饲料蛋氨酸对斜带石斑鱼生长性能、抗氧化及糖异生相关酶活性的影响
2015-02-28迟淑艳王学武谭北平杨奇慧董晓慧刘泓宇
迟淑艳 王学武 谭北平 杨奇慧 董晓慧 刘泓宇 章 双
(广东海洋大学水产学院, 湛江 524088)
饲料蛋氨酸对斜带石斑鱼生长性能、抗氧化及糖异生相关酶活性的影响
迟淑艳 王学武 谭北平 杨奇慧 董晓慧 刘泓宇 章 双
(广东海洋大学水产学院, 湛江 524088)
实验通过评价斜带石斑鱼幼鱼生长性能、血清指标和相关酶活性的变化, 探讨斜带石斑鱼获得最大生长的饲料蛋氨酸(Met)水平与Met代谢关键酶活性和氧化损伤的关系。添加DL-Met使实验饲料中Met的含量分别为0.71%、0.98%、1.26%、1.57%、1.86%和2.18%(Diet1-Diet6), 配制6组等氮等脂的饲料。选择健康实验鱼[初重(9.75±0.05) g]随机分为6组, 每天分别于8: 00和17: 00投喂实验饲料, 养殖8周。结果表明, Diet3组鱼体增重率和特定生长率显著高于Diet1和Diet6组(P<0.05); Diet4斜带石斑鱼幼鱼的肥满度显著高于Diet1、Diet5和Diet6 (P<0.05); Diet2和Diet3组血清总蛋白含量显著高于Diet5组(P<0.05), Diet3组幼鱼血糖含量显著低于Diet1组和Diet2组(P<0.05), 血清总胆固醇含量在Diet3组逐渐降低, Diet4—6组显著低于 Diet2组(P<0.05); Diet3组肝脏超氧化物歧化酶(SOD)和过氧化氢酶(CAT)活性最高, 显著高于其他各组(P<0.05), Diet 4组肝脏磷酸烯醇式丙酮酸羧激酶(PEPCK)活性与Diet 3相比差异不显著, 但是显著低于其余各组(P<0.05)。综合以上结果, 以特定生长率为判据, 经二次曲线模型拟合可得斜带石斑鱼幼鱼若获得最大特定生长率, 其饲料中 Met的最适含量为 1.42%(占饲料蛋白 3.16%)。在该水平下, 鱼体血糖、血清总胆固醇含量和PEPCK活性较低, 有利于改善鱼体对能量的利用; SOD和CAT活性升高有利于改善鱼体的氧化损伤。
蛋氨酸; 生长性能; 血清指标; 磷酸烯醇式丙酮酸羧激酶; 斜带石斑鱼
对哺乳动物的研究表明, 低蛋氨酸(Methionine restriction, MR)饮食可减少生物体线粒体活性氧的产生, 从而降低氧化损伤, 增加脂肪氧化进而减少脂肪沉积, 改善葡萄糖和胰岛素的平衡[1—4]。蛋氨酸(Met)是鱼类正常生长及其基础代谢所必需, 海水养殖鱼类饲料蛋氨酸适宜含量为0.8%—1.7%[5—9]。近期有研究表明, 蛋氨酸缺乏会导致大西洋鲑(Salmo salar)肝体比增大, 肝脏脂肪酸合成酶活性增加, 脂肪酸比例发生变化, 甘油三酯累积[10]。那么, 满足鱼类最大生长和降低生物体氧化损伤所需的蛋氨酸量是否一致呢?
本实验通过研究斜带石斑鱼(Epinephelus coioides)生长性能、血清学指标和相关酶活性, 探讨在减少鱼粉用量的条件下, 饲料中不同蛋氨酸含量对斜带石斑鱼生长相关指标的影响, 在当下蛋氨酸与鱼粉价格同时上涨的时期, 为饲料配方的合理配制提供参考。
1 材料与方法
1.1 实验饲料配方及制作
以鱼粉、豆粕和玉米蛋白粉为主要蛋白源, 豆油、鱼油和大豆磷脂为脂肪源, 通过添加 DL-Met使实验饲料中 Met的含量分别为 0.71%、0.98%、1.26%、1.57%、1.86%和2.18% (Diet1—Diet6)。除Met外, 添加晶体氨基酸使饲料中必需氨基酸组成与斜带石斑鱼肌肉氨基酸组成一致。随Met含量增加, 通过改变非必需氨基酸(天冬氨酸︰甘氨酸)相应含量, 调节饲料至等氮(表1)。

表1 实验饲料配方(%干重)Tab. 1 The formula of the experimental diets (% dry weight)
饲料原料经粉碎后过60目筛, 按配方称重, 逐级混合均匀, 用双螺杆挤压机(华南理工大学科技实业总厂, F-75)加工制粒成3.0 mm的颗粒状饲料, 晾干后于–20℃冰箱中储存备用。
1.2 实验用鱼及饲养管理
实验鱼购自雷州石斑鱼苗场, 水泥池中暂养两周, 期间投喂商业饲料(中山统一饲料有限公司, 粗蛋白 46%), 使其逐渐适应人工配合饲料和饲养环境。养殖实验在广东海洋大学东海岛生物研究基地室内海水鱼养殖系统中进行。在实验开始时, 随机挑选规格一致、健康的斜带石斑鱼[初重(9.75± 0.05) g]于玻璃钢桶(500 L)中。实验设6个处理, 每个处理 3个重复, 每个重复 30尾鱼。每天分别于8: 00和17: 00投喂直径3.0 mm 的实验饲料, 初始投喂量为3%体重, 并根据摄食情况适当调整。实验期间水温 28.5—31.5 , ℃ 盐度 26—32, 溶解氧≥7 mg/L以上, pH 7.6—8.0, 氨氮浓度0.03 mg/L以下,实验持续8周。

表2 实验饲料氨基酸组成(%干重)Tab. 2 The amino acid composition of the experimental diets (% dry weight)
1.3 样本采集及分析
养殖实验结束, 饥饿24h后称重, 计算增重率、蛋白质效率、特定生长率和饲料效率。每重复随机取3尾鱼测体长、体重后备测全鱼粗蛋白、粗脂肪、粗灰分和水分; 另取3尾鱼尾静脉取血, 4℃保存用于检测血液生化指标; 取血后解剖, 取肝脏和背肌迅速于液氮中保存, 后置于–80℃冷冻保存, 用于肝脏酶活力和肌肉氨基酸含量测定。
对饲料和全鱼样品进行常规养分分析[11], 水分测定采用 105℃烘干恒重法, 粗蛋白质采用凯氏定氮法(Kjeltec 8400凯氏定氮仪), 粗脂肪索式抽提法,粗灰分采用550℃马弗炉灼烧法。将样本(实验原料、饲料和鱼体肌肉)经由冷冻干燥处理后, 按照GB/T18246-2000进行酸水解和样本处理, 经氨基酸自动分析仪测定氨基酸含量(国家粮食局成都粮油食品饲料质量监督检验测试中心, 日立L8900)。
血清葡萄糖(GLU)、总蛋白(TP)、胆固醇(CHOL)、血清甘油三酯(TG)采用全自动生化分析仪(日立7600-110型)测定。
肝脏超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和匀浆液蛋白含量采用南京建成生物工程研究所试剂盒测定, 磷酸烯醇式丙酮酸羧激酶(PEPCK)采用武汉华美生物公司试剂盒测定。
1.4 计算方法
增重率(Weight gain, WG, %)=(末均重–初均重)/初均重×100;
特定生长率(Special growth rate, SGR, %/d)=(ln末均重–ln初均重)/饲养天数×100;
蛋白质效率(Protein efficiency ratio, PER, %)=(终末体重–初始体重)/(饲料摄食量×蛋白质含量)×100;
摄食率(Feed intake, FI, %) = l00×采食干饲料重(g) / [(实验末均重+实验初均重)/2)×天数];
成活率(Survival rate, SR, %)=末尾数/初尾数×100;
饲料系数(Feed coefficient rate, FCR)=摄食饲料干重/(末重–初重);
肥满度(Condition factor, CF, %)=体重(g)/体长(cm)3×100。
1.5 统计分析
采用SPSS Version 17.0统计软件对数据进行单因素方差分析(One-way ANOVA), 如有显著性差异(P<0.05), 则进行 Duncan’s多重比较。实验数据用“平均数±标准差”表示。
2 结果
2.1 饲料蛋氨酸水平对斜带石斑鱼的生长性能和饲料利用效率的影响
由表3 可见, 饲料中Met水平对斜带石斑鱼成活率、蛋白质效率饲料系数和摄食率未产生显著影响(P>0.05)。随着饲料Met水平的升高, 斜带石斑鱼的增重率和特定生长率均呈先升高, 后下降的趋势, Diet3组鱼体WG和SGR显著高于Diet1和Diet6组(P<0.05)。参考特定生长率数据, 经二次曲线模型拟合可得出方程y = –0.1445x2+0.4092x+2.03(R²=0.8451),即斜带石斑鱼幼鱼若获得最大特定生长率, 其饲料中Met的最适含量为1.42% (占饲料蛋白3.16%) (图1)。
2.2 饲料蛋氨酸水平对斜带石斑鱼体成分和肥满度的影响表4的结果表明, 饲料Met水平对斜带石斑鱼幼鱼全鱼体成分影响不显著(P>0.05)。Diet4斜带石斑鱼幼鱼的肥满度显著高于 Diet1、Diet5和 Diet6 (P<0.05)。饲料 Met含量对斜带石斑鱼幼鱼肌肉必需氨基酸含量影响不显著(P>0.05)(表5)。
2.3 饲料蛋氨酸水平对斜带石斑鱼血清相关指标的影响
Diet3组幼鱼血糖含量显著低于 Diet1组和Diet2组(P<0.05), 随饲料 Met含量的升高, 当高于1.26% (Diet3)时血糖变化趋于稳定 (P>0.05)。Diet2 和 Diet3组血清总蛋白含量显著高于 Diet5组(P<0.05), Diet2组总胆固醇含量显著高于 Diet4、Diet5和Diet6组, 随Met含量增加, 总胆固醇含量下降, Diet6组最低, 与Diet4和Diet5组相比差异不显著(P>0.05); 饲料 Met含量对幼鱼血清甘油三酯含量未产生显著影响(P>0.05)。

表3 蛋氨酸对斜带石斑鱼的生长性能和饲料利用率的影响Tab. 3 The effects of dietary methionine on the growth performance and feed utilization of juvenile groupers

图1 饲料蛋氨酸含量与斜带石斑鱼幼鱼SGR的二次曲线模型Fig. 1 The relationship between dietary methionine and special growth rate of juvenile grouper
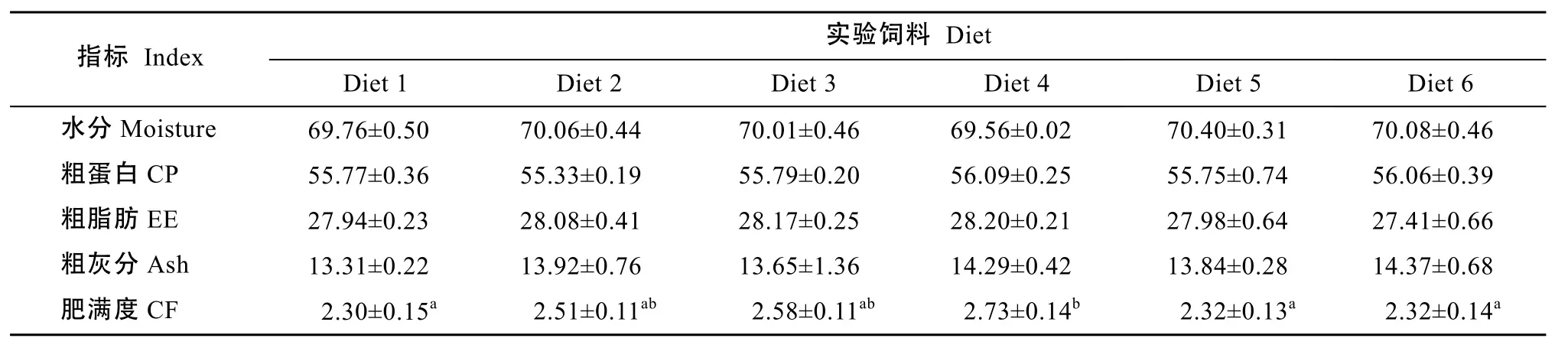
表4 蛋氨酸对斜带石斑鱼全鱼常规成分(%干重)和肥满度的影响Tab. 4 The body composition (% dry weight) and condition factor of juvenile grouper

表5 蛋氨酸对斜带石斑鱼幼鱼肌肉必需氨基酸组成的影响(%干重)Tab. 5 The effects of dietary methionine on EAA composition in the muscles of juvenile groupers (% dry weight)
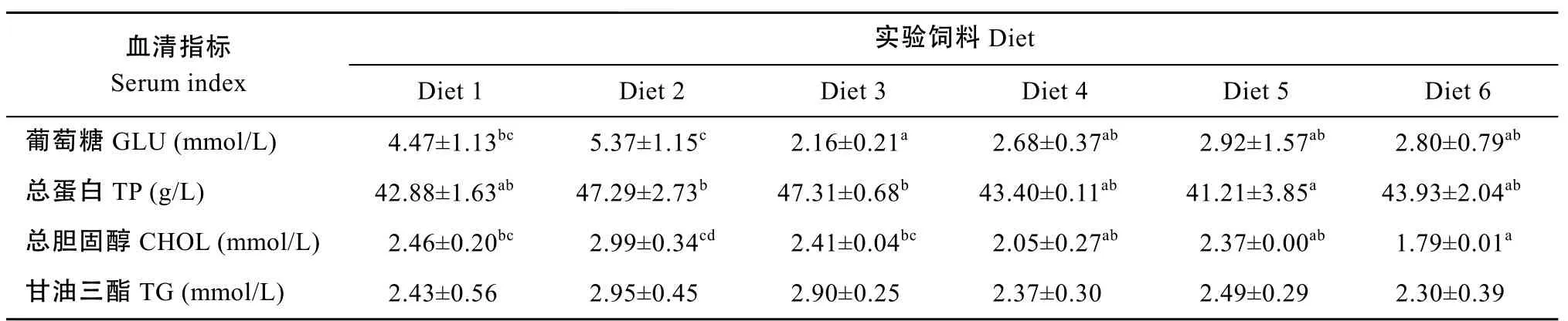
表6 蛋氨酸水平对斜带石斑鱼幼鱼血清相关指标的影响Tab. 6 The effects of dietary methionine on the serum index of juvenile groupers
2.4 饲料蛋氨酸含量对斜带石斑鱼肝脏酶活力的影响
表7显示Diet3组肝脏SOD 和CAT活性最高,其中 SOD活性与 Diet4组相比差异不显著, 但是显著高于其他各组(P<0.05); CAT 活性则显著高于其余各组(P<0.05)。Diet4组肝脏PEPCK活性与Diet3相比差异不显著, 但是显著低于其余各组(P<0.05)。
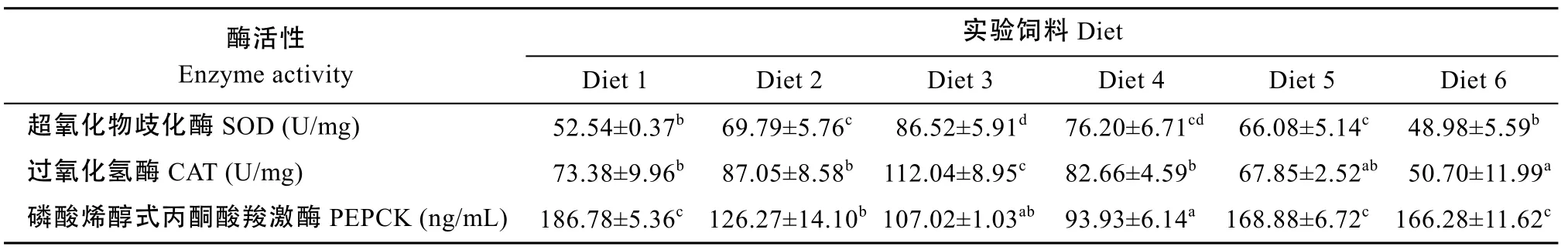
表7 饲料中蛋氨酸含量对斜带石斑鱼肝脏酶活性的影响Tab. 7 The effects of dietary methionine in diet on enzyme activities in the liver of juvenile groupers
3 讨论
3.1 饲料蛋氨酸水平对斜带石斑鱼的生长性能和饲料利用效率的影响
本实验以SGR为依据时, 经二次曲线模型拟合得出斜带石斑鱼幼鱼(9.75 g)获得最大 SGR所需饲料蛋氨酸量为1.42%(占饲料蛋白3.16%), 随Met水平进一步升高, 鱼体增重下降, 这与黑鲷(Sparus macrocephalus)[5]、建鲤(Cyprinus carpiovar Var. Jian)[6]、大黄鱼(Pseudosciaena crocea R.)[8]和欧洲鲈(Dicentrarchus labrax)[9]等研究结果一致。然而, Luo等[7]参考全卵蛋白的氨基酸组成配制饲料, 得出斜带石斑鱼(13.25 g)获得最大增重所需Met量为1.31% (胱氨酸含量0.26%), 并且当Met超过该水平后对鱼体增重影响不显著, 而本实验以斜带石斑鱼肌肉氨基酸组成为模式, 并且初重低于前者, 可能导致 Met需要量上的差异。在本实验中WG在Diet3 (1.26%)显著升高, PER有升高趋势但是未达显著性差异, FCR和 FI均有下降趋势但是同样未达显著性差异;王和伟的研究表明饲料中添加牛磺酸对斜带石斑鱼WG、PER和饲料效率要显著高于未添加组, 但是随添加水平升高各指标未出现显著性差异[12]; 植物蛋白源替代鱼粉后饲料中添加少量 Met, 可以显著提高大菱鲆(Scophthalmus maximus L.)WG, 但是对PER和FI没有显著影响[13], 和本研究结果相近。研究表明斜带石斑鱼[7]、虹鳟(Salmo gairdneri)[14]和卵形鲳 鲹(Trachinotus ovatus L.)[15]肥满度受饲料 Met水平的影响显著, 且随着Met水平的升高呈先升高,后趋于稳定的趋势。本实验饲料Met含量超过1.57% 时, 鱼体肥满度下降, 与0.71%组的状态一致, 这种变化与其增重的变化趋势一致。
3.2 饲料蛋氨酸水平对斜带石斑鱼血清相关指标的影响
鱼类血清相关指标的变化与饲料中营养水平息息相关[16]。 许氏鲆 鲉(Sebastes schlegeli)[17]和黑鲷[5]的实验表明, Met水平对血清甘油三酯均未产生显著性影响, 然而, 许氏鲆 鲉分别摄食最低Met (0.58%)和最高Met (3.08%)的饲料, 血清总胆固醇含量均为最高, 随着Met水平的升高基本呈现“W”的变化趋势[17]; 饲料Met含量为0.75%组的黑鲷血清总胆固醇显著高于1.09%—2.35%组, 血糖含量以最低Met (0.75%)和最高Met (2.35%)组显著高于其余各组,获得最大SGR其Met的最适需要量为1.71%(胱氨酸含量0.31%)[5]。Luo等[7]得出斜带石斑鱼血浆葡萄糖和总甘油三酯含量随着饲料Met含量升高而升高, 在1.34%组升至最高而后显著下降, 1.34%组血浆胆固醇含量显著高于其余各组。然而, 本实验中, 鱼体获得最大SGR时饲料Met含量在Diet3 (1.26%)和Diet4 (1.57%)之间, 血糖和总胆固醇含量也在Met 1.26%—1.57%水平间达最低, 之后趋于平稳。这是否说明这样的饲料Met含量可以促使鱼体更好地利用碳水化合物和脂类作为能量来满足生长需要呢?MR饮食在哺乳动物的研究中虽然已展现出较多优势, 但是其调控能量和新陈代谢的机制尚不清晰。Craig 和Moon[18]观察到有趣的现象, 虹鳟摄食中(12%)和高(22%)碳水化合物-MR饲料6h后, 葡萄糖被快速吸收, 肉食性鱼类的葡萄糖不耐受现象消失。随后, Carig等[19]分别对虹鳟的肝细胞进行Met和无Met培养48h的实验, 并用葡萄糖进行干预, 结果发现无Met的肝细胞对葡萄糖的吸收是增加的, 有可能是线粒体解偶联增加, 导致细胞ATP下降, 促进膜上Na-葡萄糖转运蛋白2(SGLT2)表达增加, 同时发现解偶联蛋白2α(UCP2α)在转录水平上有所升高。
3.3 饲料蛋氨酸含量对斜带石斑鱼肝脏酶活力的影响
SOD和CAT是体内清除过氧化物的关键酶, 二者具有协同作用, 与生物体的健康状况密切相关。李亮等[20]及马春桃等[21]在对小鼠的研究中发现, 饲料中添加高于1%的Met可显著降低血液中SOD活性。不同形式Met均能增强肉种鸡机体的抗氧化功能,显著提高肝脏、肾脏组织谷胱甘肽过氧化物酶、SOD活性和降低丙二醛含量[22]。Met可显著提高生长中期草鱼(Ctenopharyngodon idellus)肝脏SOD和CAT活性[23], 大菱鲆获得最大SGR时, DL-Met适宜含量为1.58%, 肝脏TBARs含量先升高随后下降, 提示Met含量升高对肝脏脂质过氧化物的生成有影响[24]。在本实验中, 随着Met水平的增高, SOD和CAT活力均呈现先升高后降低的趋势, 表明适宜的Met含量可以有效提高抗氧化酶的活性, 有效清除过氧化物,改善生长。
PEPCK是糖异生途径的关键酶, 它可催化草酰乙酸转化为磷酸烯醇式丙酮酸, 继而生成葡萄糖。当葡萄糖含量不足时PEPCK的活力会升高, 而当体内葡糖含量足够时, 该酶活力降低[25]。饲料糖源和糖水平均能显著影响大菱鲆PEPCK活性[26], 饲料添鲌加碳水化合物相比于无糖组能够显著降低翘嘴红(Eryghroculter ilishaeformis Bleeker) PEPCK活性[27]。然而, 对河鲈(Perca fkuviatilis Linnaeus)[28]、大西洋鲑[29]和虹鳟[30]的研究表明, PEPCK的mRNA水平不受日粮中碳水化合物的影响。氨基酸对PEPCK活性的研究较少, 氨基酸单独作用能够诱导糖异生、糖酵解和氨基酸分解。Met可以转化为三羧酸循环的中间产物琥珀酰辅酶A参加糖异生途径。与不添加Met相比, 大鼠摄食含有Met的饲料, 肝脏PEPCK活性显著降低[31]。在怀孕母猪饲粮中添加甜菜碱, 会通过提高新生仔猪肝脏Met代谢、后续DNA和组氨酸甲基化以及miRNA介导的转录后机制, 进而影响仔猪肝脏糖异生基因的表达。与无甜菜碱组仔猪相比,甜菜碱组仔猪肝脏PEPCK活性、PEPCK2(启动子低甲基化)在mRNA水平和PEPCK蛋白水平的表达均显著增加, 而PEPCK1在mRNA水平却显著下降(启动子高甲基化)[32]。通过监测大西洋鲑稚鱼肝脏β-同型半胱氨酸甲基转移酶、S-腺苷高半胱氨酸水解酶和胱硫醚β-合酶的mRNA表达, 发现将高Met饲料和低Met饲料交替饲喂大西洋鲑稚鱼, 可以更好地改善肝脏Met的利用效率[33]。与不加Met的处理组相比, Met显著降低了虹鳟肝细胞葡萄糖-6-磷酸酶、葡萄糖激酶和丝氨酸脱水酶的表达, 但是对PEPCK没有显著影响[34], 没有体现出Met对PEPCK的调控, 这是否与Met的浓度有关(2 mmol/L)?在本实验中斜带石斑鱼PEPCK活性随着饲料Met含量的升高呈先降低后升高的趋势, 表明获得最大SGR时, PEPCK的活性是较低的, 提示在该Met水平下, 糖异生作用不强, 当饲料Met水平低于或高于这个水平时,机体PEPCK活性上升糖异生途径加强。
本实验研究结果表明, 以 SGR为判据, 当饲料胱氨酸含量为0.46%, 斜带石斑鱼幼鱼获得最大SGR 时, 饲料Met的含量为1.42%(占饲料蛋白3.16%)。在该水平下, 鱼体血糖、血清总胆固醇含量和PEPCK 活性较低有利于改善鱼体对能量的利用, SOD和CAT活性升高有利于改善鱼体的氧化损伤。
[1] Sanz A, Caro P, Ayala V, et al. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins [J]. FASEB Journal, 2006, 20(8): 1064—1073
[2] Malloy V L, Krajcik R A, Bailey S J, et al. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction [J]. Aging Cell, 2006, 5(4): 305—314
[3] Caro P, Gomez J, Lopez-Torres M, et al. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver [J]. Biogerontology, 2008, 9(3): 183—196
[4] Ables G P, Perrone C E, Orentreich D, et al. Methioninerestricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density [J]. PLoS One, 2012, 7(12): e51357
[5] Zhou F, Xiao J X, Hua Y, et al. Dietary L-methionine requirement of juvenile black sea bream (Sparus macrocephalus) at a constant dietary cystine level [J]. Aquaculture Nutrition, 2011, 17(5): 469—481
[6] Tang L, Wang G X, Jiang J, et al. Effect of methionine on intestinal enzymes activities, microflora and humoral immune of juvenile Jian carp (Cyprinus carpio var. Jian) [J]. Aquaculture Nutrition, 2009, 15(5): 477—483
[7] Luo Z, Liu Y J, Mai K S, et al. Dietary L-methionine requirement of juvenile grouper Epinephelus coioides at a constant dietary cystine level [J]. Aquaculture, 2005, 249(1—4): 409—418
[8] Mai K S, Wan J L, Ai Q H, et al. Dietary methionine requirement of large yellow croaker, Pseudosciaena crocea R [J]. Aquaculture, 2006, 253(1—4): 564—572
[9] Tulli F, Messina M, Calligaris M, et al. Response of European sea bass (Dicentrarchus labrax) to graded levels of methionine (total sulfur amino acids) in soya protein-based semi-purified diets [J]. British Journal of Nutrition, 2010, 104(5): 664—673
[10] Espe M, Rathore R M, Du Z Y, et al. Methionine limitation results in increased hepatic FAS activity, higher liver 18:1 to18:0 fatty acid ratio and hepatic TAG accumulation in Atlantic salmon, Salmo salar [J]. Amino Acids, 2010, 39(2): 449—460
[11] Helric K. AOAC Association of Official Analytical Chemists [A]. In: Official Methods of Analysis of AOAC, 15th edn [C]. Association of Official Analytical Chemists Inc., Arlington, VA, USA. 1990, 70—80
[12] Wang H W. Effect of dietary taurine levels on the growth performance of GIFT tilapia Oreochromis nilotictus and grouper Epinephelus coioides [D]. Thesis for Master of Science.Jimei University. Xiamen. 2013 [王和伟. 饲料牛磺酸水平对吉富罗非鱼和斜带石斑鱼生长的影响. 硕士学位论文, 集美大学. 厦门. 2013]
[13] Qi G S. Effects of dietary taurine, methionine, cystine, serine and cysteamine on growth performance and metabolism of taurine synthesis in turbo [D]. Thesis for Doctor of Science.Ocean University of China. Qingdao. 2012 [齐国山. 饲料中牛磺酸、蛋氨酸、胱氨酸、丝氨酸和半胱胺对大菱鲆生长性能及牛磺酸合成代谢的影响. 博士学位论文, 中国海洋大学. 青岛. 2012]
[14] Walton M J, Cowey C B, Adron J W. Methionine metabolism in rainbow trout fed diets of differing methionine and cystine content [J]. The Journal of Nutrition, 1982, 112(8): 1525—1535
[15] Ma X K. The study on dietary protein to energy ratios and several essential amino acid of juvenile ovate pompano (Trachinotus ovatus L.) [D]. Thesis for Doctor of Science.Ocean University of China. Qingdao. 2013 [马学坤. 卵形鲳鲹幼鱼对饲料中蛋白能量比和几种必需氨基酸需求的研究. 博士学位论文, 中国海洋大学. 青岛. 2013]
[16] Regost C, Arzel J, Kaushik S J. Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima) [J]. Aquaculture, 1999, 180(1—2): 99—117
[17] Yan Q G, Xie S Q, Zhu X M, et al. Dietary methionine requirement for juvenile rockfish, Sebastes schlegeli [J]. Aquaculture Nutrition, 2007, 13(3): 163—169
[18] Craig P M, Moon T W. Methionine restriction affects the phenotypic and transcriptional response of rainbow trout (Oncorhynchus mykiss) to carbohydrate enriched diets [J]. British Journal of Nutrition, 2013, 109(3): 402—412
[19] Craig P M, Massarsky A, Moon T W. Understanding glucose uptake during methionine deprivation in incubated rainbow trout (Oncorhynchus mykiss) hepatocytes using a non-radioactive method. Comparative Biochemistry and Physiology, Part B: Biochemistry and Molecular Biology, 2013, 166(1): 23—29
[20] Li L, Yang B, Zhang J. Effects of aerobic exercise on the capability of antioxidation in methionine-induced hyperhomocysteinemia rats [J].Chinese Journal of SportsMedicine, 2006, 25(3): 282—285 [李亮, 杨波, 张钧. 有氧运动对蛋氨酸诱导高半胱氨酸血症大鼠抗氧化应激能力的影响. 中国运动医学杂志, 2006, 25(3): 282—285]
[21] Ma C T, Li M. Effect of folic acid on hyperhomocysteinemia induced by methionine in rat [J].Journal of Public Healthand Preventive Medicine, 2006, 17(1): 14—16 [马春桃, 黎明. 叶酸对大鼠蛋氨酸诱发高同型半胱氨酸水平的影响.公共卫生与预防医学, 2006, 17(1): 14—16]
[22] Liu W F, Liu W L, Zhan X A, et al. Effects of different methionine sources on performance, immune indices and antioxidant function of broiler breeders [J].Chinese Journalof Animal Nutrition, 2013, 25(9): 2118—2125 [刘文斐, 刘伟龙, 占秀安, 等. 不同形式蛋氨酸对肉种鸡生产性能、免疫指标及抗氧化功能的影响. 动物营养学报, 2013, 25(9): 2118—2125]
[23] Tang B R. Effects of dietary methionine on digestive and absorptive ability and antioxidative ability of young grass carp (Ctenopharyngodon idell) [D]. Thesis for Master of Science. Sichuan Agricultural University, Ya’an. 2012 [唐炳荣. 蛋氨酸对生长中期草鱼消化吸收能力和抗氧化能力影响的研究. 硕士学位论文, 四川农业大学. 雅安. 2012]
[24] Ma R, Hou H P, Mai K S, et al. Comparative study on the effects of L-methionine or 2-hydroxy-4-(methylthio) butanoic acid as dietary methionine source on growth performance and anti-oxidative responses of turbot (Psetta maxima) [J]. Aquaculture, 2013, 412—413(1): 136—143
[25] Wang J Y, Zhu S G, Xu C F. Biochemistry [M]. Beijing: Higher Education Press. 2002, 154—158 [王镜岩, 朱圣庚,徐长法. 生物化学. 北京: 高等教育出版社. 2002, 154—158]
[26] Nie Q, Miao H J, Miao S Y, et al. Effects of dietary carbohydrate sources and levels on the activities of carbohydrate metabolic enzymes in turbot [J]. Acta Hydrobiologica Sinica, 2013, 37(3): 425—433 [聂琴, 苗惠君, 苗淑彦, 等. 不同糖源及糖水平对大菱鲆糖代谢酶活性的影响. 水生生物学报, 2013, 37(3): 425—433]
[27] Ge X P. Effects of different carbohydrate and lipid levels in diets on carbohydrate metabolic enzymes in Topmouth Culter (Eryghroculter ilishaeformis Bleeker) [D]. Thesis for Doctor of Science. Nanjing Agricultural University, Nanjing. 2006 [戈贤平. 不同糖、脂含量日粮对翘嘴红鲌 相关糖代谢酶的调节研究. 博士学位论文, 南京农业大学. 南京. 2006]
[28] Borrebaek B, Christophersen B. Hepatic glucose phosphorylating activities in perch (Perca fluviatilis) after different dietary treatments [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2000, 125(3): 387—393
[29] Tranulis M A, Dregni O, Christophersen B, et al. A glueokinase-like enzyme in the liver of Atlantic salmon (Salmo salary) [J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 1996, 114(1): 35—39
[30] Panserat S, Plagnes-Juan E, Kaushik S. Hepatic phosphoenolpyruvate carboxykinas gene expression is notrepressed by dietary carbohydrates in rainbow trout (Oncorhynchus mykiss) [J]. The Journal of Experimental Biology, 2001, 204(1): 359—365
[31] Smith J T, Acuff R V, Bittle J B, et al. A metabolic comparison of cysteine and methionine supplements in the diet of a rat [J]. The Journal of Nutrition, 1983, 113(2): 222—227
[32] Cai D M, Jia Y M, Song H G, et al. Betaine supplementation in maternal diet modulates the epigenetic regulation of hepatic gluconeogenic genes in neonatal piglets [J]. PLoS One, 2014, 9(8): e105504
[33] Kwasek K, Terova G, Lee Bong-Joo, et al. Dietary methionine supplementation alters the expression of genes involved in methionine metabolism in salmonids [J]. Aquaculture, 2014, 433(20): 223—228
[34] Lansard M, Panserat S, Plagnes-Juan E, et al. L-leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes [J]. The Journal of Nutrition, 2011, 141(1): 75—80
EFFECTS OF DIETARY METHIONINE ON THE GROWTH PERFORMANCE,
ANTI-OXIDATION AND ACIVITIES OF GLUCONEOGENESIS-RELATED ENZYME IN JUVENILE GROUPERS, EPINEPHELUS COIOIDES
CHI Shu-Yan, WANG Xue-Wu, TAN Bei-Ping, YANG Qi-Hui, DONG Xiao-Hui, LIU Hong-Yu
and ZHANG Shuang
(Laboratory of Aquatic Economic Animal Nutrition and Feed, College of Fisheries, Guangdong Ocean University, Zhanjiang 524088, China)
In this study, we investigated the effects of dietary methionine (Met) on the growth performance, the serum index, the activities of key enzymes in Met metabolism, and the oxidation of juvenile groupers (Epinephelus coioides). Six iso-nitrogen and iso-lipid diets were prepared with DL-Met supplement at different concentrations, 0.71%, 0.98%, 1.26%, 1.57%, 1.86%, and 2.18% (Diet1-Diet6). Each treatment was randomly assigned to a triplicate of 30 fish [initial weight (9.75±0.05) g] per aquarium. Fish were fed at 8: 00 and 17: 00 every day and were maintained in a flow- through aquaria for eight weeks. The results showed that the weight gain and the specific growth rate of the Diet3 group were significantly higher than those of the Diet1 and Diet6 groups (P<0.05). The condition factor of the Diet4 group was significantly higher than that of the Diet1, Diet5 and Diet6 groups (P<0.05). The levels of serum total proteins of the Diet2 and Diet3 groups were significantly higher than that of the Diet5 group (P<0.05). The level of blood glucose of the Diet3 group was significantly lower than that of the Diet1 and Diet2 groups (P<0.05). The level of serum total cholesterol was gradually reduced in the Diet3 group, and it was significantly decreased in the Diet4 and Diet6 groups compared to the Diet2 group (P<0.05). The activities of superoxide dismutase (SOD) and catalase (CAT) in the liver of the Diet3 group were the highest (P<0.05). There was no significant difference in the activity of phosphoenolpyruvate carboxylase kinase (PEPCK) between the Diet3 and the Diet4 groups, but it was significantly lower than that in the other groups (P<0.05). Quadratic regression analysis of the specific growth rate corresponding to the level of dietary methionine indicated that the optimal concentration of dietary methionine for the growth of juvenile groupers was 1.42% of dry diet in the presence of 0.46% cystine (corresponding to 3.16% of dietary protein on a dry weight basis). At this level, the blood glucose, the serum total cholesterol and the activity of PEPCK could be relatively low, which would help improve the energy efficiency in fish metabolism. Also the increased activities of SOD and CAT may help protect the body from oxidative damages.
Methionine; Growth performance; Serum index; PEPCK; Epinephelus coioides
S965.3
A
1000-3207(2015)04-0645-08
10.7541/2015.86
2014-11-27;
2015-03-05
国家自然科学基金(31402310); 公益性行业(农业)科研专项(201003020); 广东省科技创新项目(2013KJCX0097); 广东省高等学校科技创新重点项目(粤财教2011-473)资助
迟淑艳(1977—), 女, 内蒙古赤峰人; 博士; 主要从事水产动物营养与饲料学研究。E-mail: chishuyan77@163.com
谭北平(1967—), 男, 湖北巴东人; 博士; 主要从事水产动物营养与饲料学研究。E-mail: bptan@126.com
