THE EFFECTS OF TEMPERATURE ON EGG LAYING, EGG HATCHING AND LARVAL DEVELOPMENT OF DACTYLOGYRUS VASTATOR
2015-03-01ZHANGXiaoPingSHANGBaoDiWANGGuiTangLIWenXiangYANGXingandLIZhengYou
ZHANG Xiao-Ping, SHANG Bao-Di, WANG Gui-Tang, LI Wen-Xiang, YANG Xingand LI Zheng-You
(1. Guizhou Fisheries Research Institute, Guiyang 550025, China; 2. Institute of Hydrobiology,
Chinese Academy of Sciences, Wuhan 430072, China)
THE EFFECTS OF TEMPERATURE ON EGG LAYING, EGG HATCHING AND LARVAL DEVELOPMENT OF DACTYLOGYRUS VASTATOR
ZHANG Xiao-Ping1, SHANG Bao-Di1, WANG Gui-Tang2, LI Wen-Xiang2, YANG Xing1and LI Zheng-You1
(1. Guizhou Fisheries Research Institute, Guiyang 550025, China; 2. Institute of Hydrobiology,
Chinese Academy of Sciences, Wuhan 430072, China)
The effects of water temperature on egg laying and hatching in vitro were investigated, and egg laying and development in vivo at 20℃ were also characterised. The results indicated a positive correlation between mean egg production and temperature in vitro, and the mean egg production was 5.9, 9.1, 9.2 and 13.4 eggs/worm at 10, 20, 30 and 35℃, respectively. Except 4℃, majority of the eggs were laid during the first 5h. However, egg laying of the mature worms was continuous and uniform, with a mean 6.5 eggs/worm at 20℃ per day. Although the hatching time and the duration of hatching declined with increasing temperature, with 19d, 3d, 2d, 36h and 24d, 5d, 5d, 3d at 10℃, 20℃, 30℃ and 35℃, respectively, the highest hatching success was observed at 30℃. Ninety percent of worms reached maturity within 7 days post-infection when exposed to 20℃. The life-cycle of D. vastator from egg to sexual maturity lasted for 8 to 11 days which indicated that a second treatment should be administered a week later at 20℃.
Dactylogyrus vastator; Temperature; Egg laying; Egg hatching; Larval development
Fish diseases caused by monogenean parasites arise due to intensive farming practices and the deterioration of water quality, and are among the most serious parasitic diseases in aquaculture[1, 2]. The symptoms of infected fish by gill monogeneans included inflamed gills, excessive mucous secretions, and elevated respiration rate[3]. Common carp (Cyprinus carpio carpio Linnaeus, 1758) and crucian carp (Carassius auratus Linnaeus, 1758) are farmed extensively in China, and are generally infected by an important monogenean gill parasite, Dactylogyrus vastator Nybelin, 1924. Infection with D. vastator has resulted in serious economic losses to the aquaculture industry of common carp and crucian carp in China[4, 5].
Various parasiticides, including formalin[6], trichlorfon[7], praziquantel[8]and mebendazole[9]have been used to control the parasite. However, monogenean eggs are relatively resistant to physical and chemical treatment[6, 10, 11]. Their survival and the subsequent propagation of a new generation of worms could lead to the rapid evolution of drug resistance[12]. For this reason, getting insight into the life-cycle of D. vastator was vital to formulate effective strategies to manage and control parasite populations.
Water temperature are one of important environmental factors to influence the life cycle of monogeneans[13—15]. Previous studies have shown that the development of Benedenia seriolae, Zeuxapta seriolae, Neoheterobothrium hirame and Neobenedenia girellae from egg to sexual maturity is strongly affected by temperature[16—18]. In addition, egg hatching and the maturation of larval D. vastator are also shown to be temperature-dependent under a temperature range of 4℃ to 28℃[19, 20]. To date, however, the effects of temperature on egg laying and hatching success in D. vastator have not been investigated in China. In addition, ambient temperature in the subtropics of Central China where carp are farmed is usually above 30℃during summer. The life-cycle of D. vastator in the subtropical belt is expected to be different from that in the subfrigid zone, yet the effects of higher temperatures on the life-cycle of D. vastator remain unknown. Accordingly, in this study, the effects of temperatures ranging from 4℃ to 35℃ on egg laying and egghatching were investigated in D. vastator in vitro. In addition, the egg laying and the developmental period from oncomiracidia to sexual maturity at 20℃ were characterised in vivo.
1 Materials and Methods
1.1 Collection of Dactylogyrus vastator
Goldfish (Carassius auratus Linnaeus, 1758) infected with Dactylogyrus spp. were obtained from a farmer and reared in a 40 L glass tank in the laboratory, each tank with 40 goldfish. The fish were feed per day, and 1/3 of the cultured water was changed every 2 days. To increase the sample of goldfish infected with D. vastator, the infected goldfish were stocked at a ratio of 20% to newly purchased, uninfected goldfish in 120 L tanks for a minimum of 1 mo (50 fish per tank). The water temperature was maintained at around 20℃ and the fish were fed daily on commercial pellet feed.
Subsequently, the gills of the goldfish were removed and examined for infection with Dactylogyrus spp. under a stereomicroscope (XTB-1, Shanghai Zhaoyi Photoelectric Co., LTD.). All identified Dactylogyrus spp. were collected from the gills using fine needles and placed in 24-well culture plates. Only active parasites were selected for experiments. Dactylogyrus vastator parasites were identified by the morphological features of the anchor hook and copulatory organ using a biological microscope (Nikon E200, Japan)[21].
1.2 The effect of water temperature on egg laying in vitro
Each adult D. vastator was randomly allocated to 1 well of a 24-well culture plate containing 2 mL dechlorinated tap water. The plates were incubated either at 4℃ in a refrigerator, or at 10℃, 20℃, 30℃and 35℃ in a dark incubator. Water was changed daily in each well by gentle pipetting. At least 20 adult worms were used in each temperature group (Tab. 1). Parasites were observed every hour during the initial 5h, and subsequently every 2h until all worms were dead. The number of eggs laid in each well and whether each parasite remained alive were recorded. The diameter of 10 randomly selected eggs was also measured.
1.3 The effect of water temperature on egg hatching
Eggs laid at 20℃ were collected immediately and incubated at 4℃ in a refrigerator, or at 10℃, 20℃, 30℃ and 35℃ in a dark incubator. Eggs were observed under an inverted microscope once per day, until eyespots were detected, when the frequency of monitoring increased to 6h intervals. The total number of eggs with opened opercula in each temperature group were recorded and the experiment was terminated when no egg hatching had been observed for 3 consecutive days (except for the 10℃ temperature group, which was terminated when no egg hatching had been observed for 7 consecutive days). Hatching success was expressed as the proportion of empty egg cases out of the total number of eggs incubated. Ten oncomiracidia were chosen and incubated in each temperature group, and the longest survival time in each temperature group was recorded. Furthermore, the motion of oncomiracidia incubated at 20℃ was observed. At the end of the experiment, 10 oncomiracidia were fixed in formalin and their anterior-posterior length was measured.
1.4 In vivo egg laying and larval development at 20℃
The egg laying rate and reproductive development of D. vastator larvae in goldfish were examined in this experiment. Thirty naive goldfish (3.0±0.2) g that had been starved for 48h were transferred to a 20 L tank. To obtain freshly hatched oncomiracidia for host exposures, Petri dishes containing eggs were placed in an incubator and observed daily. Oncomiracidia hatched within 2h were removed with a pipette, counted, and introduced into the container with naive goldfish in dechlorinated tapwater. To that tank, oncomiracidia that had hatched within the previous 2h were added[22]. After 24h exposure to the oncomiracidia at 20℃ with natural illumination, the fish were transferred to a 40 L tank.
At 4 days post-infection, 4 fish were removed and D. vastator observed on their gills was collected using fine needles. This continued every 3 days until all worms could lay eggs. At each occasion, 10 of the worms collected (chosen at random) were fixed in formalin and their anterior-posterior lengths were measured using an ocular micrometer under a light microscope. A further 10 worms were placed individually into the wells of a 24-well plate containing 2 mL dechlorinated tap water. The egg laying and the deposition of pigment in the worms were recorded.
To observe egg laying in vivo, on day 12 when all the worms had matured according to the results of the development experiment, the remaining 18 goldfish infected with the oncomiracidia were kept separately in 18 beakers containing 500 mL dechlorinated tap water at 20℃. Every 24h for 3 consecutive days, the beaker water was filtered through a 40 μm nylon mesh and new dechlorinated tap water was added. Eggs that adhered to the mesh were counted under a light microscope. At the end of the experiment, the goldfish were killed and the number of D. vastator on their gills was recorded.
Finally, the prevalence and mean abundance[23]of the 30 goldfish were also determined.
1.5 Statistical analysis
Significant differences in egg production among the 5 temperature groups were tested by one-way ANOVA. A Chi-square test was used to evaluate whether there were significant differences in egg hatching success among the different temperature groups. Analyses were performed using the program SPSS 13.0.
2 Results
2.1 The in vitro effect of water temperature on egg laying
Eggs (n=10) are dark brown colour and symmetrical or asymmetrically oval, with an average diameter of (45.7±3.2) μm (41.0—50.1 μm), and a short filament at posterior end (Fig. 1A). Egg laying was observed after D. vastator had been removed and incubated for about 5min in all temperature groups, except the 4℃treatment where almost no egg laying was observed. The duration of egg laying decreased with increasing temperature (Tab. 1). The mean egg production was 5.9, 9.1, 9.2 and 13.4 eggs/worm at 10, 20, 30 and 35℃, respectively. It was significantly higher at 35℃than that in the other temperature groups (P<0.05). There was no significant difference in mean egg production between at the 20℃ and 30℃ temperature groups, though production was significantly higher at 20℃ and 30℃ than that at 10℃ (P<0.05). The proportion of eggs produced during the first 5h of the total egg output also increased with increasing temperature, accounting for 79.1%, 88.8%, 94.7% and 97.5%, respectively (Tab. 1).
2.2 The in vitro effect of water temperature on egg hatching
No egg hatching was observed after a mo of incubation at 4℃. The eggs, however, had developed to an oncomiracidia-like stage after 25 days at 4℃. Both hatching time and duration of egg hatching decreased with increasing incubation temperature (Tab. 2). The hatching successes of D. vastator eggs were 57.0%, 61.7%, 65.5% and 51.1% at 10℃, 20℃, 30℃ and 35℃, respectively. No significant differences were detected among the temperature treatment groups. In contrast, the longest survival times of the oncomiracidia declined with increasing temperature (Tab. 2).
2.3 In vivo egg laying and the larval development at 20℃
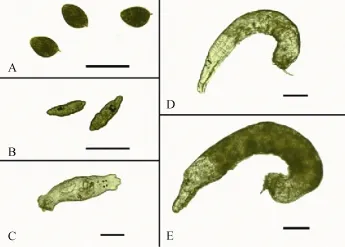
Fig. 1 Morphologic characteristics of Dactylogyrus vastator (A) eggs and larvae hatched on day (B) 1, (C) 4, (D) 7, and (E) 10 post-hatch at 20℃. Scale bar=100 μm
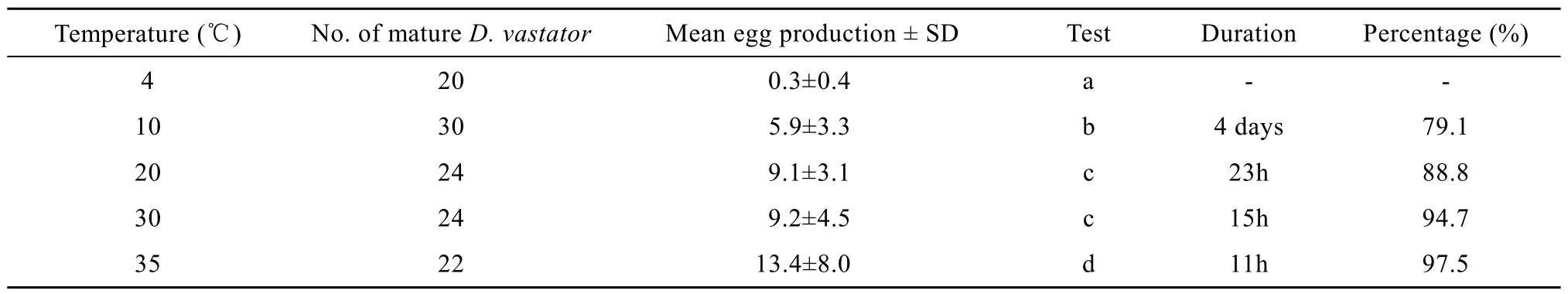
Tab. 1 Characteristics of in vitro egg laying of Dactylogyrus vastator at different temperatures

Tab. 2 In vitro effects of water temperature on egg hatching and the survival of Dactylogyrus vastator oncomiracidia
In the artificial infection experiment, all of the goldfish were infected with oncomiracidia. The meanabundance of parasites per goldfish was 10.7±3.1. Morphology of the attachment hooks and copulatory organ of the collected larvae was the same as those of D. vastator as described by Gussev[21].
Mean body length of the newly-hatched oncomiracidia was (100.3±3.7) μm (92.8—105.4 μm); Fig. 1B). The body shape of worms was pyriform with 4 black eye spots in the anterior end of the pharynx. There were 3 bands of cilia located on the anterior, middle and posterior haptor segments. The larvae travelled in straight lines, but occasionally turned in circles. After 3—4h of rapid and vigorous movement, some of the oncomiracidia sank and feebly crawled on the bottom. At 4 days post-infection, the larvae collected from the gills averaged (407.8±22.8) μm in length. The worms were transparent, with a little pigment in the middle of the body (Fig. 1C). Two pairs of head lobes were prominent, and the copulatory organ could be seen clearly under a microscope. Larvae now moved vigorously, with constant contractions and extensions, though none of the worms laid eggs at this stage. At 7 days post-infection, D. vastator worms were almost cylindrical and averaged (634.7±22.2) μm in length (Fig. 1D). By this stage, 90% of the parasites could lay eggs. Pigmentation was observed from the pharynx to posterior of the worm. At 10 days post-infection, all worms exhibited a dark body colour, with an average length of (931.3±23.0) μm, and could lay eggs (Fig. 1E).
Egg laying in vivo was continuous and uniform. Mean egg production per day at 20℃ was 6.5 eggs/ worm, with mean egg production being 6.0±0.4, 6.2±0.6 and 7.1±0.2 on days 12, 13 and 14, respectively.
3 Discussion
In this study, we have shown that the egg laying rates of D. vastator increased significantly with increasing water temperature, and also demonstrated that there was a difference in the rhythm of egg laying between in vitro and in vivo conditions. However, our results showed that the hatching time and duration of egg hatching decreased with increasing water temperature, that peak hatching success occurred at an intermediate temperature. At 20℃, the life-cycle of the D. vastator was 8—11 days.
The positive correlations we observed between mean egg production and egg laying rate and the water temperature have been previously reported for in vitro and in vivo egg laying of D. extensus[24]and Neobenedenia girellae[18]. However, mean egg production in vitro by Benedenia seriolae and Zeuxapta seriolae in the kingfish Seriola lalandi were observed to be highest not at 21℃, but at 17.5℃[17]. Similarly, the in vivo egg production rates of D. anchoratus[25], D. intermedius[26], Protopolystoma xenopodis[13]and Discocotyle sagittata[27]decreased at highest temperatures. Previously, Paperna[28]reported the oviposition rate of D. vastator significantly decreased until at 37℃ in vivo. Combined with our results that the egg production was still high at 35℃ suggested that D. vastator exhibited a broader range of tolerance to thermal extremes.
Previous studies have revealed that artificial and unfavourable conditions usually result in intensive egg laying[19], and shown that in vitro egg laying rhythm often different to those in vivo. In this study, we also observed different egg laying rhythm in vitro and in vivo: at 20℃ in vitro egg production was 9.1 eggs/ worm within 23h, with more than 88% of eggs laid during the first 5h, while egg production every day in vivo was 6.5 eggs/worm, and occurred in a continuous and uniform manner. Bychowsky[19]demonstrated that D. vastator laid 4—10 eggs/d in vivo at 12—18℃. Similarly, other studies have observed intensive egg laying when worms were removed from their hosts in D. extensus[24], Polylabroides multispinosus[10], Benedenia lutjani, B. rohdei[29], and B. seriolae[17]. Dactylogyrus vastator removed from the gills of fish might experience poor conditions as they lacked the nutrition they required. This unfavourable condition might stimulate the worm to undergo mass egg production in a short period of time. In addition, because the parasites’ nutrients were gradually depleted the longer they remained off the host, starvation was thought to be a major contributor to the reduction of egg production in vitro[30]. Therefore, it was not surprising that we observed that egg laying rate of D. vastator in vitro decreased sharply compared to the egg laying rate in vivo.
A more short hatching time with increasing temperature in D. vastator concurred with previous results recorded for D. vastator[19], D. extensus[24]and B. seriolae[17]. A similar relationship between incubation time and temperatures was also observed for Z. seriolae[17], N. girellae[31], H. okamotoi[32], N. hirame[11]and Protopolystoma xenopodis[33]. In contrast, we did not find a linear relationship between hatching success and temperature, as the highest hatching rate occurred at intermediate temperatures (>60.0% success at 20℃ and 30℃). The hatching success of several species, such as B. seriolae, Z. seriolae[17], Bivagina tai[34]and N. hirame[11], had been reported to decline at higher temperatures. Our results indicated that an intermediate temperature of between 20℃ and 30℃ was optimal for egg hatching in D. vastator.
The development of larvae has been recorded for several monogeneans. At 22℃, sexual maturation of D. vastator was attained only on the 9th—10th day[28]. Prost[25]recorded that D. anchoratus laid eggs 6 days after infection. However, maturity in B. seriolae occurred between 16 and 20 days post-infection (DPI) at 21℃, with 90% of parasites laying eggs by 20 DPI[15]. Sexual maturity of Z. seriolae[17], N. hirame[16], Heterobothrium ecuadori[35]and P. xenopodis[36]was attained by 25, 16, 38, 33 and 73 DPI at 20—23℃, respectively. In this study, when exposed to an ambient temperature of 20℃, we observed that 90% of D. vastator worms were capable of laying eggs by 7 DPI, and the egg-laying worms of D. vastator were found 4—6 days in the same conditions[20]. Hence, comparising with other monogenean species, Dactylogyrus spp. required a shorter time to attain maturity at 20—23℃.
In conclusion, based on the characteristics of in vivo egg hatching and the larval development at 20℃, the development of D. vastator from egg to sexual maturation lasted from 8 to 11 days. According to this life-cycle, we proposed that drug treatments to control parasite outbreaks be administered in 2 stages, with a second treatment administered 1 wk later after the first at 20℃.
[1] Buchmann K, Slotved H C, Dana D. Gill parasites from Cyprinus carpio in Indonesia [J]. Aquaculture, 1995, 129(1—4): 437—439
[2] Bondad-Reantaso M G, Subasinghe R P, Arthur J R, et al. Disease and health management in Asian aquaculture [J]. Veterinary Parasitology, 2005, 132(3—4): 249—272
[3] Reed P A, Francis-Floyd R, Klinger R C. FA28/FA033: Monogenean parasites of fish. EDIS—Electronic Data Information Source—UF/IFAS Extension. University of Florida. http://edis.ifas.ufl.edu/FA033. 2009
[4] Rahanandeh M, Jalali B, Sharifpour I, et al. Survey on Dactylogyrusis in Caspian Frisian roach (Rutilus fissi ktum) caused by Dactylogyrus fissi [J]. Global Veterinaria, 2010, 4(5): 515—518
[5] Galli P, Stefni F, Benzoni F, et al. Introduction of alien host–parasite complexes in a natural environment and the symbiont concept [J]. Hydrobiologia, 2005, 548(1): 293—299
[6] Sharp N J, Diggles B K, Poortenaar C W, et al. Efficacy of Aqui-S, formalin and praziquantel against the monogeneans, Benedenia seriolae and Zeuxapta seriolae, infecting yellowtail kingfish Seriola lalandi lalandi in New Zealand [J]. Aquaculture, 2004, 236(1—4): 67—83
[7] Buchmann K, Mellergaard S, Koie M. Pseudodactylogyrus infections in eel: A review [J]. Diseases of Aquatic Organisms, 1987, 3: 51—57
[8] Schmahl G, Mehlhorn H. Treatment of fish parasites: 1. Praziquantel effective against Monogenea (Dactylogyrus vastator, Dactylogyrus extensus, Diplozoon paradoxum) [J]. Parasitology Research, 1985, 71(6): 727—737
[9] Katharios P, Papandroulakis N, Divanach P. Treatment of Microcotyle sp. (Monogenea) on the gills of cage-cultured red porgy, Pagrus pagrus following baths with formalin and mebendazole [J]. Aquaculture, 2006, 251(2—4): 167—171
[10] Diggles B K, Roubal F R, Lester R J G. The influence of formalin, benzocaine and hyposalinity on the fecundity and viability of Polylabroides multispinosus (Monogenea: Microcotylidae) parasitic on the gills of Acanthopagrus australis (Pisces: Sparidae) [J]. International Journal for Parasitology, 1993, 23(7): 877—884
[11] Yoshinaga T, Segawa I, Kamaishi T, Sorimachi M. Effects of temperature, salinity and chlorine treatment on egg hatching of the monogenean Neoheterobothrium hirame infecting Japanese flounder [J]. Fish Pathology, 2000, 35(2): 85—88
[12] Buchmann K, Roepstorff A, Waller P J. Experimental selection of mebendazole resistant gill monogeneans from the European eel, Anguilla Anguilla [J]. Journal of Fish Diseases, 1992, 15(5): 393—400
[13] Jackson J A, Tinsley R C. Effects of temperature on oviposition rate in Protopolystoma xenopodis (Monogenea: Polystomatidae) [J]. International Journal for Parasitology, 1998, 28(2): 309—315
[14] Cecchini S, Saroglia M, Berni P, et al. Influence of temperature on the life cycle of Diplectanum aequans (Monogenea, Diplectanidae), parasitic on sea bass, Dicentrarchus labrax (L.) [J]. Journal of Fish Diseases, 1998, 21(1): 73—75
[15] Lackenby J A, Chambers C B, Ernst I, et al. Effect of water temperature on reproductive development of Benedenia seriolae (Monogenea: Capsalidae) from Seriola lalandi in Australia [J]. Diseases of Aquatic Organisms, 2007, 74(3): 235—242
[16] Tsutsumi N, Yoshinaga T, Kamaishi T, et al. Effects of water temperature on the development of the monogenean Neoheterobothrium hirame on Japanese flounder Paralichthys olivaceus [J]. Fish Pathology, 2003, 38: 41—47 [17] Tubbs L A, Poortenaar C W, Sewell M A, et al. Effects of temperature on fecundity in vitro, egg hatching and reproductive development of Benedenia seriolae and Zeuxapta seriolae (Monogenea) parasitic on yellowtail kingfish Seriola lalandi [J]. International Journal for Parasitology, 2005, 35(3): 315—327
[18] Hirazawa N, Takano R, Hagiwara H, et al. The influence of different water temperatures on Neobenedenia girellae (Monogenea) infection, parasite growth, egg production and emerging second generation on amberjack Seriola dumerili (Carangidae) and the histopathological effect of this parasiteon fish skin [J]. Aquaculture, 2010, 299(1): 2—7
[19] Bychowsky B E. Monogenetic Trematodes, their Classification and Phylogeny [M]. Moscow: Leningrad. Academy of Science. USSR. 1957, 85—107 (in Russian)
[20] Tomnatik V E. The influence of water temperature on the sexual maturation of Dactylogyrus vastator [J]. Parazitologiya, 1990, 24(3): 235—238
[21] Gussev A V. Keys to Parasites of Freshwater Fish of the USSR [M]. Vol 2, Parasitic Metazoa. Akad Nauk USSR. 1985, 15—251 (in Russian)
[22] Chigasaki M, Nakane M, Ogawa K, et al. Standardized method for experimental infection of tiger puffer Takifugu rubripes with oncomiracidia of Heterobothrium okamotoi (Monogenea: Diclidophoridae) with some data on the oncomiracidial biology [J]. Fish Pathology, 2000, 35(4): 215—221
[23] Bush A O, Lafferty K D, Lotz J M, et al. Parasitology meets ecology on its own terms: Margolis et al. revisited [J]. Journal of Parasitology, 1997, 83(4): 575—583
[24] Turgut E. Influence of temperature and parasite intensity on egg production and hatching of the monogenean Dactylogyrus extensus [J]. Bamidgeh, 2012, 729: 1—5
[25] Prost M. Investigations on the development and pathogenicity of Dactylogyrus anchoratus (Duj., 1845) and D. extensus Mueller et v. Cleave, 1932 for breeding carps [J]. Acta Parasitologica Polonica, 1963, 11(1/4): 17—47
[26] Zhang X P, Li W X, Ai T S, et al. Effects of temperature on egg production and hatching of Dactylogyrus intermidius [J]. Acta Hydrobiologica Sinica, 2013, 37(3): 495—500 [张效平,李文祥, 艾桃山, 等. 温度对中型指环虫产卵和孵化的影响. 水生生物学报, 2013, 37(3): 495—500]
[27] Gannicott A M, Tinsley R C. Environmental effects on transmission of Discocotyle sagittata (Monogenea): Egg production and development [J]. Parasitology, 1998, 117(5): 499—504
[28] Paperna I. Some observations on the biology and ecology of Dactylogyrus vastator in Israel [J]. Bamidgeh, 1963, 15(1): 8—28
[29] Ernst I, Whittington I D. Hatching rhythms in the capsalid monogeneans Benedenia lutjani from the skin and B. rohdei from the gills of Lutjanus carponotatus at Heron Island, Queensland, Australia [J]. International Journal for Parasitology, 1996, 26(11): 1191—1204
[30] Whittington I D. Reproduction and host-location among the parasitic platyhelminthes [J]. International Journal for Parasitology, 1997, 27(6): 705—714
[31] Bondad-Reantaso M G, Ogawa K, Fukudome M, et al. Reproduction and growth of Neobenedenia girellae (Monogenea: Capsalidae), a skin parasite of cultured marine fishes of Japan [J]. Fish Pathology, 1995, 30(3): 227—231
[32] Ogawa K. Egg hatching of the monogenean Heterobothrium okamotoi, a gill parasite of cultured tiger puffer (Takifugu rubripes), with a description of its oncomiracidium [J]. Fish Pathology, 1998, 33: 25—30
[33] Tinsley R C, York J E, Everard A L. E, et al. Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on egg development [J]. Parasitology, 2011, 138(8): 1029—1038
[34] Ogawa K. Development of Bivagina tai Monogenea: Microcotylidae [J]. Nippon Suisan Gakkaishi, 1988, 54(1): 61—64
[35] Grano-Maldonado M, Roque A, Fajer-Avila E J. Development of Heterobothrium ecuadori (Monogenea: Diclidophoridae) in bullseye puffer fish Sphoeroides annulatus under experimental conditions [J]. Fish Pathology, 2010, 45(4): 175—178
[36] Tinsley R C, York J E, Stott L C, et al. Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on development within the host [J]. Parasitology, 2011, 138(8): 1039—1052
10.7541/2015.154
Received date: 2014-12-02; Accepted date: 2015-03-15
Foundation item: the Earmarked Fund for China Agriculture Research System (CARS-46-49); the National Natural Science Foundation of China (No. 31272695); the science and techndogy fund of Guizhou provime (QKH-LH, NO. [2014]7698)
Brief introduction of author: Zhang Xiao-Ping (1987—), male, Xintai city in Shandong province; PhD with specialty in ichthyopathology; E-mail: zhangxiaoping_0311@163.com
Wang Gui-Tang, E-mail: gtwang@ihb.ac.cn
S941.52
A
1000-3207(2015)06-1177-07
