UV-B辐射增强对拟南芥表皮蜡质的影响
2015-03-11李加纳
倪 郁,宋 超,李加纳
西南大学农学与生物科技学院,南方山地农业教育部工程技术中心,重庆 400716
UV-B辐射增强对拟南芥表皮蜡质的影响
倪 郁,宋 超,李加纳*
西南大学农学与生物科技学院,南方山地农业教育部工程技术中心,重庆 400716
以野生型拟南芥、蜡质不同程度缺失突变体CER1、CER3、CER4、CER6、CER10、CER20及KCS1为试验材料,通过施加50 μW/cm2、长达10 d的UV-B辐射,研究了拟南芥表皮蜡质晶体结构、组分及蜡质基因对UV-B辐射的响应机制。结果表明:UV-B辐射增强改变了拟南芥表皮蜡质晶体结构,表皮蜡质松针状(CER1)、柱状、杆状(CER3、CER10与KCS1)晶体结构显著减少,球状蜡质晶体类型出现在CER6表面,无规则片状、膜状结构覆盖在KCS1与CER10茎表面。野生型拟南芥蜡质晶体结构类型无明显变化,但在部分区域积累了大量水平杆状、管状结构,增加了蜡质层厚度。UV-B辐射增强也改变了拟南芥表皮蜡质组分的分泌量。野生型在UV-B处理后一级醇、酸、醛含量显著上升,烷、次级醇及酮含量显著下降,蜡质总量增加不显著。一级醇含量的增加及酮和次级醇含量的减少在拟南芥各材料响应UV-B辐射中具有普遍性。UV-B辐射增强诱导了野生型CER3、CER4、KCS1基因表达的上调,其中CER4大量表达,促进了蜡质组分中一级醇、酸和醛含量的积累;CER1在UV-B处理后表达量下调,可能导致烷合成下游分支途径相关产物(烷类、次级醇及酮类)的减少。WIN1表达量的下调对蜡质总量没有显著影响。UV-B辐射增强使蜡质前体从烷合成分支途径更多地转向一级醇分支途径。
拟南芥;表皮蜡质;基因;UV-B辐射;蜡质突变体
大气臭氧层减薄导致的紫外线B(UV-B,280—320 nm)辐射增强是当今人类面临的全球性重大环境问题之一[1- 4]。研究UV-B 辐射增强对植物的伤害作用以及植物响应UV-B辐射的防御与修复机制,对于促进UV-B辐射环境下植物的正常生长发育以及作物的高产、稳产均具有重要的理论意义。植物叶片形态特征的变化是植物对环境胁迫的防御反应之一。前人研究表明,植物叶表皮毛的结构与密度、蜡质层及表皮细胞层的厚度均影响紫外线辐射能否穿透到叶肉细胞[5];蜡质含量高的植物叶片比含量低的叶片能吸收更多的紫外线[6]。表皮蜡质主要由可溶性的超长链脂肪酸、烷烃、一级醇、二级醇、脂肪醛、酮类和酯类组成。前人对UV-B辐射与植物表皮蜡质互作研究主要集中在对表皮蜡质组分及含量的化学分析上[7- 11],而对表皮蜡质响应UV-B辐射变化的分子机制了解甚少。拟南芥作为研究遗传学和分子生物学的一种模式植物,具有典型的角质层结构及组份。因此,拟南芥在植物角质层的生态学特性研究上也应该是个模式系统。在包括拟南芥在内的大多数植物中,角质层蜡生物合成主要依靠蜡质前体超长链脂肪酸(very long chain fatty acids, VLCFAs)通过脱羰基途径(烷合成途径)与酰基还原途径(一级醇合成途径)进行[12]。在拟南芥蜡质合成途径中,一些蜡质相关基因陆续被克隆,如蜡质前体VLCFAs合成基因KCS1[13]、催化生成一级醇的脂肪酰-CoA还原酶基因CER4[14]、醛类合成相关基因CER3[15]、烷类合成基因CER1[16,17]及表皮蜡质过量表达转录因子WIN1[18]等。这些蜡质基因的分离鉴定为揭示蜡质组分在植物中的功能奠定了分子基础。本试验以野生型拟南芥以及蜡质不同程度缺失突变体为试验材料,研究UV-B辐射增强条件下表皮蜡质含量、组分及蜡质晶体结构的变化规律;分析UV-B辐射诱导表达的主要蜡质基因、特异性蜡质组分,为深入揭示植物表皮蜡质与UV-B辐射互作机制积累基础资料。
1 材料与方法
1.1 试验材料
哥伦比亚野生型(Columbia- 0 ecotype) 拟南芥种子来源于西南大学植物生理生化研究室,其余表皮蜡质突变体CER1、CER3、CER4、CER6、CER10、CER20及KCS1种子均购于拟南芥资源中心(ABRC,USA)。突变体特征如表1所示。蛭石、珍珠岩和有机土按1∶1∶1的比例混匀,装入花盆(7 cm×8 cm),将低温春化后的种子播在花盆中,每盆4 株,然后用塑料薄膜覆盖5 d以利于出苗。试验材料放置在人工气候培养箱中培养,温度为21 ℃/23 ℃(黑夜/白天),光照每天16 h,空气湿度为75%。待植株生长至5—6 周时进行UV-B胁迫处理。
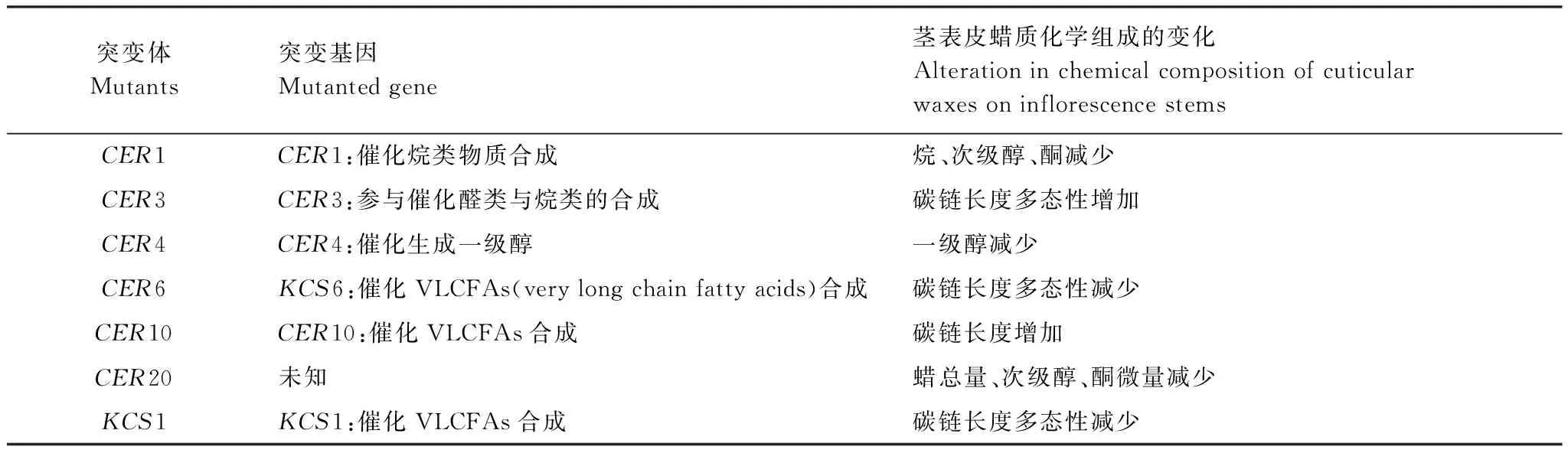
表1 拟南芥表皮蜡质突变体特征Table 1 Characteristics of the Arabidopsis thaliana cuticular wax mutants
1.2 UV-B辐射处理
在植物培养室内使用日光灯管提供正常光照,使用Philips 公司的UV-B灯管(TL30W/03,波峰308 nm)进行UV-B辐射处理,UV-B灯管用0.13 mm厚度的醋酸纤维素膜滤除少量UV-C,每隔3 d更换1 次。UV-B 处理的拟南芥植株每天10:00—12:00 增施UV-B辐射,辐射强度50 μW/cm2。通过调节紫外灯管与材料间的高度控制紫外辐射强度。紫外辐射剂量用UV- 340A紫外光照度计(Lutron, 台湾) 测定。连续处理10 d后,采集茎秆用于表皮蜡质提取、电镜扫描分析及基因表达检测。每种材料不同处理各设3 个重复。
1.3 表皮蜡质形态结构观察
采用扫描电子显微镜(S3000-N,Hitachi)观察蜡质晶体结构。将植株茎秆分成1 cm长小段,间隔贴于电镜载物台上,进行离子溅射镀金膜,镀金后材料进行电镜扫描观察、拍照。
1.4 表皮蜡质含量和组分分析
利用气相色谱-质谱联用仪(GCMS-QP2010 plus)测定分析蜡质组成与含量。采集植株茎杆放入含有10 mL氯仿的试管中浸提30 s,以提取表面蜡质,样品中加入C16烷作为内部标准。氮吹仪吹干浸提液,在100 ℃下用80 μL BSTFA衍生20 min,再经氮气吹干后,溶于200 μL正己烷中,进行色谱-质谱分析。程序升温方式:初温80 ℃,保持2 min;每分钟15 ℃升温至260 ℃,保持10 min。然后每分钟5 ℃升温至320 ℃,保持5 min。基于FID峰值量化蜡质,根据内部标准物(C16烷)的浓度计算各蜡质组分含量。蜡质含量用单位面积上的微克数(μg/cm2)进行表示。茎秆面积测定采用数字化扫描仪(EPSON V750)和WinFOLIA专业叶片图像分析系统(Regent Instrument Inc, Canada)进行分析并记录。
1.5 qRT-PCR分析
1.5.1 样品RNA的提取与cDNA合成
采用Trizol试剂盒(invitrogen)分别提取对照与UV-B处理野生型拟南芥茎秆总RNA,DNase I(宝生物工程有限公司)消化去除基因组DNA。样品mRNA的反转录依照北京全式金生物技术有限公司的TransScript First-Strand cDNA Synthesis SuperMix反转录试剂盒进行。
1.5.2 qPCR 分析
qPCR反应在Mx3000P荧光定量PCR仪(StrataGene, USA)上进行。反应体系按照iTaq Universal SYBR Green Supermix试剂盒(BIO-RAD)说明配制,设3 次重复。待分析的蜡质基因有:参与烷类物质合成的CER1、参与醛类物质合成的CER3、催化VLCFAs还原生成一级醇的脂肪酰-CoA 还原酶基因CER4、催化VLCFAs合成的第一步缩合反应的β-酮脂酰-COA合酶KCS1、控制表皮蜡质积累的转录因子WIN1,Actin7为内参基因。本实验所使用引物序列如表2所示。数据收集和处理在MXPro v4.10软件(StrataGene, USA)中进行。UV-B胁迫下蜡质基因表达水平的变化以相对表达量表示,即UV-B胁迫下蜡质基因的表达量与对照植株蜡质基因表达量的比值。

表2 qPCR相关引物Table 2 Sequences of the primers used in qPCR
1.6 数据分析
采用SPSS13.0软件分析UV-B处理对拟南芥茎表皮蜡质含量、蜡质基因表达量的影响。显著水平为P<0.05(LSD检验)。
2 结果与分析
2.1 UV-B辐射增强对拟南芥蜡质晶体结构的影响

野生型拟南芥茎蜡质结构主要由垂直柱状结构与水平片状结构组成,此外还存在少量杆状、管状、和伞状结构。与野生型相比较,各蜡质突变体总体上表现出蜡质晶体分布密度减少,晶体结构的形状与大小发生改变。UV-B辐射处理后,拟南芥各材料表现出不同程度的茎干与莲座叶枯黄现象,其中突变体材料受UV-B辐射影响普遍较野生型严重。扫描电镜显示(图1,图2),CER1在受到UV-B辐射处理后,松针状晶体结构减少并显著变短。CER3、CER10与KCS1在UV-B辐射处理后柱状、杆状晶体结构显著减少;CER10局部出现边界不清的“无定型”膜状结构覆盖茎表面;而KCS1则出现许多水平无规则片层结构。CER20蜡质晶体体积减小,但数量增加。UV-B辐射处理对一级醇减少突变体CER4结构无显著影响,处理前后均以垂直片状结构为主。CER6在UV-B辐射处理后蜡质晶体形状发生变化,一些小型球状蜡质晶体出现。UV-B辐射处理后,野生型拟南芥局部区域积累了大量水平杆状、管状结构。

图1 增强UV-B辐射下拟南芥野生型(WT)和蜡质突变体(CER1、CER3、CER4)茎表皮蜡质晶体结构的变化(10 μm)Fig.1 Effects of enhanced UV-B radiation on crystalloid structures of epicuticular wax on stem in Arabidopsis thaliana wild type (WT) and mutants (CER1, CER3, CER4)

图2 增强UV-B辐射下拟南芥突变体(CER6、CER10、CER20、KCS1)茎表皮蜡质晶体结构的变化(10 μm)Fig.2 Effects of enhanced UV-B radiation on crystalloid structures of cuticular wax on stem in Arabidopsis thaliana mutants(CER6, CER10, CER20, KCS1)
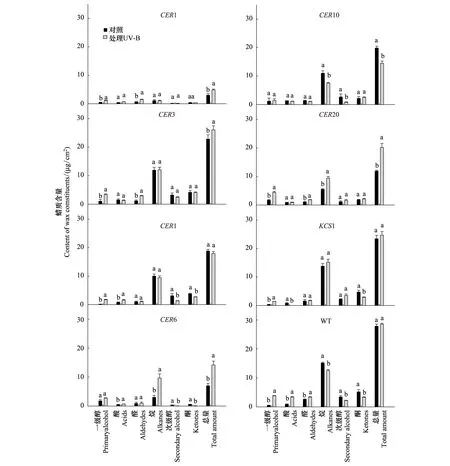
图3 增强的UV-B辐射对拟南芥野生型和蜡质突变体茎表皮蜡质含量的影响Fig.3 Effects of enhanced UV-B radiation on contents of wax constituents and total wax in stems of Arabidopsis wild type and mutants
2.2 UV-B辐射增强对拟南芥表皮蜡质组分含量的影响
拟南芥各材料经UV-B处理(图3),一级醇含量显著上升(CER6、CER10增加不显著),醛类与酸类含量增加或无显著变化(KCS1酸类含量显著减少),次级醇、酮含量下降或无显著变化,烷及蜡总量在不同材料中变化不一致。野生型在UV-B处理后一级醇、酸、醛含量显著上升,烷、次级醇及酮含量显著下降,蜡质总量无显著变化(图3)。
2.3 蜡质合成相关基因表达对UV-B辐射的响应
本试验对蜡质前体VLCFAs合成基因KCS1、催化生成一级醇的脂肪酰-CoA还原酶基因CER4、醛类合成相关基因CER3、烷类合成基因CER1、及表皮蜡质过量表达转录因子WIN1对UV-B辐射的响应进行了调查。结果表明,这5个蜡质基因在野生型拟南芥中均有表达。在增强的UV-B辐射下,蜡质相关基因CER3、CER4、KCS1表达量上调,其中CER4表达量是对照植株的13.64倍。而UV-B处理植株的CER1与WIN1表达量下调,分别是对照植株的0.09倍和0.01倍(图4)。

图4 增强的UV-B辐射对野生型拟南芥相关蜡质基因表达的影响Fig.4 Effects of enhanced UV-B radiation on the transcript of wax-related gene in Arabidopsis wild type
3 讨论
强紫外线辐射下,植物采取一系列防御措施以适应环境条件的改变,如减少叶面积、节间长度及生物量[19];叶片表面玻璃化[20];合成大量类黄酮类物质以提高对紫外线的吸收能力等[21- 22]。植物表皮蜡质在减少紫外线辐射伤害方面也发挥着积极作用,如玉米蜡质缺失突变体出现了比野生型更多的卷叶及DNA损伤现象[6,23]。各拟南芥材料经UV-B处理后表现出不同程度的茎干与莲座叶枯黄现象,其中蜡质突变体材料枯黄、萎蔫程度大于野生型拟南芥。
前人研究报道,叶表皮蜡质晶体结构不同,其叶片对光的反射率也不尽相同,如水平片状蜡质结构比垂直片状结构及光滑表面有更强的反射率[24]。本实验中UV-B辐射处理除了对CER4与CER20蜡质晶体结构无显著影响外,其余突变体材料蜡质晶体结构均发生了一定的变化。例如,UV-B辐射处理后,表皮蜡质松针状(CER1)、柱状、杆状(CER3、CER10与KCS1)晶体结构显著减少;CER10与KCS1不同程度出现无规则膜状、片状结构覆盖在茎表面等。野生型拟南芥蜡质结构类型虽然无明显变化,但在部分区域积累了大量水平杆状、管状结构,增加了蜡质层厚度。紫外线辐射下,植物蜡质层厚度是影响紫外线辐射到达叶肉细胞的因素之一[5]。UV-B辐射诱导的拟南芥蜡质晶体结构变化反映了植物的一种主动调节适应过程。
蜡质总量对UV-B辐射的响应在不同植物中是不一样的。UV-B处理条件下大麦(Hordeumvulgare)、黄瓜(Cucumissativus)等蜡质含量增加[25],云杉(Piceaasperata)蜡质含量变化不显著[10],烟草(Nicotianatabacum)蜡质含量降低[26]。UV-B处理后的不同材料间蜡质总量总体上呈现出不变或增加趋势(CER10蜡质总量在UV-B处理后下降)。蜡质组分中一级醇含量在UV-B处理后普遍上升,酮和次级醇含量普遍下降,暗示了拟南芥植株可通过改变特定蜡质组分的分泌量来响应UV-B胁迫。在增强的UV-B辐射下,挪威云杉叶表蜡质中二十九烷含量显著增加,烷基酯含量显著下降[10];黄瓜子叶的主要蜡质组分——烷和一级醇的种类和含量发生改变[11]。
拟南芥角质层蜡生物合成主要依靠蜡质前体VLCFAs通过一级醇分支途径与烷合成分支途径进行[12]。野生型拟南芥qRT-PCR结果表明,CER3、CER4、KCS1在UV-B辐射处理后表达量上调,增强的UV-B辐射诱导了CER4基因的大量表达。这3个基因的表达上调解释了蜡质组分中一级醇、酸和醛含量在受到UV-B胁迫后的显著增加。另外一方面,CER1与WIN1在UV-B辐射处理后表达量下调。CER1催化长链醛到烷的转化,其表达量的降低可能会导致烷合成下游分支途径相关产物的减少及醛的积累。野生型拟南芥在受到UV-B胁迫后烷类、次级醇及酮类的显著下降证明了这一点。WIN1作为表皮蜡质过量表达时的转录因子,其表达量的降低将影响蜡质总量的积累。但在本实验中,野生型拟南芥蜡质总量无显著变化,说明蜡质数量的积累受蜡质合成途径中上、下游相关蜡质基因的共同作用。综合分析蜡质组分与基因表达结果表明,增强UV-B辐射使蜡质前体从烷合成分支途径更多地转向了一级醇分支途径。
[1] Caldwell M M, Bornman J F, Ballaré C L, Flint S D, Kulandaivelu G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochemical and Photobiological Sciences, 2007, 6(3): 252- 266.
[2] Mckenzie R L, Aucamp P J, Bais A F, Björn L O, Ilyas M. Changes in biologically-active ultraviolet radiation reaching the earth′s surface. Photochemical and Photobiological Sciences, 2007, 6(3): 218- 231.
[3] Shindell D T, Rind D, Lonergan P. Increased polar stratospheric ozone losses and delayed eventual recovery owing to increasing greenhouse-gas concentrations. Nature, 1998, 392(6676): 589- 592.
[4] Jansen M A K, Gaba V, Greenberg B M. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends in Plant Science, 1998, 3(4): 131- 135.
[5] Karabourniotis G, Papadopoulos K, Papamarkou M, Manetas Y. Ulrraviolet-B radiation absorbing capacity of leaf hairs. Physiologia Plantarum, 1992, 86(3): 414- 418.
[6] Long L M, Patel H P, Cory W C, Stapleton A E. The maize epicuticular wax layer provides UV protection. Functional Plant Biology, 2003, 30(1): 75-81.
[7] Gonzalez R, Paul N D, Percy K, Ambrose M, McLaughlin C K, Barnes J D, Areses M, Wellburn A R. Responses to ultraviolet-B radiation (280- 315 nm) of pea (Pisumsativum) lines differing in leaf surface wax. Physiologia Plantarum, 1996, 98(4): 852-860.
[8] Pilon J J, Lambers H, Baas W, Tosserams M, Rozema J, Atkin O K. Leaf waxes of slow-growing alpine and fast-growing lowlandPoaspecies: inherent differences and responses to UV-B radiation. Phytochemistry, 1999, 50(4): 571- 580.
[9] Holmes M G, Keiller D R. Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wave bands: a comparison of a range of species. Plant Cell and Environment, 2002, 25(1): 85- 93.
[10] Gordon D C, Percy K E, Riding R T. Effects of UV-B radiation on epicuticular wax production and chemical composition of four Picea species. New Phytologist, 1998, 138(3): 441- 449.
[11] Fukuda S, Satoh A, Kasahara H, Matsuyama H, Takeuchi Y. Effects of ultraviolet-B irradiation on the cuticular wax of cucumber (Cucumissativus) cotyledons. Journal of Plant Research, 2008, 121(2): 179- 189.
[12] Millar A A, Clemens S, Zachgo S, Giblin M, Taylor D C, Kunst L.CUT1, anArabidopsisgene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. The Plant Cell, 1999, 11(5): 825-838.
[13] Todd J, Post-Beittenmiller D, Jaworski J G. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis inArabidopsisthaliana. The Plant Journal, 1999, 17(2): 119- 130.
[14] Rowland O, Zheng H, Hepworth S R, Lam P, Jetter R, Kunst L.CER4 encodes an alcohol-forming fatty acyl-coenzyme a reductase involved in cuticular wax production inArabidopsis. Plant Physiology, 2006, 142(3): 866-877.
[15] Chen X B, Goodwin S M, Boroff V L, Liu X L, Jenks M A. Cloning and characterization of theWAX2 gene ofArabidopsisinvolved in cuticle membrane and wax production. The Plant Cell, 2003, 15(5): 1170- 1185.
[16] Bourdenx B, Bernard A, Domergue F, Pascal S, Leger A, Roby D, Pervent M, Vile D, Haslam R P, Napier J A, Lessire R, Joubes J. Overexpression ofArabidopsisECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiology, 2011, 156(1): 29- 45.
[17] Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure J D, Haslam R, Napier J, Lessire R, Joubès J. Reconstitution of plant alkane biosynthesis in yeast demonstrates thatArabidopsisECERIFERUM1 andECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. The Plant Cell, 2012, 24(7): 3106- 3118.
[18] Broun P, Poindexter P, Osborne E, Jiang C, Riechmann J L. WIN1, a transcriptional activator of epidermal wax accumulation inArabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(13): 4706- 4711.
[19] Nogués S, Allen D J, Morison J I L, Baker N R. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiology, 1998, 117(1): 173- 181.
[20] Braun J, Tevini M. Regulation of UV-protective pigment synthesis in the eipermal layer of rye seedings (Secale cereale L. cv. Kustro). Photochemistry and Photobiology, 1993, 57(2): 318- 323.
[21] Huttunen S, Lappalainen N M, Turnen J. UV-absorbing compounds in subarctic herbarium bryophytes. Environmental Pollution, 2005, 133(2): 303- 314.
[22] Murai Y, Takemura S, Takeda K, Kitajima J, Iwashina T. Altitudinal variation of UV-absorbing compounds inPlantagoasiatica. Biochemical Systematics and Ecology, 2009, 37(4): 378- 384.
[23] Sturaro M, Hartings H, Schmelzer E, Velasco R, Salamini F, Motto M. Cloning and characterization ofGLOSSY1, a maize gene involved in cuticle membrane and wax production. Plant Physiology, 2005, 138(1): 478- 489.
[24] Grant R H, Heisler G M, Gao W, Jenks M. Ultraviolet leaf reflectance of common urban trees and the prediction of reflectance from leaf surface characteristics. Agricultural and Forest Meteorology, 2003, 120(1): 127- 139.
[25] Steinmüller D, Tevini M. Action of ultraviolet radiation (UV-B) upon cuticular waxes in some crop plants. Planta, 1985, 164(4): 557- 564.
[26] Barnes J D, Percy K E, Paul N D, Jones P, McLaughlin C K, Mullineaux P M, Creissen G, Wellburn A R. The influence of UV-B radiation on the physicochemical nature of tobacco (NicotianatabacumL) leaf surfaces. Journal of Experimental Botany, 1996, 47(1): 99- 109.
Effect of enhanced ultraviolet-B radiation on epicuticular wax inArabidopsisthaliana
NI Yu, SONG Chao, LI Jiana*
CollegeofAgronomyandBiotechnology,SouthwestUniversity,EngineeringResearchCenterofSouthUplandAgriculture,MinistryofEducation,Chongqing400716,China
Due to the depletion of stratospheric ozone (O3), the quantity of UV-B radiation reached the earth′s surface has increased significantly. Enhanced UV-B radiation influenced plant growth, development and productivity by damaging DNA, RNA, and proteins. Plants are thought to employ a variety of UV-B protective mechanisms, including accumulation of a range of secondary metabolites and adaptive changes in plant morphological features. As the outer surface of aerial plant tissues, the epicuticular wax is believed to play an important role in protecting plants against ultraviolet-B radiation. Epicuticular wax was composed primarily of saturated free fatty acids, aldehydes, alkanes, primary alcohols, secondary alcohols, ketones, and wax esters.Arabidopsis, as a model system to study the genetics and the molecular biology, has the typical structure and composition of cuticle, and it should be a model system to study the ecology of epicuticular waxes. However, little is known as to the responsees of different wax constituents to enhanced UV-B radiation, and the role of epicuticular waxes in providing protection forArabidopsisfrom UV-B has not been examined either. Therefore, in the current study, seven wax mutants (CER1,CER3,CER4,CER6,CER10,CER20 andKCS1) and wild type ofA.thalianawere selected to analyze the responses of epicuticular wax in crystalloid structure, content and constituents, and wax related gene to UV-B radiation (50 μW/cm2) . The plants accepted UV-B radiation 2 h every day for consecutive 10 days. The results showed that enhanced UV-B radiation altered the crystalloid structure of epicuticular wax onArabidopsisstems. Under the enhanced UV-B radiation, amounts of pine needle-shaped crystalloids (CER1), columnar-shaped crystalloids and rods (CER3、CER10 andKCS1) decreased significantly. Some small globular-shaped crystalloids appeared on the surface ofCER6 mutant. Some irregular plate- and membrane-shaped structures covered the surface ofKCS1 andCER10. No significant change of crystalloid types was observed on the stem of theArabidopsiswild type; however, many horizontal rod and tube crystalloids accumulated in specific zones, which increased the thickness of cuticle. Enhanced UV-B radiation also altered the secretion quantities of wax constituents. Under UV-B radiation, the contents of primary alcohols, fatty acids and aldhydes inArabidopsiswild type increased significantly, the contents of alkanes, secondary alcohols and ketones decreased significantly, while the contents of total wax changed insignificantly. Under UV-B radiation, the increase of primary alcohols and the decrease of secondary alcohols and ketones were universal inArabidopsismutants. Enhanced UV-B radiation upregulated the expression ofCER3,CER4 andKCS1, which promoting the accumulation of primary alcohols, fatty acids and aldehydes. The decreased expression ofCER1 under UV-B radiation might lead to the reduction of products from alkane-synthesizing branch of the pathway, including alkanes, secondary alcohols and ketones. The decreased expression ofWIN1 under UV-B radiation had no effect on the content of total wax, implying that the accumulation of total wax was a result of comprehensive effects of multi genes involved in wax synthesis pathway. Enhanced UV-B radiation shunted wax precursors away from the alkane-synthesizing branch to primary alcohol branch of the pathway.
Arabidopsisthaliana; epicuticular wax; gene; UV-B radiation; wax mutants
国家自然科学基金(31000122, 31270450); 重庆市自然科学基金(cstc2012jjA80022); 教育部作物资源利用创新引智基地(B12006)
2013- 05- 07;
日期:2014- 04- 17
10.5846/stxb201305070972
*通讯作者Corresponding author.E-mail: ljn1950@swu.edu.cn
倪郁,宋超,李加纳.UV-B辐射增强对拟南芥表皮蜡质的影响.生态学报,2015,35(5):1505- 1512.
Ni Y, Song C, Li J N.Effect of enhanced ultraviolet-B radiation on epicuticular wax inArabidopsisthaliana.ActaEcologicaSinica,2015,35(5):1505- 1512.
