than microporous carbons[10,11].MCs are mainly made by the template method,during which the template needs to be synthesized beforehand andto be removed by acid or base washing after the synthesis of MCs,resulting in a high production cost,tedious and environment-hazardous preparative process of the templated MCs[12-14].Thus facile and templatefree methods to prepare MCs are highly desired.
In this paper,the abundant and renewable rice husk(RH)with low cost has been selected as precursor of MC.Silicon-free MC was obtained by a method combining a H3PO4activation with a pretreatment with NaOH solution and a pre-oxidation in air.Symmetric supercapacitors were fabricated with the resulting MC as electrode material.Their electrochemical performance was evaluated by a two-electrode configuration.
2 Experimental
2.1 Preparation and characterization of mesoporous carbon
The synthesis schematic of MC is described in Fig.1.NaOH,RH and deionized water were sufficiently mixed at a mass ratio of 1∶5∶12.5,the resulting mixture was heated to 110℃ and then kept for 24 h.Then the mixture was fully washed by deionized water and dried.The obtained material was grinded and mixed with a H3PO4solution(85 wt%)and deionized water at a mass ratio of 1∶2∶2.5,the mixture was first heated to 200℃ and kept for 6 h in air, and then activated at 800℃ for 1 h.After the product was washed with deionized water and dried at 110℃for 1 h,the MC was obtained.For a comparison,activated carbon(AC) was prepared by the same process except without the treatment of RH with NaOH solution.
X-ray fluorescence(XRF,EIGAKS,Japan) was used to characterize the contents of silicon in RH and the MC.The surface morphology and microstructure of the samples were investigated by field emission scanning electron microscopy(FE-SEM,S4800,Japan)and transmission electron microscopy(TEM, JEM-2100UHR,Japan).The pore structure characterization was performed on the basis of low temperature nitrogen adsorption-desorption isotherms on a sorptometer(Micromeritics,ASAP 2020,America).

Fig.1 A schematic illustration of MC synthesis from rice husk.
2.2 Preparation of electrodes and electrochemical measurements
The samples were mixed with acetylene black and polytetrafluoroethylene(PTFE)binder at a mass ratio of 85∶5∶10 in ethanol to form a slurry.The slurry was coated onto the nickel foam to make the electrodes,which was finally dried at 100℃ for 1 h under vacuum.Two symmetric electrodes separated by a thin porous polymer separator(Celgard 2400)in a 6 mol/L KOH aqueous electrolyte were sandwichedin a CR2032 coin cell.The electrochemical properties of supercapacitors were studied by cyclic voltammetry (CV)on a Princeton electrochemical workstation (PARSTAT 4000,Princeton,USA)and galvanostatic charge-discharge test on a Land cell tester(Land, CT-2001A,China).
3 Results and discussion
The content of silicon in RH was evaluated by X-ray fluorescence(XRF).The silicon content of raw RH is 6.65 wt% whilethesilicon content is 0.33 wt%for the NaOH treated one,indicating that the silicon can be effectively removed by the NaOH treatment.
The N2adsorption-desorption isotherms and pore size distributions of the samples are shown in Fig.2. Both of the nitrogen adsorption-desorption isotherms show clearly hysteresis loops,indicating the existence of abundant mesopores.The pore structure parameters of the samples are presented in Table 1.The SBETand mesoporosity of MC are 2 009 m2·g-1and 90.8%, respectively,much higher than those of AC,illustrating that the NaOH treatment benefits the formation of mesopores.

Fig.2 (a)N2adsorption-desorption isotherms and(b)pore size distributions of AC and MC.

Table 1 Pore structural parameters of MC and AC.
Fig.3(a)shows the SEM image of RH.Fig.3 (b)and Fig.3(c)are SEM and TEM images of the prepared MC,respectively.MC in Fig.3(b)has a cracked and pitted surface,indicating that RH has been seriously corroded by H3PO4.A great deal of mesopores centering at 2-10 nm can be seen in Fig.3 (c),which is consistent with averaged pores of 3.3 nm given in Table 1.

Fig.3 SEM images of(a)RH and(b)MC,(c)TEM image of MC.
Fig.4 gives the discharge curves of MC electrode at different current densities.The charge-discharge curve at the current density of 50 mA·g-1exhibits a symmetrical triangular shape and all discharge curves are nearly linear,implying a good capacitive behavior[15-17].
Fig.5 presents the CV curves of MC electrode at different scan rates in the 6 mol/L KOH aqueous electrolyte.The CV curves show rectangular shapes without redox peaks,denoting that the capacitancecomes from electric double layer capacitance.The CV curve keeps high rectangular degree even at high scan of 10 mV·s-1,which should be attributed to the high ion diffusion rate in mesopores[18-20].

Fig.4 Discharge curves of MC electrode at different current densities.
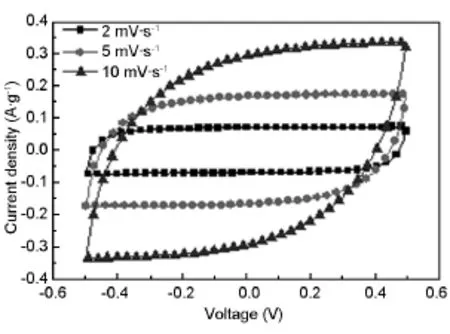
Fig.5 CV curves of MC electrode at different scan rates.
Fig.6 shows the variation in the specific capacitance vs.current density.It can be observed that the specific capacitance of MC can reach as high as 176 F·g-1at the current density of 50 mA·g-1,and retains 126 F·g-1even at 1 000 mA·g-1,indicating an excellent rate capability of the MC electrode.The high capacitive behavior of MC electrode is originated from its high mesoporosity and large specific surface area as shown in Table 1,both of which benefit the formation of double-layer capacitance.

Fig.6 Specific capacitance of MC electrode at different current densities.
Electrochemical cycling stability is well known a key requirement for the application of supercapacitors,the specific capacitance change of MC under 1 000 cycles at 200 mA·g-1is shown in Fig.7.It can be easily seen that MC electrode has a good cycle stability,it has a stable specific capacitance of about 150 F/g,no capacitance fading is observed even after 1 000 cycles,indicating a good electrochemical stability of MC electrode.

Fig.7 Specific capacitance of MC electrode at a constant current density of 200 mA·g-1as a function of cycle number.
4 Conclusions
MC with a specific surface area of 2 009 m2·g-1and a mesoporosity of 90.8%was prepared from RH by a facile and template-free method.This templatefree method combines a H3PO4activation with a pretreatment of RH with a hot NaOH solution and a preoxidation in air.The pretreatment of RH with a hot NaOH solution can remove silicon and damage the crystal structure of cellulose in RH,which are crucial and beneficial to the preparation of MC with a high electrochemical performance.The specific capacitance of MC electrode can reach 176 F·g-1at 50 mA·g-1, and the electrode exhibits an excellent rate capability and cycle stability.
[1] He X,Ling P,Yu M,et al.Rice husk-derived porous carbons with high capacitance by ZnCl2activation for supercapacitors[J].Electrochimica Acta,2013,105:635-641.
[2] Conway B E.Electrochemical Supercapacitors[M].New York: Kluwer-Plenum Pub.Co.,1999.
[3] DENG Mei-gen,WANG Ren-qing.The effect of the HClO4oxidization of petroleum coke on the properties of the resulting activated carbon for use in supercapacitors[J].New Carbon Materials,2013,28(4):262-266.
(邓梅根,王仁清.HClO4氧化对石油焦基活性炭超级电容器性能的影响[J].新型炭材料,2013,28(4):262-266.)
[4] Zhai D,Li B,Du H,et al.The preparation of graphene decorated with manganese dioxide nanoparticles by electrostatic adsorption for use in supercapacitors[J].Carbon,2012,50(14): 5034-5043.
[5] Wu M,Ai P,Tan M,et al.Synthesis of starch-derived mesoporous carbon for electric double layer capacitor[J].Chemical Engineering Journal,2014,245:166-172.
[6] He X,Ling P,Qiu J,et al.Efficient preparation of biomassbased mesoporous carbons for supercapacitors with both high energy density and high power density[J].Journal of Power Sources,2013,240(0):109-113.
[7] Tanaka S,Fujimoto H,Denayer J F M,et al.Surface modification of soft-templated ordered mesoporous carbon for electrochemical supercapacitors[J].Microporous and Mesoporous Materials,2015,217:141-149.
[8] Zhang Q,Li L,Wang Y L,et al.Uniform fibrous-structured hollow mesoporous carbon spheres for high-performance supercapacitor electrodes[J].Electrochimica Acta,2015,176:542-547.
[9] Kim K S,Park S J.Electrochemical performance of graphene/ carbon electrode contained well-balanced micro-and mesopores by activation-free method[J].Electrochimica Acta,2012,65: 50-56.
[10] TANG Li,ZHAN Liang,YANG Guang-zhi,et al.Preparation of mesoporous carbon microsphere/activated carbon composite for electric double-layer capacitors[J].New Carbon Materials, 2011,26(3):237-240.
(唐 丽,詹 亮,杨光智,等.双电层电容器用中孔炭微球活性炭复合电极的制备[J].新型炭材料,2011,26(3): 237-2407.)
[11] Zheng C,Qi L,Yoshio M,et al.Cooperation of micro-and meso-porous carbon electrode materials in electric double-layer capacitors[J].Journal of Power Sources,2010,195(13): 4406-4409.
[12] Sui W,Zheng J,Yang Z,et al.A simple method of preparing ordered and three-dimensionally interconnected macroporous carbon with mesoporosity by using silica template[J].Materials Letters,2011,65(15):2534-2536.
[13] Momĉiloviĉ M Z,Raneloviĉ M S,Zarubica A R,et al.SBA-15 templated mesoporous carbons for 2,4-dichlorophenoxyacetic acid removal[J].Chemical Engineering Journal,2013,220 (0):276-283.
[14] Xie X,Zhang C,Wu M B,et al.Porous MnO2for use in a high performance supercapacitor:replication of a 3D graphene network as a reactive template[J].Chemical Communications, 2013,49(94):11092-11094.
[15] Huang Q,Wang X,Li J,et al.Nickel hydroxide/activated carbon composite electrodes for electrochemical capacitors[J]. Journal of Power Sources,2007,164(1):425-429.
[16] Rakhi R B,Chen W,Cha D,et al.High performance supercapacitors using metal oxide anchored graphene nanosheet electrodes[J].Journal of Materials Chemistry,2011,21(40): 16197-16204.
[17] Wang W,Hao Q,Lei W,et al.Graphene/SnO2/polypyrrole ternary nanocomposites as supercapacitor electrode materials[J].RSC Advances,2012,2(27):10268-10274.
[18] Ma X,Liu M,Gan L,et al.Synthesis of micro-and mesoporous carbon spheres for supercapacitor electrode[J].Journal Solid State Electrochemistry,2013,17(8):2293-2301.
[19] Wang D W,Li F,Liu M,et al.3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage.Angewandte Chemie-International Edition,2008,47(2):373-376.
[20] Yan Y,Cheng Q,Pavlinek V,et al.Controlled synthesis of mesoporous carbon nanosheets and their enhanced supercapacitive performance[J].Journal of Solid State Electrochemistry, 2013,17(6):1677-1684.
Template-free preparation of mesoporous carbon from rice husks for use in supercapacitors
WU Ming-bo1, LI Ling-yan1,2, LIU Jun1, LI Yang1, AI Pei-pei1, WU Wen-ting1, ZHENG Jing-tang1
(1.State Key Laboratory of Heavy Oil Processing,China University of Petroleum,Qingdao 266580,China; 2.China Construction Installation Engineering Co.,Ltd,Nanjing 210046,China)
Mesoporous carbon(MC)was prepared from rice husk(RH)by a simple and template-free method which combines H3PO4activation with a pretreatment of the RH with a NaOH solution and pre-oxidation in air.The pretreatment of RH with NaOH removes silicon and damages the crystal structure of the cellulose in the RH,both of which are beneficial to the preparation of MC with a high surface area and high mesoporosity.The MC has a specific surface area of 2 009 m2·g-1and a mesoporosity of 90.8%. Its specific capacitance can reach 176 F·g-1at a current density of 50 mA·g-1,and a value of 126 F·g-1is retained at 1 000 mA·g-1,indicating an excellent rate capability.A MC electrode has a stable specific capacitance of about 150 F/g at 200 mA·g-1with no apparent capacitance fade after 1 000 cycles,indicating good electrochemical stability.
Rice husk;Mesoporous carbon;Alkali pretreatment;Supercapacitor
TQ127.1+1
A
国家自然科学基金(51172285,51372277,21302224);中央高校基本科研业务费专项资金(CX-1217,14CX06045A, 14CX02060A).
吴明铂,教授,博士.E-mail:wumb@upc.edu.cn.
1007-8827(2015)05-0471-05
10.1016/S1872-5805(15)60201-3
