AP与TP方案在EGFR-TKI耐药的非小细胞肺癌患者中的疗效比较
2015-03-13张洁霞李时悦占杨清周承志秦茵茵欧阳铭
张洁霞 蔡 迪 李时悦 占杨清 周承志 秦茵茵 欧阳铭
(广州医科大学附属第一医院广州呼吸疾病研究所 呼吸疾病国家重点实验室,广东 广州 510120)
·论著·
AP与TP方案在EGFR-TKI耐药的非小细胞肺癌患者中的疗效比较
张洁霞 蔡 迪 李时悦 占杨清 周承志 秦茵茵 欧阳铭*
(广州医科大学附属第一医院广州呼吸疾病研究所 呼吸疾病国家重点实验室,广东 广州 510120)
目的:探讨AP(培美曲塞+顺铂)和TP(多西紫杉醇+顺铂)方案对EGFR-TKIs耐药的晚期非小细胞肺癌(NSCLC)患者的疗效。方法:回顾性分析2009年2月至2010年3月广州医科大学附属第一医院收治的232例EGFR-TKIs耐药的晚期NSCLC患者的临床资料,根据化疗方案不同分为AP组(培美曲塞+顺铂)和TP组(多西紫杉醇+顺铂),每组各116例。根据RECIST标准评价近期疗效(RR、DCR)、总生存期(OS),无进展生存期(PFS)。结果:TP组和AP组有效率、自靶向药物进展后总生存期分别为34.5%和24.1%、12.4个月和12.0个月,两组比较,差异无统计学意义(P>0.05)。TP组和AP组PFS分别为8.0个月和6.2个月,两组比较,差异有统计学差异(P<0.01)。AP组中不吸烟与吸烟患者的总生存期、PFS分别为12.0和9.0个月、6.5和5.6个月,两者比较,差异有统计学意义(P<0.01)。两组吸烟与不吸烟患者疾病控制率比较,差异无统计学意义(P>0.05)。TP组中不吸烟与吸烟患者的总生存期分别为14和8.8个月,两者比较,差异无统计学意义(P=0.725);TP组中不吸烟与吸烟患者的PFS分别为8.0和6.0个月,两者比较,差异有统计学意义(P<0.01)。结论:AP和TP方案对EGFR-TKI靶向治疗耐药进展的晚期NSCLC患者疗效相似;吸烟者疗效较不吸烟者差。
非小细胞肺癌;表皮生长因子受体酪氨酸激酶抑制剂;耐药;培美曲塞;多西紫杉醇
肺癌的治疗目前已进入个体化时代,分子靶向药物给患者带来了新的希望。吉非替尼(商品名易瑞沙)对于非小细胞肺癌(non-mall-cell carcinoma,NSCLC)EGFR突变阳性患者的疗效较为确切,且起效时间迅速,但患者平均1~2年则会出现耐药,需要接受化疗。新型化疗药物(如培美曲塞、多西紫杉醇)的合理联用能显著改善患者生活质量,且耐受性良好。2009年2月至2010年3月本科对232例曾经口服EGFR-TKI有效的NSCLC患者采用AP(培美曲塞+顺铂)与TP(多西紫杉醇+顺铂)进行治疗,现报道如下。
1 资料与方法
1.1 一般资料
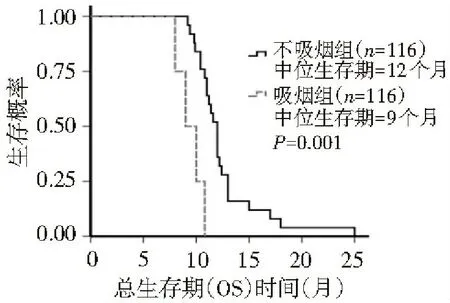
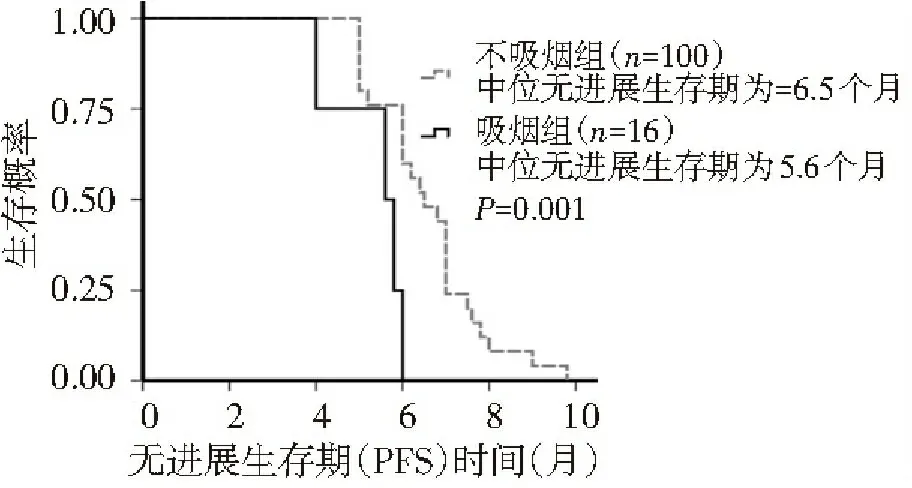
本组患者232例,根据化疗方案的不同分为AP组(培美曲塞+顺铂)和TP组(多西紫杉醇+顺铂),每组各116例。患者均经组织学或细胞学检查确诊为NSCLC;按国际抗癌联盟(UICC)标准分期为局部晚期至晚期(ⅢB~Ⅳ期);经EGFR-TKI治疗获缓解3个月以上又出现进展者[1,2];体力状况评分(ECOG 1.2 治疗方法 AP组给药方法:培美曲塞500 mg/m2、顺铂80 mg/m2,静脉滴注,21 d为1个周期。TP组给药方法:多西紫杉醇75 mg/m2、顺铂80 mg/m2,静脉滴注,21 d为1个周期。预防用药:在使用培美曲塞前1~2周开始给予叶酸和维生素B12预处理。每2个周期化疗结束后进行评价疗效,共4~6个疗程。 1.3 疗效及毒性评价标准 按照世界卫生组织(WHO)制定的RECIST疗效评价标准及抗癌药物不良反应评价标准,其中疗效分为完全缓解(CR)、部分缓解(PR)、稳定(SD)和进展(PD),CR+PR为总有效率(RR);CR+PR+SD为临床获益率(DCR);不良反应分为0~Ⅳ度。无疾病进展时间(PFS)是指化疗开始到肿瘤出现进展的时间。总生存期(OS)是指口服靶向药疾病进展起至死亡时间的中位数。 1.4 统计学分析 应用SPSS 20.0统计软件进行分析,计量资料以中位数表示,计量资料的比较使用非参数检验,生存期比较使用Log-rank检验法,并绘制Kaplan-Meier曲线,计数资料的比较采用χ2检验,P<0.05为差异有统计学意义。 2.1 近期疗效 AP组中PR 35例、SD 68例、PD 13例,有效率为34.5%;TP组中PR 28例、SD 68例、PD 20例,有效率为24.1%,两组比较,差异无统计学意义(P=0.301),见表1。AP组和TP组自靶向药物进展后总生存期分别为12.4个月和12.0个月,两组比较,差异无统计学意义(P=0.133),见图1。AP组和TP组PFS分别为8.0个月和6.2个月,两组比较,差异有统计学差异(P<0.01),见图2。 2.2 疗效与吸烟的关系 TP组中不吸烟与吸烟患者的总生存期、PFS分别为12.0和9.0个月、6.5和5.6个月,两者比较,差异有统计学意义(P<0.01),见表2,图3,4。TP组吸烟患者疾病控制率为75%,低于不吸烟患者的84%,AP组吸烟患者疾病控制率为85%,低于不吸烟患者的93%,但两者比较,差异无统计学意义(P>0.05),见表2。AP组中不吸烟与吸烟患者的总生存期分别为14和8.8个月,两者比较,差异无统计学意义(P=0.725);TP组中不吸烟与吸烟患者的PFS分别为8.0和6.0个月,两者比较,差异有统计学意义(P<0.01),见图5、6。 表1 AP组与TP组患者的一般资料比较 图1 多西紫杉醇+顺铂组与培美曲塞+顺铂组总生存期(OS)比较 图2 多西紫杉醇+顺铂组与培美曲塞+顺铂组无进展生存期(PFS)比较 表2 吸烟与不吸烟患者非水细胞肿癌的临床特征比较 AP组临床特征不吸烟(100例)吸烟(16例)P值TP组临床特征不吸烟(96例)吸烟(20例)P值年龄(岁)58(51~64)71.5(65.5~77.75)0.039性别(男/女)44/648/80.751性别(男/女)32/6415/50.455总生存期(月)12.0(11.7~12.3)9(8.0~9.9)<0.001总生存期(月)14(12.5~15.4)8.8(8.4~9.2)0.725无进展生存期(月)6.5(6.0~7.0)5.6(4.7~6.4)<0.001无进展生存期(月)8.0(7.4~8.6)6.0(5.7~6.2)<0.001PR(例)2440.931PR(例)3230.104SD(例)6080.451SD(例)57140.375PD(例)1640.376PD(例)730.250RR(%)0.240.250.931RR(%)0.330.180.104DCR(%)0.840.750.376DCR(%)0.930.850.250 图3 多西紫杉醇+顺铂吸烟组与多西紫杉醇+顺铂不吸烟组总生存期比较 图4 多西紫杉醇+顺铂吸烟组与多西紫杉醇+顺铂不吸烟组无进展生存期比较 图5 培美曲塞+顺铂吸烟组与培美曲塞+顺铂不吸烟组总生存期比较 图6 培美曲塞+顺铂吸烟组与培美曲塞+顺铂不吸烟组无进展生存期比较 大量研究结果证实EGFR-TKI在EGFR突变人群一线治疗中显示出较高的肿瘤反应率和长PFS[3-8]。2010年ASCO报道EGFR突变患者接受EGFR-TKI治疗的有效率为66%,而接受EGFR-TKI+化疗者为69%,由此可见,对于EGFR突变患者,在TKI基础上加用化疗并不能改善有效率,PFS和OS亦无改善。同步化疗和EGFR-TKI尚缺乏充足证据。一个Ⅱ期研究显示联合EGFR-TKI和化疗能改善PFS,但仍需Ⅲ期研究。 培美曲塞是一个新的多靶点抗叶酸剂,它通过干扰细胞复制过程中叶酸依赖性代谢过程而发挥作用。2002年,澳大利亚学者Clarke应用培美曲塞治疗59例首次采用化疗治疗NSCLC患者,其中体力状况评分为2分的患者占32%(19例),结果显示约16%的患者达PR,中位缓解期为4.9个月,中位生存期为7.2个月[9]。培美曲塞联合顺铂是晚期非小细胞肺癌一线标准治疗[10],且低TS酶者预测培美曲塞的疗效好[11]。“培美曲塞联合顺铂对比吉西他滨和顺铂在初治肺癌”和另一个“对比培美曲塞对安慰剂维持治疗”两个随机研究分析了上千例高龄患者,结果显示,培美曲塞可使年轻和老年患者同样获益,剂量强度和毒性均可接受[12]。Patrick[13]报道1例患者在接受过厄罗替尼、多西紫杉醇化疗后病情进展,且ECOG评分为3分时接受14个疗程3线单药培美曲塞化疗能够获益且疗效持续20个月。 多西紫杉醇通过使细胞在有丝分裂过程中不能形成正常的有丝分裂纺锤体,从而抑制了细胞的分裂和增殖。2000年,Shepherd等[14]通过一个随机Ⅲ期临床试验比较多西紫杉醇单药与最佳支持治疗,结果显示,多西紫杉醇能明显提高患者生存期(6.8个月和4.8个月)和体能状态评分。38.8%TKI继发抗药患者进行EGFR突变检测,其中28.4%为T790M突变;12.3%行c-met拷贝数;7.1%行c-met扩增,3组T790M和c-met扩增差异无统计学意义。尽管临床上没有比通过T790M突变的获得性抗药更惰性的过程。EGFR突变患者EGFR-TKI 获得性耐药的主要机制HGF、c-met、IGFR、HER3和50%~60%是T790M[15-19]。针对这些靶点的药物有afatinib单独或联合西妥昔单抗、c-met单抗OAM4558g、ARQ197等。 针对EGFR靶向治疗耐药问题,现行策略主要有以下几点:(1)继续原分子靶向药物治疗;(2)靶向联合单抗或化疗,易瑞沙联合紫杉醇有一定疗效,但很难判定是化疗还是联合的协同作用;(3)多靶点药物连用;多靶点酪氨酸激酶抑制剂能从不同环节抑制肿瘤细胞生长和微环境的形成。 本研究中AP组和TP组自靶向药物进展后总生存期分别为12.4个月和12.0个月,两组比较,差异无统计学意义,但均较既往单纯化疗的总生存期(8~10个月)长,可能与对TKI敏感的肿瘤对化疗也相对敏感有关,故生存期和疾病进展时间较长。本研究中TP组中不吸烟与吸烟患者的总生存期、PFS分别为12.0和9.0个月、6.5和5.6个月,两者比较,差异有统计学意义。AP组中不吸烟与吸烟患者的总生存期分别为14和8.8个月,两者比较,差异无统计学意义;TP组中不吸烟与吸烟患者的PFS分别为8.0和6.0个月,两者比较,差异有统计学意义。何卫国等[20]的研究表明P53蛋白和mRNA基因高表达可以预测非小细胞肺癌的耐药性。吸烟者疗效较不吸烟者差,可能与吸烟患者存在TP53突变有关,且突变与吸烟量有关[20,21]。总之,培美曲塞及多西紫杉醇含铂双药对EGFR-TKI耐药的NSCLC患者有一定疗效,可推迟疾病进展,延长中位生存期,提高1年生存率。 [1] Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with non-small-cell lung cancer[J]. Cancer, 2010,116(5):1336-1343. [2] Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer[J]. J ClinOncol,2010,28(2):357-360. [3] Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor under lying responsiveness of non-small-cell lung cancer to gefitinib[J]. N Engl J Med,2004,350(21):2129-2139. [4] Mok TS, Wu YL, Thongprasert S, et al. Gefitinibor carboplatin-paclitaxel in pulmonary adenocarcinoma[J]. N Engl J Med,2009,361(10):947-957. [5] Han JY, Park K, Kim SW, et al. First-SIGNAL:firstlinesingle-agent iressa versus gemcitabine and cisplatin trial in never-smokerswith adenocarcinoma of the lung[J]. J ClinOncol,2012,30(10):1122-1128. [6] Maemondo M, Inoue A, Kobayashi K, et al. Gefitinibor chemotherapy for non-small-cell lung cancer with mutated EGFR[J]. N EnglJ Med,2010,362(25):2380-2388. [7] Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinibversus cisplatin plus docetaxel in patients with non-small-cell lung cancerharbouring mutations of the epidermal growth factor receptor (WJTOG3405):an open label, randomised phase 3 trial[J]. Lancet Oncol,2010,11(2):121-128. [8] Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positivenon-small-cell lung cancer (OPTIMAL, CTONG-0802):a multicentre, openlabel,randomised, phase 3 study[J]. Lancet Oncol,2011,12(8):735-742. [9] Clarke SJ,Abratt R,Goedhals L,et al.Phase II trial ofpemetrexeddisodium(ALIMTA,LY23 1 514)in chemotherapynaive patients with advanced non-smallcelllung cancer[J].Ann Oncol,2002,13(5):737-741. [10] Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatinplusgemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients withadvanced-stage non-small-cell lung cancer[J]. J ClinOncol,2008, 26(21):3543-3545. [11] Gomez HL, Santillana SL, Vallejos CS, et al. A phase II trial of pemetrexedinadvanced breast cancer:clinical response and association with molecular targetexpression[J]. Clin Cancer Res,2006, 12(3ptl):832-838. [12] Gridelli C, Brodowicz T, Langer CJ. Pemetrexed therapy in elderly patients with good performance status:analysis of two phase III trials of patients with nonsquamous non-small-cell lung cancer[J]. Clin Lung Cancer,2012,13(5):340-346. [13] Manson GV,MaPC. Response to Pemetrexed Chemotherapy in Lung adenocarcinoma-Bronchioloalveolar Carcinoma.Insensitive to Erlotinib[J] . Clinical Lung Cancer, 2010,11(1):57-60. [14] Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer:distinct natural history of patients with tumors harboring the T790M mutation[J].Clin Cancer Res,2011,17(6):1616-1622. [15] Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib[J]. N Engl J Med, 2005, 352(8):786-792. [16] Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain[J]. PLoS Med, 2005,2(3):73. [17] Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling[J]. Science, 2007, 316(5827):1039-1043. [18] Cappuzzo F, Janne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients[J].Ann Oncol,2009, 20(2) 298-304. [19] Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib[J]. Clin Cancer Res, 2007, 13(9):2795-2803. [20] 何卫国,马 军,张梅春,等.P53表达预测非小细胞肺癌化疗的耐药性及养肺解毒方的干预治疗[J].中华生物医学工程杂志,2010,16(3):210-213. [21] Hainaut P, Pfeifer GP. Patterns of p53 G->T transversions in lung cancers reflect the primary mutagenic signature of DNA-damage by tobacco smoke[J]. Carcinogenesis, 2001, 22(3):367-374. [22] Subramanian J, Govindan R.Molecular profile of lung cancer in never smokers[J].EJC Suppl,2013, 11(2):248-253. (本文编辑:欧阳菁) Effect of AP protocol versus TP protocol in non-small-cell lung cancer patients with EGFR-TKI resistance ZhangJiexia,CaiDi,LiShiyue,ZhanYangqing,ZhouChengzhi,QinYinyin,OuyangMing (GuangzhouInstituteofRespiratoryDiseases,FirstAffiliatedHospitalofGuangzhouMedicalUniversity,StateKeyLaboratoryofRespiratoryDisease,Guangzhou510120,China) Objective:To investigate the effect of AP (pemetrexed+cisplatin) protocol versus TP (docetaxel+cisplatin) protocol in advanced non-small-cell lung cancer (NSCLC) patients with EGFR-TKI resistance.Methods:The clinical data of 232 advanced NSCLC patients with EGFR-TKI resistance hospitalized in First Affiliated Hospital of Guangzhou Medical University between February 2009 and March 2010 were retrospectively analyzed. Based on different protocols of chemotherapy, all patients were divided into two groups (n=116 each):the AP(premetrex+cisplatin) group and TP (docetaxel+cisplatin) group. The short-term efficacy [response rate (RR) and disease control rate (DCR)], overall survival (OS), progression free survival (PFS) were evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Results:The RR and OS after the targeted drug developed in TP group and AP group were 34.5% vs. 24.1% and 12.4 months vs. 12.0 months, respectively. And there were no statistically significant differences between the two groups (P>0.05). The PFS in TP group and AP group was 8.0 months vs. 6.2 months, with statistically significant difference (P<0.01). The OS and PFS in non-smokers and smokers of the AP group were 12.0 months vs. 9.0 months and 6.5 months vs. 5.6 months, respectively, with statistically significant differences between the two groups (P<0.01). No statistically significant difference was found in DCR between smokers and non-smokers of the two groups (P>0.05). The OS in non-smokers and smokers of the TP group was 14 months vs. 8.8 months, respectively, with statistically significant difference (P=0.725); while the PFS in non-smokers and smokers of the TP group was 8.0 months vs. 6.0 months, respectively, with statistically significant difference(P<0.01).Conclusion:The effect of AP protocol and TP protocol in advanced NSCLC patients was similar in the development of EGFR-TKI resistance in targeted therapy. And the efficacy in smokers was worse than non-smokers. non-small-cell lung cancer; epidermal growth factor receptor tyrosine kinase inhibitor; drug resistance; pemetrexed; docetaxel 10.3969/j.issn.2095-9664.2015.01.002 张洁霞(1971-),女,博士,主任医师。 R734.2 A 2095-9664(2015)01-0004-05 2014-04-09) 研究方向:肺癌、乳腺癌等肿瘤的基础与临床研究。 *通讯作者:E-mail:Ouyang1135@126.com。2 结 果





3 讨 论
