Radiofrequency ablation-assisted liver resection: a step toward bloodless liver resection
2015-02-07
Nicosia, Cyprus
Radiofrequency ablation-assisted liver resection: a step toward bloodless liver resection
Athanasios Petrou, Kyriakos Neofytou, Constantinos Mihas, Jessamy Bagenal, Michael Kontos, John Griniatsos and Evangelos Felekouras
Nicosia, Cyprus
BACKGROUND:Liver resection is currently the most effcient curative approach for a wide variety of liver tumors. The application of modern techniques and new surgical devices has improved operative outcomes. Radiofrequency ablation is used more often for liver parenchymal transection. This study aimed to assess the effcacy and safety of radiofrequency ablation-assisted liver resection.
METHODS:A retrospective study of 145 consecutive patientswho underwent radiofrequency ablation-assisted liver resection was performed. Intraoperative blood loss, need for transfusion or intraoperative Pringle maneuver, the duration of liver parenchymal transection, perioperative complications, and postoperative morbidity and mortality were all evaluated.
RESULTS:Fifty minor and ninety-fve major liver resections were performed. The mean intraoperative blood loss was 251 mL, with a transfusion rate of 11.7%. The Pringle maneuver was necessary in 12 patients (8.3%). The mean duration for parenchymal transection was 51.75 minutes. There were 47 patients (32.4%) with postoperative complications. There is no mortality within 30 days after surgery.
CONCLUSIONS:Radiofrequency ablation-assisted liver resection permits both major and minor liver resections with minimal blood loss and without occlusion of hepatic infow. Furthermore it decreases the need for blood transfusion and reduces morbidity and mortality.
(Hepatobiliary Pancreat Dis Int 2015;14:69-74)
bleeding; complications; hepatectomy; radiofrequency ablation; Pringle maneuver
Introduction
Liver resection is the only potentially curative treatment for liver tumors[1-5]and perioperative blood loss is one of the well identifed factors affecting morbidity and mortality.[6-11]
The Pringle maneuver is a valuable tool for controlling intraoperative bleeding but places the patient at a high risk of liver damage because of ischemia-reperfusion injury or other well documented complications.[12-18]
In an attempt to reduce the complications related to major vascular occlusion, several techniques for liver parenchymal transection without the need of clamping have been described. These surgical techniques combined with anesthetic and critical care improvements have signifcantly contributed to the reduction of morbidity and mortality associated with major liver resection.[1-5]
Since the late 1990s, radiofrequency ablation (RFA) has been broadly used forin situthermal destruction of the liver and other solid organ tumors. Subsequently, its use has been expanded to liver parenchymal transection. The standard technique consists of applying the RFA needle along the intended line of parenchymal transection, aiming to create a bloodless plane of coagulative necrosis.[19-22]Furthermore, these devices can be used not only for liver resection but also for synchronous tumor ablation within the same procedure.
Despite the fact that partial and total vascular occlusions are effcient in controlling intraoperative hemorrhage, their application may cause liver damage due to ischemia-reperfusion injury. Liver ischemia-reperfusion injury have been investigated in depth, and several strat-egies to minimize their severity have been developed.
This study aimed to determine the effcacy and safety of RFA-assisted liver resection through the analysis of 145 consecutive patients with primary or metastatic liver malignancies and benign liver tumors.
Methods
All patients who had undergone liver resection with the Cool-Tip radiofrequency device at the First Department of Surgery, University of Athens Medical School, LAIKO Teaching Hospital, from August 2001 to August 2008 were included in the study. All patients were evaluated preoperatively with spiral CT, MRI (plus magnetic resonance angiography/magnetic resonance cholangiopancreatography when necessary) and/or positron emission tomography.[23]
Demographic details, histological type and number of liver tumors, type of procedure, overall operative time, parenchymal transection time, overall amount of intraoperative blood loss and quantity of blood loss during the liver parenchymal transection, were retrospectively collected from patients' notes. Perioperative liver function tests, intra/postoperative complications, length of hospital stay, mortality, and fnal outcome were also included. The operative time was counted from knife-toskin time to the closure of skin incision. Liver resection time was defned as the time from the start of radiofrequency probe application to the liver tissue until the conclusion of parenchymal transection. The overall blood loss was calculated by the weight of surgical swabs in addition to the quantity of blood in the suction system for the whole surgical procedure. Blood loss during the parenchymal transection was measured in a similar way.
Techniques
RFA-assisted liver resection was performed as previously described.[21]Briefy, with the patient in approximately 15 to 20 degree Trendelenburg supine position and the central venous pressure below 5 mmHg, the liver was routinely mobilized. The preferred operation for the liver mobilization was performed as described by Iwatsuki et al.[24]
The hepatodudenal ligament and corresponding hepatic veins in the case of right or left liver resection and the ipsilateral branches of the hepatic artery, portal vein and common bile duct were encircled with vessel loops when an anatomical hepatectomy was planned. When segmental or other non-anatomical resections of the liver were planned, the liver incision line was determined through intraoperative ultrasonography. It is essential to mark the resection line before the application of the RFA probe as RFA coagulates the hepatic tissue, consequently causing signifcant image alterations on intraoperative ultrasonography. Thus, the visualization of tumor margins and the correlation with the surrounding vascular structures is hindered.[25]
Following complete liver mobilization, the parenchymal transection commenced with the application of a single 15-cm needle electrode with a 2-cm exposure tip (Radionics Cooltip RFA System-Valleylab, Boulder, Co, USA; a subsidiary of Tyco Healthcare Group LP). The preferred setting of the radiofrequency energy generator was established at 95 watts on the 480 kHz scale and the electro surgical generator was set to the manual control mode.
The RFA needle electrode was inserted superfcially into the liver parenchyma and gradual progression to deeper lying tissues follows. Hemostasis of the liver tissue by a single RFA application was achieved in less than one minute and followed by sharp tissue transection (Fig.). For optimal exposure of the cut surfaces of the liver, the open book technique was used. The assistant gently retracted the hepatic lobe or segment that was being resected. This ensured clear visualization of intrahepatic vessels and adequate coagulation before sharp transection. When minor hemorrhage occurred, the cut surfaces were approximated and pressed together in order to minimize the local blood supply and thus enhance the ablation effect (heat-sink phenomenon). Persistent minor hemorrhages or bile leak was controlled with sutures or clips.
The ipsilateral hepatic vein was ligated at the end of parenchymal dissection when an anatomical or extended hepatectomy was intended. The main extrahepatic bile duct structures were preserved and protected by radiofrequency energy. Typically, they were sharply divided and ligated with suture at the end of the liver parenchyma division. Packed red blood cells were transfused as needed to maintain hemoglobin levels above 8%-9% during the intraoperative and immediate postoperative period (24 hours).
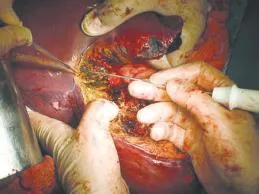
Fig.RFA-assisted liver parenchymal transection.
Statistical analysis
The SPSS statistical software (Version 12, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Common statistical methods were applied to estimate the signifcance of the results. The Chi-square test, the Mann-WhitneyUtest, and Fisher's exact test were used where appropriate. Differences were considered to be signifcant ifP<0.05.
Results
One hundred and forty-fve consecutive patients underwent RFA-assisted liver resection from August 2001 through August 2008. Patients and tumor characteristics are shown in Table 1. All patients with hepatocellular carcinoma had underlying cirrhosis (29 Child-Pugh A, 15 Child-Pugh B). Thirty-one of them were hepatitis virus B or C or B and C positive.
Ninety-fve patients underwent major (involving three or more segments) and 50 patients underwent minor (two or fewer segments or hepatic wedge resections/ tumorectomies) liver resections (Table 2). In this study, major liver resections were more common among the patients with primary malignant liver tumors than those with metastatic liver disease (85.0% vs 59.7%,P=0.002) or benign liver tumors (85.0% vs 22.2%,P<0.001). The mean overall operative time was 194 minutes (range 90 to 550). The mean parenchymal transection time was 51.75 minutes (range 18 to 120). The mean overall intraoperative blood loss was 251.1 mL, and the mean blood loss during parenchymal transection was 144.7 mL. The operative time needed for liver parenchymal transection and the mean blood loss during parenchymal transection were determined by the extent of the raw liver surface and the presence of cirrhosis (Tables 3 and 4). Four patients were complicated with major intraoperative hemorrhage. Three of them had hemorrhage during a challenging hilar dissection and one had major intraoperative hemorrhage because of inadequate ablation of vessels during RFA-assisted parenchymal transection. Seventeen patients underwent blood transfusion either intraoperatively or within 24 hours after operation.
Pringle maneuver was performed for 12 patients (Table 3). The mean blood loss during parenchymal transection was higher in patients who required Pringlemaneuver than in those who did not (292 vs 131 mL,P<0.001). This is probably explained by the fact that Pringle maneuver was utilized as the last resort in patients where bleeding could not be controlled with less invasive measures.
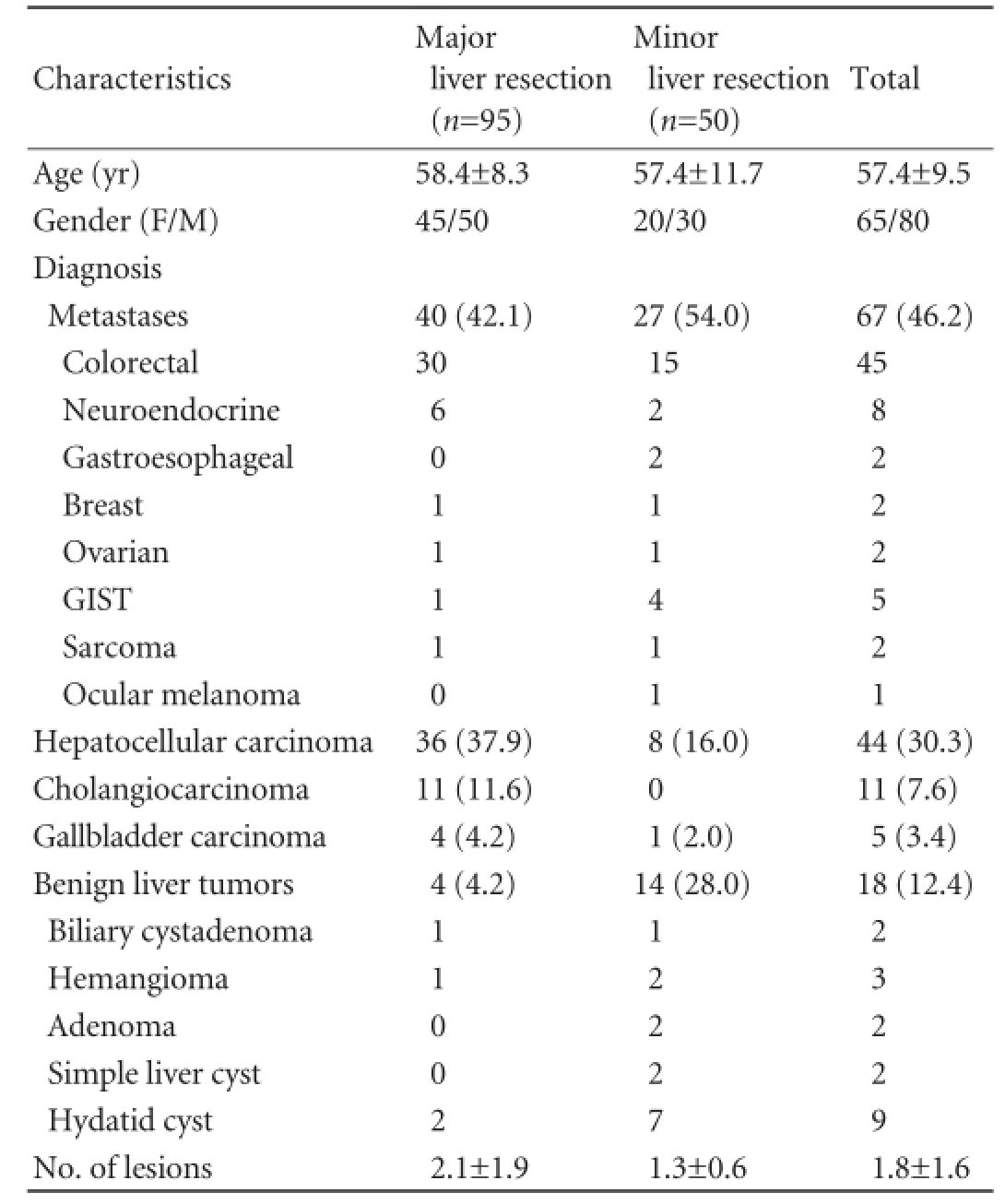
Table 1.Characteristics of patients and tumors (n, %)
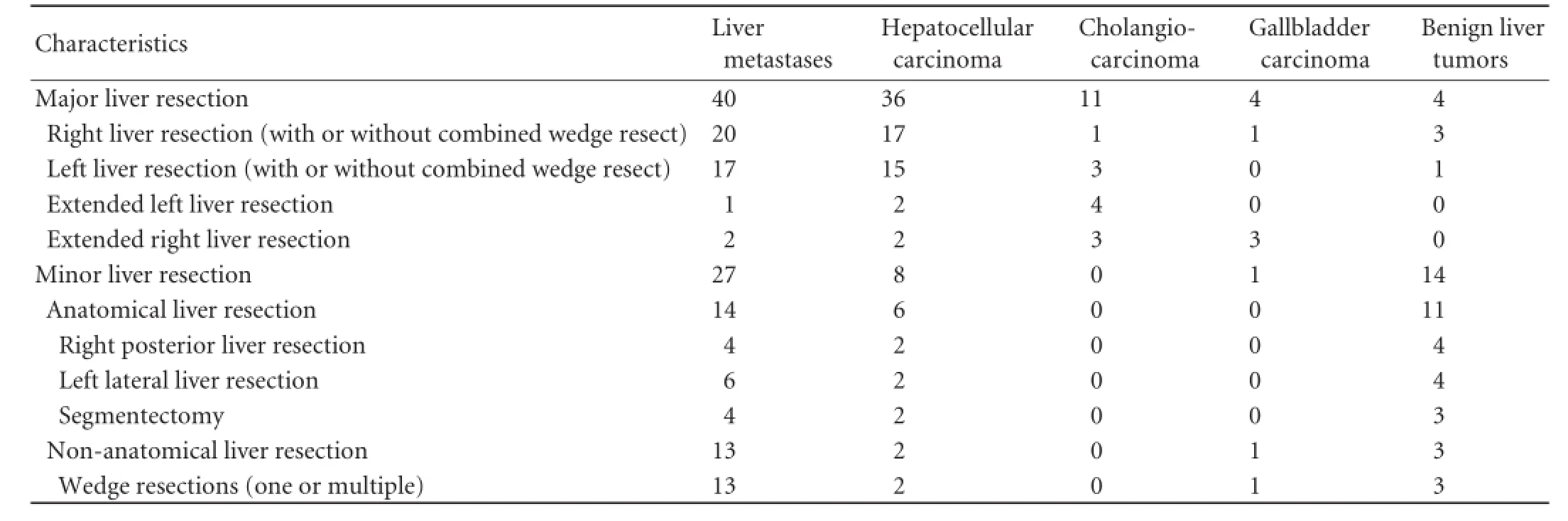
Table 2.Operation characteristics
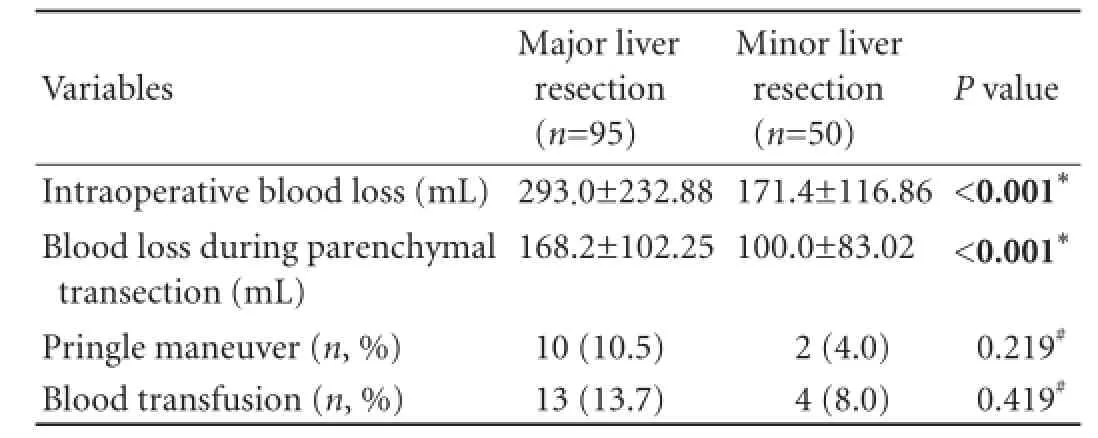
Table 3.Blood loss and liver vascular occlusion
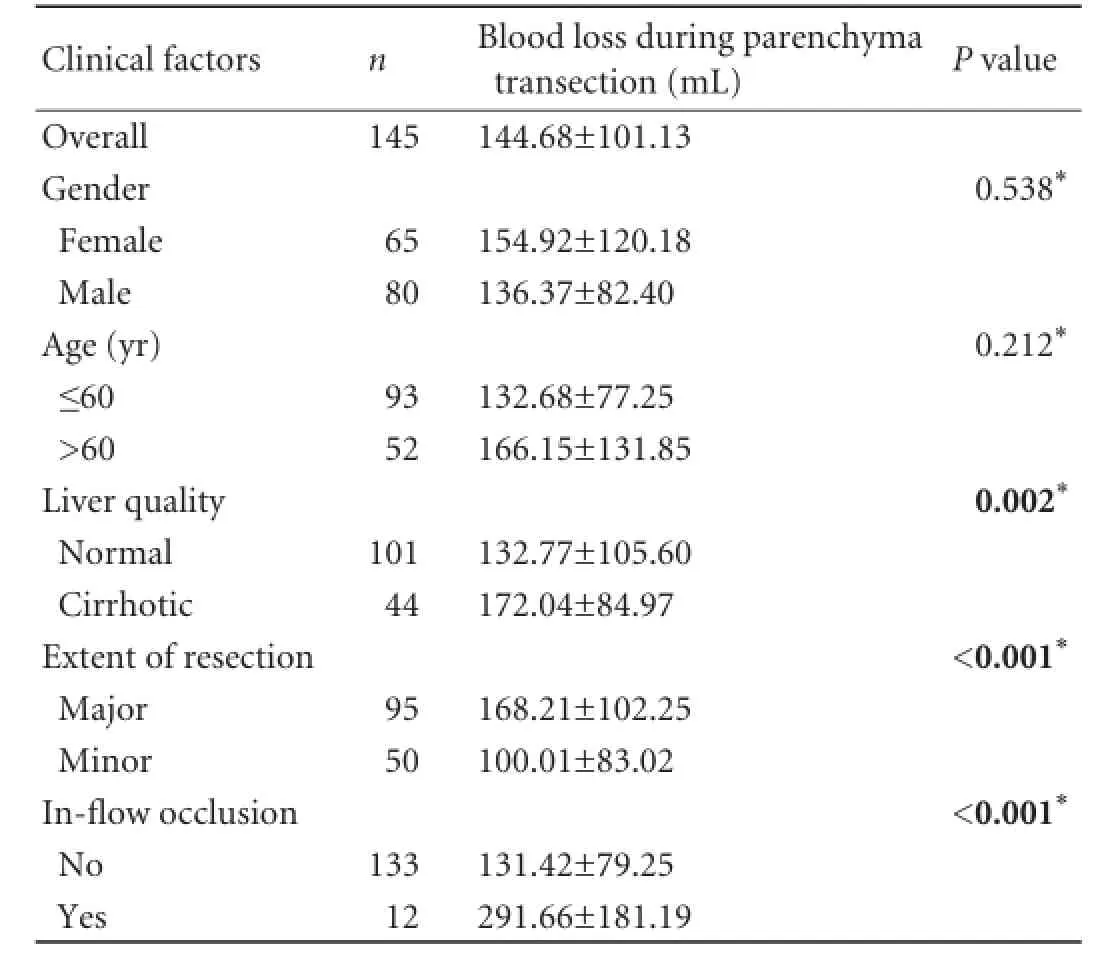
Table 4.Factors affecting blood loss during RFA-assisted liver transection
Liver function test rates were slightly elevated during the early postoperative period. In the vast majority of patients, the liver function tests were normalized within seven days after operation. In patients with cancerous pathology (n=127), a complete R0 resection was achieved in 114 patients and R1 resection in 13. The length of hospitalization ranged from 3 to 42 days (mean 8). Thirtyeight patients were admitted to the intensive care unit postoperatively.
Morbidity occurred in 47 patients. Complications and their management are summarized in Table 5. No patient required re-operation. Regarding the most severe complications, ffteen patients had transient liver insuffciency (all recovered completely). Three out often patients who developed postoperative bile leak had a biliary anastomosis as part of the frst surgery. The mean duration of bile leak was 17 days (5-32 days). The overall incidence of complications among patients undergoing major or minor liver resection was comparable in this study (27.4% vs 42.0%,P=0.074). Finally, none of each complication was more frequent in patients who underwent major liver resection than those who underwent minor excision (Table 5). The hospital mortality was zero.
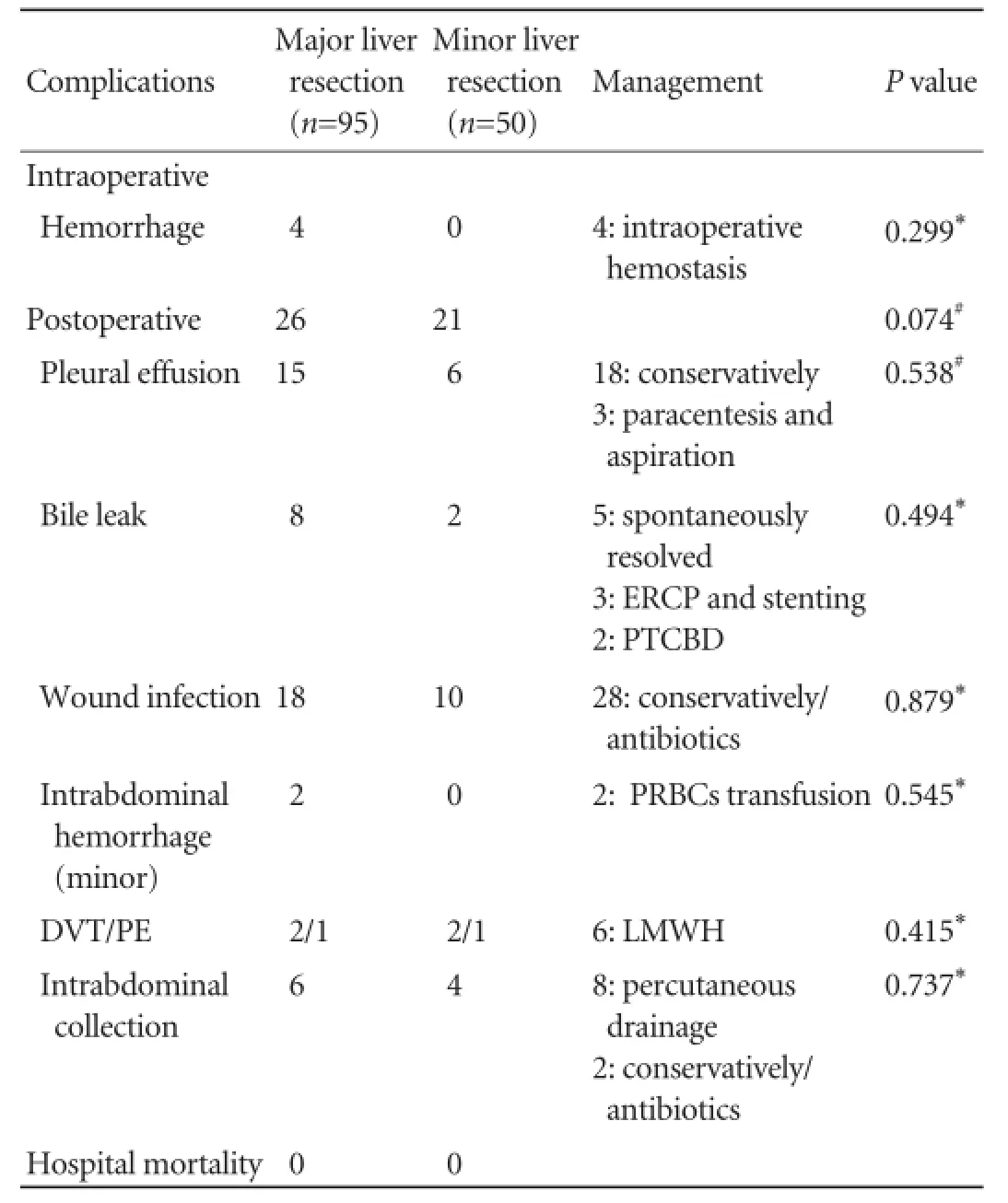
Table 5.Perioperative and postoperative complications
Discussion
During the past twenty years, hepatobiliary surgery has rapidly progressed. The methods and surgical technique of liver parenchymal transection have dramatically transformed from basic scalpels and scissors to refned devices such as the Argon beam coagulator, microwave generators, cavitron ultrasound surgical aspirator, waterjet scalpels, different types of harmonic scalpels, vascular staplers and radiofrequency. The use of these advanced instruments with concomitant improvements in anesthesia and intensive care unit have minimized the mortalityassociated with these complex surgeries.[1-5]
Despite these advancements, liver surgery remains one of the most technically demanding felds with a relatively high morbidity and mortality rate from complications such as hemorrhage, liver insuffciency/failure, bile leaks, and collection formations (serous, bilomas, hematomas, abscess, etc).
The patients in our series had a low rate of blood transfusions (11.7%), that was comparable with the rates from other groups.[26-32]Most patients avoided the risk of hepatic infow occlusion.[14-18]Only twelve (8.3%) patients required Pringle maneuver. Before adopting this technique for parenchymal transection, we performed the Pringle maneuver routinely. These data suggested that RFA-assisted liver resection with the use of a single needle is a useful and effcient technique toward bloodless liver resection without the use of vascular occlusion. The avoidance of whole-liver ischemic damage caused by Pringle maneuver is believed to be the main reason for the low rate of transient liver insuffciency in our series (10.34%, 15/145).
There are concerns on biliary tree injury following RFA liver resection in the literature.[33]It is hypothesized that the diffculty in recognizing the anatomical structures during RFA-assisted liver resection contributes to this type of complication. In the present study, seven of such cases occurred in the frst 70 liver resections. Increased experience in the use of this technique minimizes the risk of unrecognized iatrogenic injury to intrahepatic bile ducts. RFA devices coagulate nearly all the anatomical structures and surrounding tissue. Thus, the use of intraoperative ultrasonography to identify important structures along the predetermined resection line is crucial. This is particularly important when a vascular-biliary bundle, vital for the function of the posthepatectomy remaining liver tissue runs in proximity to the transection plane.
Concerns have been reported about the possible risk of infective complications following RFA-assisted liver resections because of necrotic tissue left behind.[34]However, our rate of symptomatic collections requiring intervention was very low (6.9%). This is equivalent to the rate reported by other groups.[27,30,31]
In our series, 127 patients had malignant pathology: a complete pathological resection (R0) was performed in 114 patiens and R1 resection in 13. In addition, a coagulated zone remained alongside the resection margin at the end of liver resection and it is believed that this may confer an improved locoregional oncological outcome. As reported, there is the possibility of tissue ablation beyond the histological margins. The clearance of this margin (resulting from the RFA effect on both sides of the transection plane) may reduce the incidence of local recurrence and anticipate an even better R0 vs R1 rate. There is growing evidence that radiofrequency is implicated in the enzymatic mechanism of cell division causing cell apoptosis.[35]
RFA is the only device that allows a combined/onestage procedure of tumor ablation and liver resection. The necessity of liver resections with synchronous RFA of the lesions encountered in the remaining liver is common. The described method offers the fexibility to perform (one-stage) liver resections and/or liver ablation in patients with complicated metastatic liver disease with a single device.
Despite the limitations associated with a retrospective, single-institution study, the fairly large number of patients included in this series and their favorable postoperative course allow the conclusion that RFA-assisted liver resection is an effcient and safe method for both major and minor liver resections. The method is strongly associated with decreased blood loss, reduced postoperative morbidity and minimized mortality. We believe that RFA-assisted liver resection is a useful, versatile and effective surgical technique for the transection of liver parenchyma.
Contributors:GJ and FE proposed the study. PA, NK, BJ and KM performed the research. MC, GJ and FE performed quality control of data. PA, NK and MC performed data analysis and interpretation. PA and NK wrote the frst draft. PA, BJ and KM performed manuscript editing. All authors reviewed the fnal manuscript. FE is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Fortner JG, Silva JS, Golbey RB, Cox EB, Maclean BJ. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg 1984;199:306-316.
2 Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992;216:493-505.
3 Castaing D, Kunstlinger F, Habib N, Bismuth H. Intraoperative ultrasonographic study of the liver. Methods and anatomic results. Am J Surg 1985;149:676-682.
4 Nagasue N, Yukaya H, Ogawa Y, Hirose S, Okita M. Segmental and subsegmental resections of the cirrhotic liver under hepatic infow and outfow occlusion. Br J Surg 1985;72:565- 568.
5 Buell JF, Rosen S, Yoshida A, Labow D, Limsrichamrern S, Cronin DC, et al. Hepatic resection: effective treatment for pri-mary and secondary tumors. Surgery 2000;128:686-693.
6 Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancerfree survival after curative resection for hepatocellular carcinoma. Cancer 1993;72:1866-1871.
7 Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994;115:303-309.
8 Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 2001;234:63-70.
9 Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery 1989;106:740-749.
10 Shimada M, Matsumata T, Akazawa K, Kamakura T, Itasaka H, Sugimachi K, et al. Estimation of risk of major complications after hepatic resection. Am J Surg 1994;167:399-403.
11 Makuuchi M, Takayama T, Gunvén P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg 1989;13: 644-648.
12 Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-350.
13 Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-549.
14 Grace PA. Ischaemia-reperfusion injury. Br J Surg 1994;81:637-647.
15 Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. Total versus selective hepatic vascular exclusion in major liver resections. Am J Surg 2002;183:173-178.
16 Smyrniotis VE, Kostopanagiotou GG, Contis JC, Farantos CI, Voros DC, Kannas DC, et al. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World J Surg 2003;27:765-769.
17 Torzilli G, Makuuchi M, Midorikawa Y, Sano K, Inoue K, Takayama T, et al. Liver resection without total vascular exclusion: hazardous or benefcial? An analysis of our experience. Ann Surg 2001;233:167-175.
18 Wu CC, Hwang CR, Liu TJ, P'eng FK. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br J Surg 1996;83:121-124.
19 Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg 2002;236:560-563.
20 Stella M, Percivale A, Pasqualini M, Profeti A, Gandolfo N, Serafni G, et al. Radiofrequency-assisted liver resection. J Gastrointest Surg 2003;7:797-801.
21 Felekouras E, Prassas E, Kontos M, Papaconstantinou I, Pikoulis E, Giannopoulos A, et al. Liver tissue dissection: ultrasonic or RFA energy? World J Surg 2006;30:2210-2216.
22 Delis S, Bakoyiannis A, Tassopoulos N, Athanassiou K, Papailiou J, Brountzos EN, et al. Clamp-crush technique vs. radiofrequency-assisted liver resection for primary and metastatic liver neoplasms. HPB (Oxford) 2009;11:339-344.
23 Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-391.
24 Iwatsuki S, Sheahan DG, Starzl TE. The changing face of hepatic resection. Curr Probl Surg 1989;26:281-379.
25 Ayav A, Jiao L, Dickinson R, Nicholls J, Milicevic M, Pellicci R, et al. Liver resection with a new multiprobe bipolar radiofrequency device. Arch Surg 2008;143:396-401.
26 Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-407.
27 Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46.
28 Ercolani G, Ravaioli M, Grazi GL, Cescon M, Del Gaudio M, Vetrone G, et al. Use of vascular clamping in hepatic surgery: lessons learned from 1260 liver resections. Arch Surg 2008;143: 380-388.
29 Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698-710.
30 Feng ZQ, Huang ZQ, Xu LN, Liu R, Zhang AQ, Huang XQ, et al. Liver resection for benign hepatic lesions: a retrospective analysis of 827 consecutive cases. World J Gastroenterol 2008;14:7247-7251.
31 Huang ZQ, Xu LN, Yang T, Zhang WZ, Huang XQ, Cai SW, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 2009;122:2268-2277.
32 Pai M, Frampton AE, Mikhail S, Resende V, Kornasiewicz O, Spalding DR, et al. Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol 2012;38: 274-280.
33 DeMatteo RP, Fong Y, Jarnagin WR, Blumgart LH. Recent advances in hepatic resection. Semin Surg Oncol 2000;19:200-207.
34 Torzilli G, Donadon M, Montorsi M, Makuuchi M. Concerns about ultrasound-guided radiofrequency-assisted segmental liver resection. Ann Surg 2010;251:1191-1193.
35 Di Carlo I, Barbagallo F, Toro A, Sofa M, Guastella T, Latteri F. Hepatic resections using a water-cooled, high-density, monopolar device: a new technology for safer surgery. J Gastrointest Surg 2004;8:596-600.
Received November 1, 2013
Accepted after revision June 25, 2014
AuthorAffliations:Nicosia Surgical Department, Division of Hepatobiliary Pancreatic Surgery, Nicosia General Hospital, Nicosia, Cyprus (Petrou A and Neofytou K); Bristol Heart Institute, Severn School of Surgery, Bristol, UK (Bagenal J); First Department of Surgery, University of Athens Medical School, LAIKO Teaching Hospital, Athens, Greece (Mihas C, Kontos M, Griniatsos J and Felekouras E)
Kyriakos Neofytou, MD, Nicosia Surgical Department, Division of Hepatobiliary Pancreatic Surgery, Nicosia General Hospital, Nicosia, Cyprus (Tel: +357-97648458; Email: kneophy2@gmail. com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60304-0
Published online November 14, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- p38 MAPK inhibition alleviates experimental acute pancreatitis in mice
- Development of hybrid-type modifed chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells
- Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
- Predictors of incidental gallbladder cancer in elderly patients
- Primary non-Hodgkin's lymphoma of the liver: sonographic and CT fndings
- Enlarged pancreas: not always a cancer
