两种沉水植物对间隙水磷浓度的影响
2015-03-10王立志
王立志
山东省水土保持与环境保育重点实验室,临沂大学,临沂 276000
两种沉水植物对间隙水磷浓度的影响
王立志*
山东省水土保持与环境保育重点实验室,临沂大学,临沂 276000
为研究两种根系特征的沉水植物在生长过程中对间隙水中磷浓度的影响,选取根系较多的沉水植物苦草和根系相对较少的沉水植物黑藻作为实验材料,监测底泥中间隙水各形态磷含量及环境因子的变化,探讨不同根系特征沉水植物对间隙水中磷的影响。结果表明:黑藻和苦草实验组沉积物间隙水中各形态磷的浓度均呈不同程度的降低,黑藻和苦草对于稳定水质,减少底泥中磷向水中转移具有明显的效果;沉水植物不同,底泥间隙水中溶解性总磷(DTP)和溶解性活性磷(SRP)存在明显差异。实验结束时黑藻组和苦草组间隙水中DTP的浓度分别为0.24,0.01 mg/L,SRP的浓度分别为0.22 mg/L,0.004 mg/L。间隙水中磷的形态主要以DTP和SRP为主,溶解性有机磷(DOP)的含量相对较低。沉水植物对间隙水中磷的吸收是降低间隙水中磷含量的重要原因,苦草的吸收能力大于黑藻。沉水植物根系通过降低底泥pH值,提高氧化还原电位(Eh)的方式抑制了底泥中磷的释放。
沉水植物;根系;磷;间隙水
沉水植物占据着浅水水体生态系统的关键界面,以自身的形态特征、群落结构特征及生理活动影响着其周围的环境,对水体磷循环具有十分重要的影响[1]。近百年来,湖泊富营养化现象在世界范围内普遍发生,而磷是湖泊富营养化的重要控制因子[2]。大量研究和实践表明,治理浅水湖泊,仅依靠削减外源负荷的措施,经常未能取得预期的降低湖水磷浓度的效果[3- 4],外源消减后,沉积物中的营养盐将释放出来,抵消外源负荷的消减,沉积物对湖泊生态环境的影响与其间隙水密切相关[5],间隙水中氮、磷的含量直接影响沉积物与上覆水之间氮和磷的交换,间隙水中可溶态营养物质氮、磷穿过水-泥界面向上覆水传送是沉积物中营养盐释放的重要途径[6- 7]。沉水植物生长过程中通过根系和茎叶吸收间隙水和水中的营养物质,从而影响氮磷等营养盐的循环过程,因此开展不同沉水植物对间隙水磷的影响显得尤为重要,鉴于此,本研究通过选取根系较少的沉水植物黑藻和根系相对较发达的沉水植物苦草作为实验材料,研究两种沉水植物在生长过程中对间隙水中磷的影响,为湖泊富营养化治理提供理论依据。
1 实验材料与设计
1.1 材料与设计
底质采自富营养化水华爆发水体,采集后样品低温风干后过100目筛,去除粗粒及动植物残体,然后充分混匀。将混匀后的底质加入高密度聚乙烯桶(顶直径×底直径×高=55 cm×45 cm×75 cm,预先经过5% 的HCl处理后用蒸馏水冲洗干净),底质平均厚度为10 cm,底质干重为4821.00 g。然后缓慢注入蒸馏水100 L。
按照植物的顶冠特征和根系状况的不同,分别选取根系较少沉水植物黑藻和根系相对较发达的沉水植物苦草作为实验植物,以说明沉水植物生长期不同根系特征对底泥间隙水磷浓度的影响。
实验设置实验桶装置总计9桶,其中沉水植物苦草(30 g 鲜重)和黑藻(30 g鲜重)休眠芽分别种植3桶,另外3桶不种植沉水植物作为对照组。沉水植物黑藻和苦草采用性状均一的休眠芽,均匀种植于实验桶。实验时间为2012年5—9月。
实验在温室玻璃房内进行,实验温室内部月平均光照及温度变化情况如图1所示。
实验期间水温按照室外的温度控制在5—25 ℃,各桶间水温差异小于2 ℃。将底质均匀铺设于实验桶底后,缓慢注入蒸馏水,待实验装置稳定10 d后,均匀种植沉水植物,并采集水样进行测定,同时采用探头测定底质物理指标,上覆水及底泥初始理化指标如表1所示。由于底质中氮磷等营养物质在稳定期间存在向水中的释放过程,因此,水中氮磷的含量分别为1.25和0.05 mg/L。
1.2 取样与分析
水样采集采用虹吸管抽取的方式,采集水面以下5 cm、20 cm和45 cm处的等体积水(50 mL)混匀。底泥采用微型柱状采泥器采集,底泥样品每次均匀采集5个微型柱状样(横切面直径2 cm),采集后的样品室温(25 ℃)风干,然后对风干前后的样品称重以计算由采样带来的总体磷和水量的损耗。将风干后底泥样品与植物根系分离,过100目筛后充分混匀,然后进行底泥中各形态磷分析。间隙水采用自行研制的原位渗滤器进行采集,将原位渗滤器在实验种植植物之前埋置均匀与底泥中,在需要采集时打开渗滤器进行间隙水渗滤,并收集间隙水,用0.45μ的醋酸纤维素滤膜过滤采集到的间隙水,采用钼锑抗比色法直接测定磷含量得到SRP的浓度,将滤液采用过硫酸钾消解法测定磷的含量得到DTP的浓度,DOP浓度为DTP和SRP之差。底泥样品磷分析采用国际通用的SMT法[8]。上覆水中磷浓度按照钼锑抗比色法分析。所有样品分析采用意大利连续流动分析仪FLOWSYS III 完成。
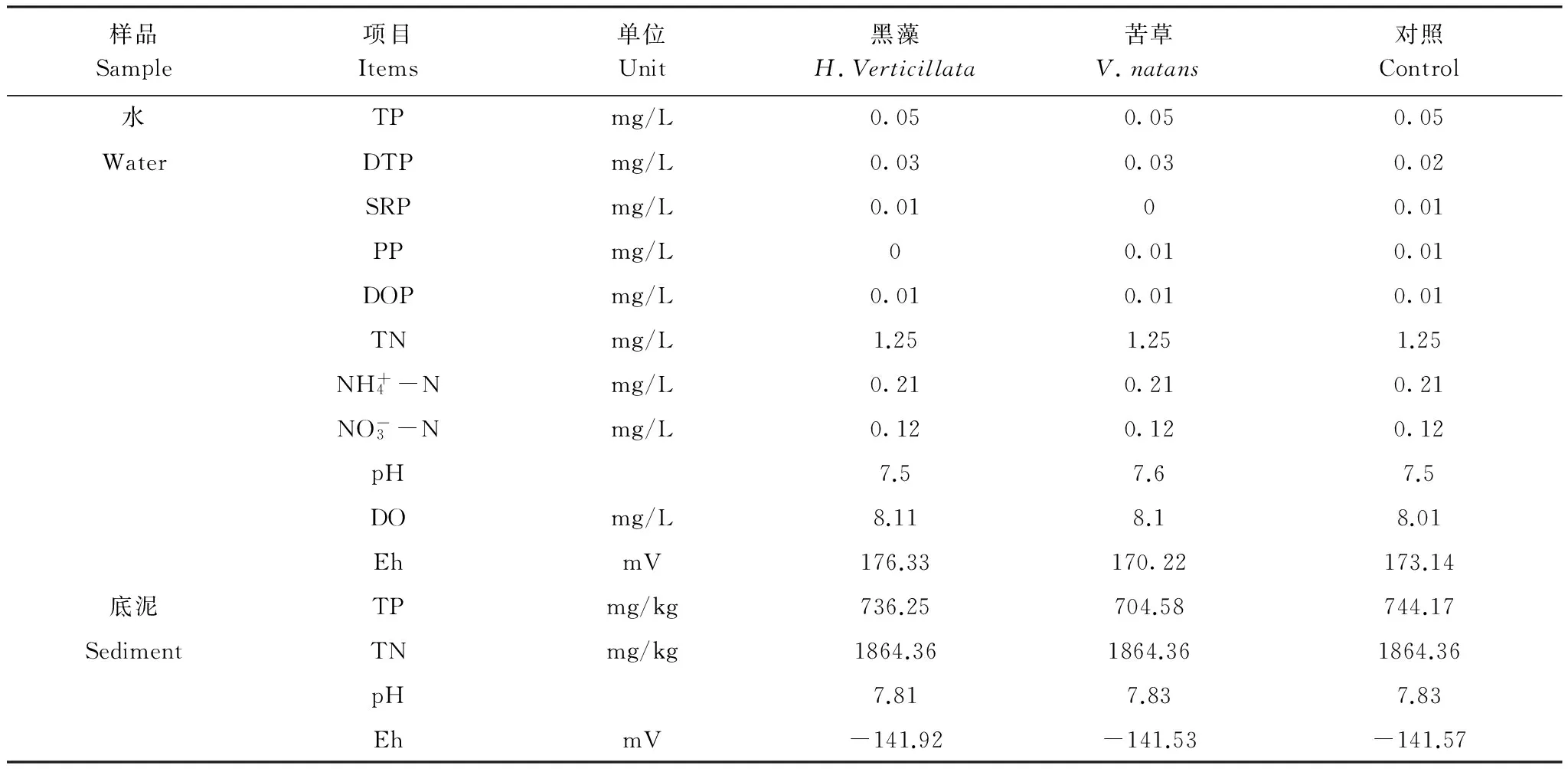
表1 底泥和上覆水初始理化指标Table 1 Physical and chemic items of sediment and water

1.3 生物量统计
另外选取不同生长期沉水植物,统计黑藻不同生长阶段的株高(X1/cm)和生物量(鲜重,W/g),生物量统计采用整株挖出的方法,将植物根系的底泥小心冲洗干净后,采用吸水纸吸收植物表面残留水分,吸干后进行称量。苦草统计叶片长度(X1/cm)、叶片宽度(X2/cm)和生物量(W/g),苦草生物量的测定方法与黑藻一致。建立不同植物的生物量模型,以推算沉水植物在生长过程中的生物量。
不同沉水植物生长期的生物量模型为:
W黑藻=0.0198X1+0.5479R2=0.86P<0.05
W苦草=0.0230X1+0.2029 X2-0.2576R2=0.76P<0.05
1.4 数据处理
实验所得数据采用SPSS16.0统计软件进行方差分析,处理组和对照组之间采用单因素方差分析法,P<0.05为差异性显著,P<0.01为差异性极显著。
2 实验结果
2.1 间隙水形态磷含量的变化
实验期间黑藻和苦草组间隙水中各形态磷的浓度均呈不同程度的降低。DTP和SRP的浓度在实验期间总体呈下降趋势,在实验第120天黑藻组间隙水中DTP和SRP的浓度均呈上升趋势,浓度分别为0.24和0.22 mg/L(图2)。方差分析表明实验期间黑藻组间隙水中DTP和SRP的浓度显著低于对照组(P<0.05)。黑藻组间隙水中DOP在整个实验期间呈下降趋势,但是方差分析表明黑藻组和对照组之间DOP浓度无显著差异(P>0.05)。
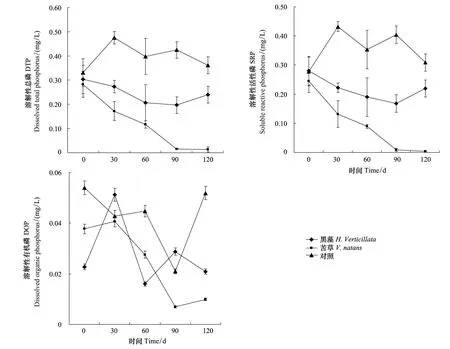
图2 实验期间间隙水中各形态磷的含量Fig.2 Phosphorus concentrations in pore water during the experiment course
苦草组间隙水中DTP和SRP的浓度在实验0至120天均呈下降趋势,在实验第120天达到最低值分别是0.01和0.004 mg/L(图2)。方差分析表明实验期间苦草组间隙水中DTP和SRP的浓度显著低于对照组(P<0.05)。苦草组间隙水中DOP在整个实验期间呈下降趋势,但是方差分析表明苦草组和对照组之间DOP浓度无显著差异(P>0.05)。
黑藻、苦草和对照组间隙水中DTP、SRP和DOP的浓度大小表明,间隙水中磷的形态主要以DTP和SRP为主,DOP的含量相对较低。
2.2 底泥及水中磷含量的变化
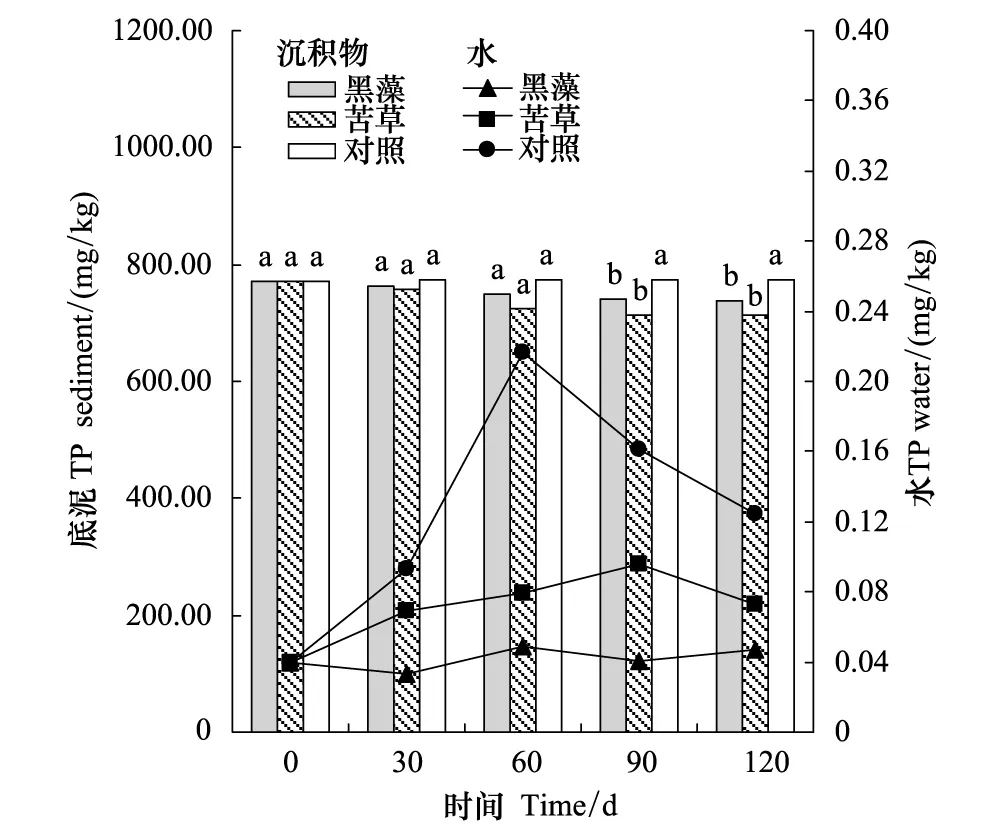
图3 实验期间底泥和水中磷含量变化(不同字符标准表示差异性显著(P < 0.05))Fig.3 Phosphorus concentrations in water and sediment during the experiment course,different letters above bars indicate a significant difference between treatments (P<0.05)
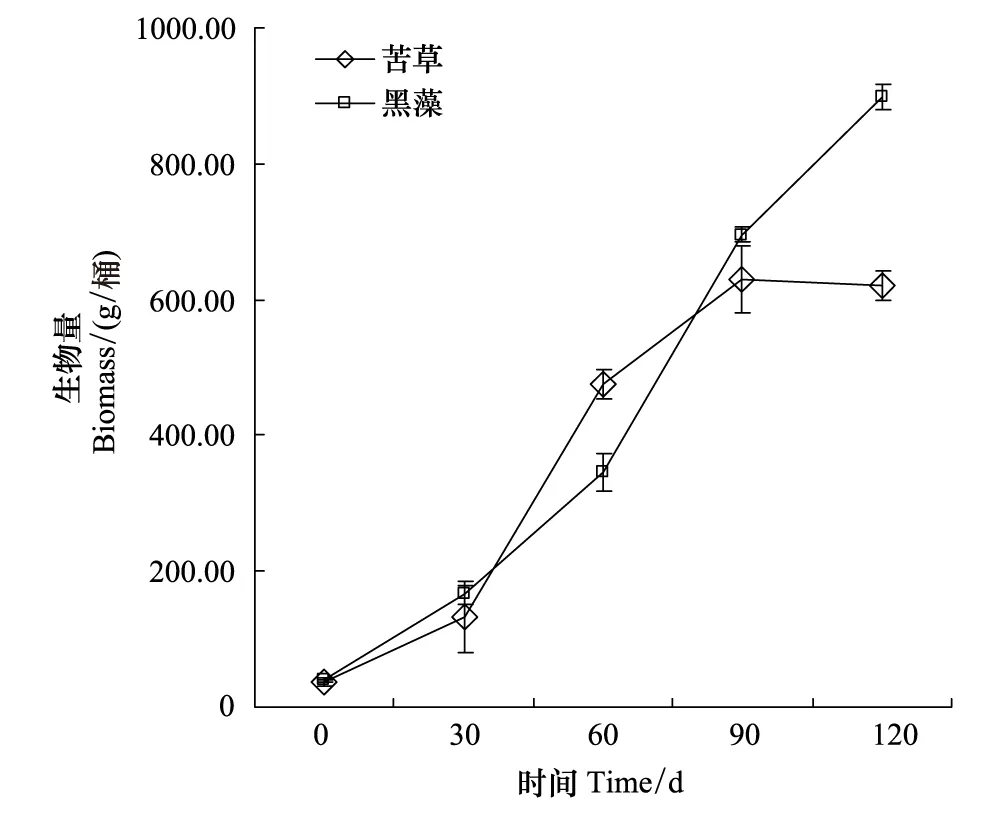
图4 实验期间每实验桶植物生物量变化Fig.4 Biomass changes in the experiment bucket
实验结果表明沉水植物组底泥中各形态磷的含量均呈现不同程度的降低,其中苦草组各形态磷含量降低幅度均较大。黑藻和苦草底泥TP的含量最大降低幅度分别为35.34、60.67 mg/kg(图3)。
苦草组水中磷的浓度在实验期间保持在相对较低的水平(0.04—0.10 mg/L)。从对照组水中磷含量呈先升高后下降的趋势中可以看出实验组水中磷浓度的上升与底泥中较高的磷含量有关,在实验开始时配水中磷含量较低,导致了底泥向水体中磷的释放,从而使得水体中磷的含量呈偏升高趋势。黑藻组水体中磷浓度总体保持在相对较稳定的水平,磷浓度在0.03—0.05 mg/L之间波动。
3 讨论
3.1 沉水植物生长对间隙水磷浓度的影响
在实验中实验第30 天后黑藻和苦草进入旺盛生长期(图4)。沉水植物在旺盛生长期对营养盐的需求量大,沉水植物在生长、繁殖过程中,吸收水体及底泥中氮、磷等营养物质,其中底质吸收是植物组织矿质营养的主要来源[9]。沉水植物可以直接从水体或底泥中吸收 N、P,然后分配到枝条。实验结束时,采集沉水植物植物样本进行植物体内磷含量分析(将烘干后植物进行粉碎,分析样品为植物茎叶和根系的混合样),然后将植物干重乘植物体内的磷含量,获得植物富集的磷总磷,试验结束时黑藻和苦草分别聚集的磷总量为,159.07和249.06 mg。在本实验系统中,处于一个相对封闭的环境,没有外源磷的输入,因此沉水植物体内富集的磷只来自于水和底泥。这是沉水植物降低上覆水和间隙水中磷的浓度的一个重要原因[10]。从本实验结果看根系发达的沉水植物苦草对间隙水中磷的吸收能力要大于黑藻,同时,沉水植物对间隙水磷的吸收主要作用于DTP和SRP。
沉水植物的生长对间隙水中磷的浓度具有重要的影响[11],因此,将实验桶中生物量和间隙水中各形态磷含量进行函数拟合,可以反映沉水植物生长过程对间隙水中磷的影响。黑藻生物量和间隙水中各形态磷的函数拟合表明,生物量的变化和间隙水中DTP和SRP的变化呈显著相关,与DOP含量的变化呈若相关(图5)。DTP和SRP随着的生物量的变化呈先降低后升高的抛物线趋势变化,说明黑藻在快速生长期能快速降低间隙水中DTP和SRP的含量,但是,当黑藻生物量达到稳定阶段时间隙水中DTP和SRP的含量有一定的反弹,呈上升趋势。函数模拟推算当生物量达到0.5614和0.5478 kg时间隙水中DTP和SRP的含量分别达到最低值。
苦草生物量的变化和间隙水中DTP和SRP的变化呈显著相关,间隙水中各形态磷含量随着生物量呈指数降低(图5)。在苦草生物量最大时间隙水中各形态磷含量并未出现如黑藻组的反弹现象,这和苦草对底泥间隙水各形态磷较高的吸收效率及对底泥环境的影响有重要关系。较高的吸收效率使得间隙水中各形态磷含量在实验后期均保持在较低的水平,苦草在生长过程中对底泥环境因子的改变同时也抑制了底泥向间隙水中磷的释放过程,因此根系较为发达的沉水植物苦草能使得间隙水中磷含量保持在较低的水平。
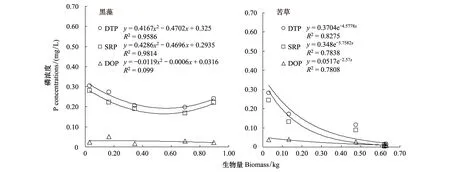
图5 实验组生物量和间隙水磷含量之间函数模拟Fig.5 Function simulation between biomass and phosphorus concentrations in pore water DTP: 溶解性总磷; SRP: 溶解性活性磷; DOP: 溶解性有机磷
3.2 底泥环境因子的变化对间隙水磷浓度的影响
影响底泥磷释放的因素很多[12],包括生物的(细菌活动、生物扰动等)、化学的[氧化还原电位(Eh)、pH值、溶解氧(DO)、铁结合态磷含量比等]以及物理的(风浪扰动等)等因素[13]。研究表明,DO、pH值、Eh、温度及水动力条件等是影响底泥中磷的释放与吸收的主要因素[14]。
在本实验条件下,由于是室内培养实验,对温度和水动力均做了限制,因此底泥中环境因子pH值和Eh的变化是影响底泥磷释放的主要因子。

pH值是水质的重要指标,它对水体物理化学反应有重要影响。碱性条件下,pH值升高时底泥磷释放增加;在中性范围内,释磷量最小;酸性条件下促使磷的释放。温度对水体磷的循环也会产生一定的影响。研究表明[7],随温度的升高,底泥磷释放增加。
在本实验中,黑藻和苦草组底泥pH值在实验期间均低于对照组,且苦草组底泥pH值的降低幅度要大于黑藻组,因此黑藻和苦草均能降低沉积物的pH值,但是各植物组pH值的变化范围在7—8之间(图6),因此,沉积物pH值保持在中性范围之内,底泥磷释放量最小。
苦草组沉积物Eh在实验第30天就显著低于黑藻组和对照组(P<0.05),并在后期实验中呈上升趋势(图6),所以,苦草组沉积物中较高的Eh抑制了底泥磷的释放是间隙水中磷浓度较低另外一个重要原因。黑藻组底泥Eh较对照组虽然有升高趋势,但在实验大部分时间均无显著差异(P>0.05),因此,黑藻底泥中较低的Eh导致了底泥中磷的释放,是间隙水中磷浓度相对偏高的另外一个重要原因。

图6 实验期间环境因子的变化(不同字符标准表示差异性显著P < 0.05)Fig.6 Environmental factor changes in the experiment,different letters above bars indicate a significant difference between treatments (P < 0.05)
4 结论
沉水植物黑藻和苦草对于稳定水质,减少底泥中的磷向水体转移有明显的效果,沉水植物不同,底泥间隙水中DTP和SRP存在明显差异。沉水植物黑藻和苦草在生长期均能降低间隙水中磷的浓度,苦草对间隙水中DTP和SRP的降低能力要大于黑藻,实验第120天黑藻和苦草组间隙水中DTP和SRP的含量均显著降低,黑藻和苦草对DOP具有一定的降低作用,但和对照组相比无显著差异。
沉水植物黑藻和苦草在生长期均能降低底泥中磷的含量,并降低底泥pH值,提高底泥Eh,苦草对底泥Eh的提高能力要大于黑藻。
[1] Søndergaard M, Phillips G, Hellsten S, Kolada A, Ecke F, Mäemets H, Mjelde M, Azzella M M, Oggioni l. Maximum growing depth of submerged macrophytes in European lakes. Hydrobiologia, 2013, 704(1): 165- 177.
[2] Wang S G, Jin X C, Pang Y, Zhao H C, Zhou X N, Wu F C. Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of shallow lakes in the middle and lower reaches of Yangtze River region, China. Journal of Colloid and Interface Science, 2005, 289(2): 339- 346.
[3] Van Nes E H, Scheffer M, van den Berg M S, Coops H. Charisma: a spatial explicit simulation model of submerged macrophytes. Ecological Modelling, 2003, 159(2/3): 103- 116.
[4] Ye C, Yu H C, Kong H N, Song X F, Zou G Y, Xu Q J, Liu J. Community collocation of four submerged macrophytes on two kinds of sediments in Lake Taihu, China. Ecological Engineering, 2009, 35(11): 1656- 1663.
[5] Wang F, Liang L L, Zhang Y S, Gao R H. Eco-hydrological model and critical conditions of hydrology of the wetland of Erdos Larus Relictus Nature Reserve. Acta Ecologica Sinica, 2009, 29(5): 307- 313.
[6] Sun S J, Huang S L, Sun X M, Wen W. Phosphorus fractions and its release in the sediments of Haihe River, China. Journal of Environmental Sciences. 2009, 21(3): 291- 295.
[7] Haygarth P M, Condron L M, Heathwaite A L, Turner B L, Harris G P. The phosphorus transfer continuum: Linking source to impact with an interdisciplinary and multi-scaled approach. Science of the total environment. 2005, 344(1/3): 5- 14.
[8] Ruban V, López-Sánchez J F, Pardo P, Rauret G, Muntau H, Quevauviller P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments -A synthesis of recent works. Fresenius Journal of Analytical Chemistry, 2001, 370(2/3): 224- 228.
[9] Schorer A, Schneider S, Melzer A. The importance of submerged macrophytes as indicators for the nutrient concentration in a small stream (Rotbach, Bavaria). Limnologica - Ecology and Management of Inland Waters, 2000, 30(4): 351- 358.
[10] Horppila J, Nurminen L. Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland). Water Research, 2003, 37(18): 4468- 4474.
[11] Sorrell B K, Downes M T, Stanger C L. Methanotrophic bacteria and their activity on submerged aquatic macrophytes. Aquatic Botany, 2002, 72(2): 107- 119.
[12] Wang S R, Jin X C, Bu Q Y, Jiao L X, Wu F C. Effects of dissolved oxygen supply level on phosphorus release from lake sediments. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 316(1/3): 245- 252.
[13] Asaeda T, Trung V K, Manatunge J. Modeling the effects of macrophyte growth and decomposition on the nutrient budget in Shallow Lakes. Aquatic Botany, 2000, 68(3): 217- 237.
[14] Jin X C, Wang S R, Pang Y, Wu F C. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environmental Pollution, 2006, 139(2): 288- 295.
[15] Wang S R, Jin X C, Zhao H C, Wu F C. Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 273(1/3): 109- 116.
Influence of two submerged macrophytes on pore water phosphorus concentration
WANG Lizhi*
ShandongProvincialKeyLaboratoryofSoilConservationandEnvironmentalProtection,LinyiUniversity,Linyi276000,China
Submerged macrophytes play an important role in nutrient cycling, especially in shallow lakes. Submerged macrophytes can acquire significant amounts of nutrients from the water via shoots and from the sediment via roots. In most natural situations, root uptake is the primary pathway for nutrients, because the absorbable nutrient concentrations are much higher in the sediment than in the water column. However, submerged macrophyte species vary in their root traits. Some submerged macrophytes, such asVallisnerianatans, have large root systems, while other species, such asHydrillaverticillata, grow only a few roots per plant. Phosphorus (P) is the most critical nutrient limiting lake productivity, and while submerged macrophytes play an important role in P cycling, little is known about the effects of different submerged macrophyte species on the behavior of P. Therefore, studies of the effects of different root characters of submerged macrophytes on P concentrations are important for understanding lake ecosystems. The purpose of this work was to identify how two different typical submerged macrophytes,H.verticillataandV.natans, affect the behavior of sediment P. We examined P concentrations and environmental factors in aquatic systems growing each of these plant species from May to September, 2012. During that time, we collected samples of sediment pore water, sediment, and column water on days 0, 30, 60, 90, and 120 of the experiment to determine P concentrations. The environmental factors of pH and redox potential (Eh) of the sediment were also measured. The results indicated that P concentrations in pore water of theH.verticillataandV.natanstreatments were lower than that of the control group. BothH.verticillataandV.natanshad obvious effects on water stabilization and reducing P release from sediment. Pore water concentrations of dissolved total P (DTP) and soluble reactive P (SRP) in theH.verticillataandV.natansgroups were significantly different, with 0.24 mg/L DTP and 0.22 mg/L SRP in theH.verticillatatreatment and 0.01 mg/L DTP and 0.004 mg/L SRP in theV.natanstreatment. P concentration increased after the 90thday of the experiment in theH.verticillatagroup, but it remained at a low level in theV.natansgroup. The pH was lower in theH.verticillatatreatment than in theV.natanstreatment, while Eh was higher in theH.verticillatagroup than in theV.natansgroup, which might explain why P levels fluctuated differently in the two treatment groups. The main P fractions in pore water were DTP and SRP, while the amount of dissolved organic P (DOP) was relatively low. The submerged macrophytes reduced the P concentration in water, sediment, and pore water during their growth periods. Their absorption of pore water P was one of the main reasons for the decreased P levels.Vallisnerianatanscould absorb more P thanH.verticillata. BothH.verticillataandV.natanscould reduce the DOP concentration, but there was no significant difference between the two submerged macrophyte species. There was no significant difference between treatments and control groups in DOP, indicating thatH.verticillataandV.natansmainly absorbed DTP and SRP. Submerged macrophyte inhibited the release of P from the sediment into the water column by decreasing the pH and increasing the Eh of the sediment. Overall, the submerged macrophytesH.verticillataandV.natanssignificantly stabilized water quality and reduced the release of P from the sediment to the water.
submerged macrophyte; root system; phosphorus; pore water
国家自然科学基金项目(41303061);山东省科技攻关项目(2011GGH21704,2013GSF11701);临沂市重大科技创新项目(201211027),山东省水土保持与环境保育重点实验室开发基金(stkf201206)
2013- 05- 01;
日期:2014- 04- 11
10.5846/stxb201305010879
*通讯作者Corresponding author.E-mail: wanglizhi@lyu.edu.cnHT
王立志.两种沉水植物对间隙水磷浓度的影响.生态学报,2015,35(4):1051- 1058.
Wang L Z.Influence of two submerged macrophytes on pore water phosphorus concentration.Acta Ecologica Sinica,2015,35(4):1051- 1058.
