Extended central hepatectomy with preservation of segment 6 for patients with centrally located hepatocellular carcinoma
2015-02-07
Kaohsiung, Taiwan, China
Extended central hepatectomy with preservation of segment 6 for patients with centrally located hepatocellular carcinoma
Mahmoud Abdelwahab Ali, Jung-Fang Chuang, Chee-Chien Yong, Chih-Chi Wang, Chi-Ying Lin and Chao-Long Chen
Kaohsiung, Taiwan, China
BACKGROUND:In order to preserve functional liver parenchyma, extended central hepatectomy (segments 4, 5, 7 and 8 resection) was proposed for the management of centrally located hepatocellular carcinoma invading the right and middle hepatic veins, reconstructing segment 6 outfow in the absence of the thick inferior right hepatic vein. The present study was to describe our surgical techniques of extended central hepatectomy.
METHODS:Between 2008 and 2012, 5 patients with centrally located hepatocellular carcinoma invading or in the vicinity of the right and middle hepatic veins underwent extended central hepatectomy. The thick inferior right hepatic vein was preserved during dissection. Gore-Tex graft was used for segment 6 outfow reconstruction in the absence of the thick inferior right hepatic vein.
RESULTS:The mean future remnant liver volume for segments 2 and 3 was 28% versus 45% on segment 6 preservation. The mean tumor diameter was 7.4 cm. The thick inferior right hepatic vein was found in 1 patient. Outfow reconstruction from segment 6 was performed in 4 patients. Postoperative complications included bile leakage (1 patient), pleural effusion (2) and liver failure (1). The rate of graft patency was 75%. There was no perioperative mortality.
CONCLUSION:Extended central hepatectomy is a safe alternative for extended hepatic resection in selected patients attempting to preserve the functional liver parenchyma.
(Hepatobiliary Pancreat Dis Int 2015;14:63-68)
hepatectomy;hepatic vein thrombosis; hepatocellular carcinoma; liver cirrhosis; liver imaging
Introduction
Surgical management of centrally located hepatocellular carcinoma (HCC) is a challenging issue. Extended hemihepatectomy is considered to be the frst curative option for the treatment of centrally located liver tumors.[1,2]However, hepatic resection for HCC in cirrhotic liver is associated with a high mortality rate primarily because of inadequate hepatocellular reserve and limited capacity for regeneration in case of cirrhosis.[3,4]Even with normal non-tumorous parenchyma, posthepatectomy liver failure (PHLF) may occur after extensive resection.[5,6]Hence, hepatic parenchyma preservation should be considered in each case, provided that oncological aspect is not severed. Extended central hepatectomy (resection of Couinaud's segments 4, 5, 7 and 8[7]) was proposed by Makuuchi et al[8]for the management of centrally located HCC involving the right hepatic vein (RHV) and middle hepatic vein (MHV), depending on the thick inferior right hepatic vein (IRHV) for drainage of segment 6. However, the thick IRHV is present only in 20%-24% of cases.[7,9]Hence segment 6 venous outfow should be reconstructed when the RHV is divided and the IRHV is absent or not suffcient for drainage.[10]Lessons learnedfrom the anterior section drainage of right liver graft in living donor liver transplantation were the motive for outfow reconstruction in those patients with hepatic venous congestion that may result in serious complications like sepsis, liver failure, and even death.[11]In this study, we described our techniques and outcomes of extended central hepatectomy with or without reconstruction of segment 6 outfow performed in 5 consecutive patients.
Methods
Between June 2008 and February 2012, 5 patients underwent central liver resections including Couinaud's segments 4, 5, 7 and 8[7]in Kaohsiung Chang Gung Memorial Hospital by the same experienced surgeon. Data of the patients were collected prospectively and analyzed retrospectively. The mean follow-up period of the patients was 2 years.
Preoperative diagnosis and assessment
The preoperative work up for HCC in our patients was discussed in a previous report.[12]Abdominal ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and hepatic angiography were used for the diagnosis of the patients. In patients without hepatitis, either serum alpha fetoprotein (AFP) level of >400 ng/mL or histopathological confrmation was needed in addition to typical imaging fndings. After resection, diagnosis of HCC was confrmed by histopathological examination.
Criteria of resectability and eligibility
Criteria of resectability were absence of distant metastases, anatomically resectable lesion on preoperative imaging, absence of the main portal vein or inferior vena cava tumor thrombus and suffcient functional reserve as estimated by indocyanine green retention rate at 15 minutes (ICGR-15). Future remnant liver volume was estimated using CT volumetry and then standardized to the total liver volume (TLV), which was calculated from the patient's body surface area (BSA) using a mathematical formula TLV (mL)=706.2×BSA (m2)+2.4.[13]
Patients with tumors located in the right anterior and/or left medial section, invading or in the vicinity of the RHV and MHV with tumor-free Glissonian pedicle of the posterior section as confrmed on preoperative imaging and/or intraoperative Doppler ultrasonography (DUS) were considered for extended central hepatectomy (Figs. 1A and B, 2A and B). Patients were also selected for the procedure when the standardized future remnant liver volume (FRLV) was less than 30% if extended right hepatectomy was the selected procedure[14]and this cutoff value was increased in case of cirrhosis.
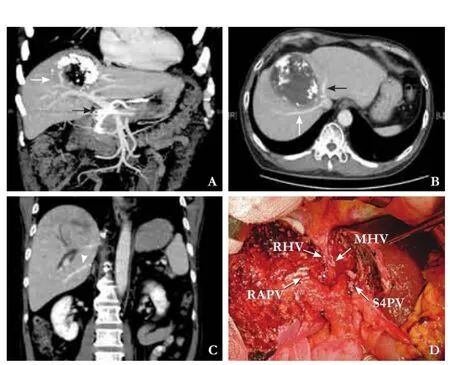
Fig. 1.Patient 1.A: Incomplete response to intra-arterial chemotherapy of tumor was shown (white arrow). The right and left portal veins were not invaded. The black arrow points to an intra-arterial chemotherapy catheter;B: The tumor in contact with the right hepatic vein (white arrow) and middle hepatic vein (black arrow);C: The thick inferior right hepatic vein (arrow head) shown by preoperative contrast enhanced CT;D: Vascular stumps after extended central hepatectomy. RHV: right hepatic vein; MHV: middle hepatic vein; RAPV: right anterior portal vein; S4PV: segment 4 portal vein.
No reconstruction of RHV was performed in case 1 because the thick IRHV was suffcient for segment 6 drainage (Fig. 1C). Outfow reconstruction from segment 6 was considered in cases lacking the thick IRHV, when cyanotic changes of segment 6 were noticed after transection of the RHV and when the absence of fow in segment 6 RHV remnant was confrmed by DUS.
Method of hepatectomy
Our surgical techniques for liver resection were described in a previous report.[12]A reverse-L incision was used in all cases. We routinely performed DUS to defne the limits of the tumor and to identify major vascular structures, diameter of the IRHV if existed, vascular invasion and lesions missed on preoperative evaluation. Mobilization of the right hemiliver was performed with preservation of the thick IRHV which was encircled and tapped. Hilar dissection was performed to identify right and left Glissonian pedicles. The area drained by the IRHV was estimated by inspecting the non-congested area on the posterior section on simultaneous clamping of the RHV and the right hepatic artery (RHA).
Because double transection plane usually takes more time than a single one, alternate clamping for infow control was used in 3 cases, including the left Glissonianpedicle for the left transection between segments 4 and 2-3, and the right Glissonian pedicle for the right transection, 15 minutes each.[15]Pringle's maneuver was used in case of persistent bleeding under Glissonian pedicle clamping. Parenchymal transection was performed via a clamp fracture technique, facilitated by CUSA (Valleylab Inc., Boulder, CO, USA), bipolar diathermy with irrigation, and individual ligation of larger blood vessels and bile ducts. The left parenchymal transection plane was carried just to the right of the umbilical ligament. On the right side, simultaneous clamping of the RHV and the RHA identifes the transection plane between anterior and posterior sections. After considerable transection, the two branches of the portal triad of S6 and 7 were exposed and only segment 7 branches were ligated while segment 6 branches were left intact. A further transection was made along the plane between segments 6 and 7.[16]The right anterior sectional portal pedicle and segment 4 pedicle were divided. The MHV and RHV were clamped and divided at the end of transection.
When cyanotic changes of segment 6 were noticed after transection of the RHV and absent fow in the RHV remnant in segment 6 was confrmed by DUS, Gore-Tex graft “8 mm×10 cm“ was interposed between the RHV stump at segment 6 and the stump of the RHV or MHV at inferior vena cava (IVC). The RHV or MHV stump at the IVC side was made smaller by approximating adjacent venous edges by using polypropylene (Prolene) 5-0 until the diameter was 10 mm, 2 mm wider than the Gore-Tex graft diameter. The graft was anastomosed to IVC side using 5-0 Prolene then to hepatic side using 6-0 Prolene, both in continuous fashion (Fig. 2C). No venous bypass was used. The patency of the graft was confrmed by DUS and the relief of congestion and cyanosis.
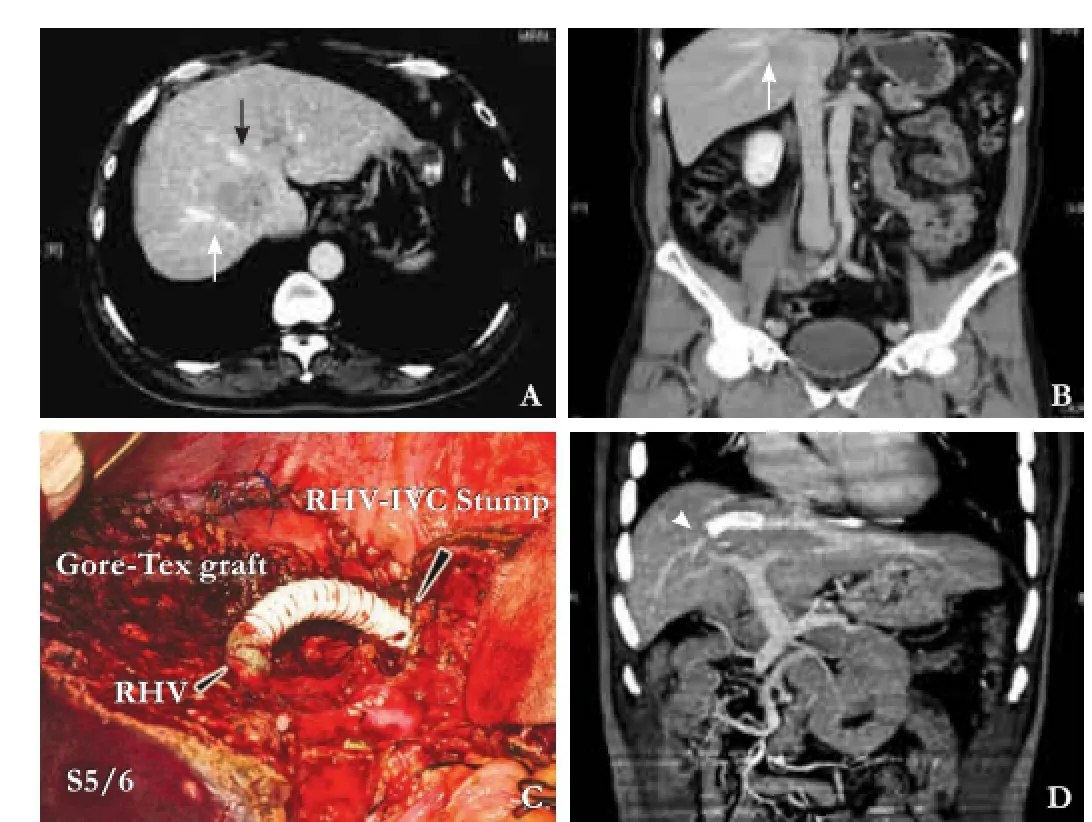
Fig. 2.Patient 3.A,B: The tumor is in contact with the right hepatic vein (white arrow) and middle hepatic vein (black arrow);C: Eight mm Gore-Tex graft (black arrow) was used to reconstruct the outflow of segment 6;D: Follow-up of enhanced computed tomography at 5 months postoperatively shows patent graft (arrow head).
Postoperative care and follow-up
All patients were routinely admitted to the intensive care unit for 1 or 2 days, or longer as necessary. Posthepatectomy liver failure (PHLF) was defned and graded according to the International Study Group of Liver Surgery (ISGLS).[6]The patients were followed up at 1 month and then every 3 months for the next 5 years. Graft patency was assessed by DUS prior to discharge and during routine follow-up. When graft obstruction was suspected, CT angiography was performed for confrmation (Fig. 2D). Non-visualized grafts shown by CT angiography were considered occluded grafts. Follow-up assessment for recurrence included liver function test, serum AFP level, and abdominal ultrasound. Further studies using CT scan or MRI were performed to confrm the diagnosis when there was a high index of suspicion of recurrence. Follow-up was recorded as time from surgery to date of the last visit or death.
Results
The baseline characteristics of the 5 patients are listed in Table 1. The patients were males with a mean age of 57 years. Four patients were positive for hepatitis B, but one was negative for both hepatitis B and C. Before surgery, 2 patients were treated with trans-arterial embolization (TAE) and 1 received intra-arterial chemotherapy. If extended right hepatectomy was performed, mean standardized FRLV for segments 2 and 3 would be 27.7% (range 21.2%-31.6%) as estimated by preoperative CT volumetry, whereas on segment 6 preservation it was 44.7% (range 38.8%-48.5%).
Peri-operative outcome and follow-up results are shown in Table 2. Extended central hepatectomy was performed in all patients. Their mean operative time was 487 minutes (range 428-595) and the mean parenchy-mal transection time was 126 minutes (range 95-150). Vascular infow control with alternate clamping of right and left Glissonian pedicles, 15 minutes for each clamping episode, was used in 3 patients, of whom one needed Pringle's maneuver for the control of bleeding. In the other 2 patients, intermittent vascular infow was occluded with clamping for 15 minutes and declamping for 5 minutes. The thick IRHV (>5 mm) was found in 1 patient, for whom outfow reconstruction of S6 wasnot needed (Fig. 1D). Congestion of S6 after division of the RHV was noted in 4 patients, so outfow reconstruction was performed using Gore-Tex graft between the RHV stump in S6 and the RHV stump (3 patients) or MHV stump (1 patient) on the IVC side. Intraoperative blood transfusion was needed in 1 patient. The mean tumor diameter was 7.4 cm (range 3.1-11.5), and the mean resection margin was 3 mm (range 1-10). Micro-vascular invasion was found in 4 patients, of whom one had major vascular invasion into the RHV and the right anterior portal vein. Histologically proven chronic hepatitis in non-tumorous liver parenchyma was found in three patients and cirrhosis in one. Two patients suffered from postoperative complications: patient 4 suffered from bile leakage and pleural effusion, whereas patient 5 suffered from PHLF grade B with ascites and pleural effusion. One of the patients with pleural effusion was subjected to drainage. The patient with PHLF was discharged on postoperative day 17 with a good recovery of liver functions. The mean hospital stay of the patients was 13 days (range 11-17). There were no perioperative mortalities. The patients were followed up for a mean period of 739 days (range 208-1502). The rate of Gore-Tex graft patency was 75% at 1 month. The mean disease-free survival of the patients was 21 months (range 3-50) and the mean overall survival was 23 months (range 7-50). Three patients had tumor recurrence in the liver (1 patient), in the lung (1) and in both lung and liver (1). However,none of the paients with hepatic recurrence had margin related recurrence. At the time of the last follow-up, 1 patient died, 2 were alive with tumor recurrence, and 2 were alive without tumor recurrence.
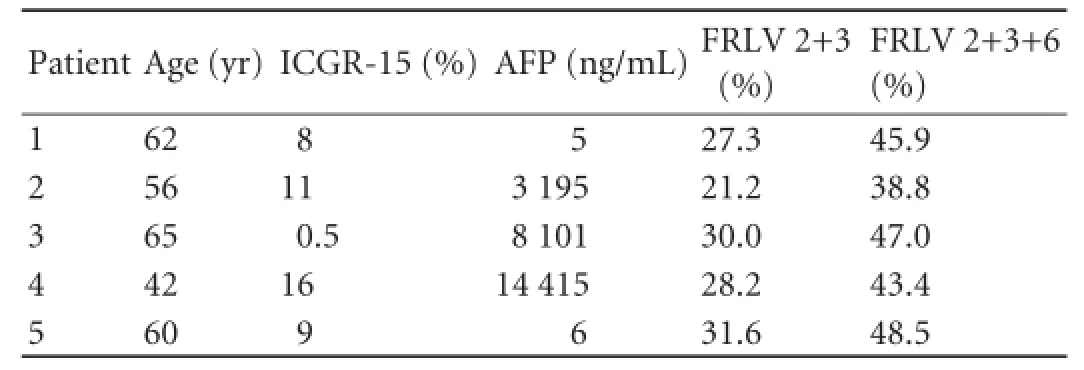
Table 1.Baseline characteristics
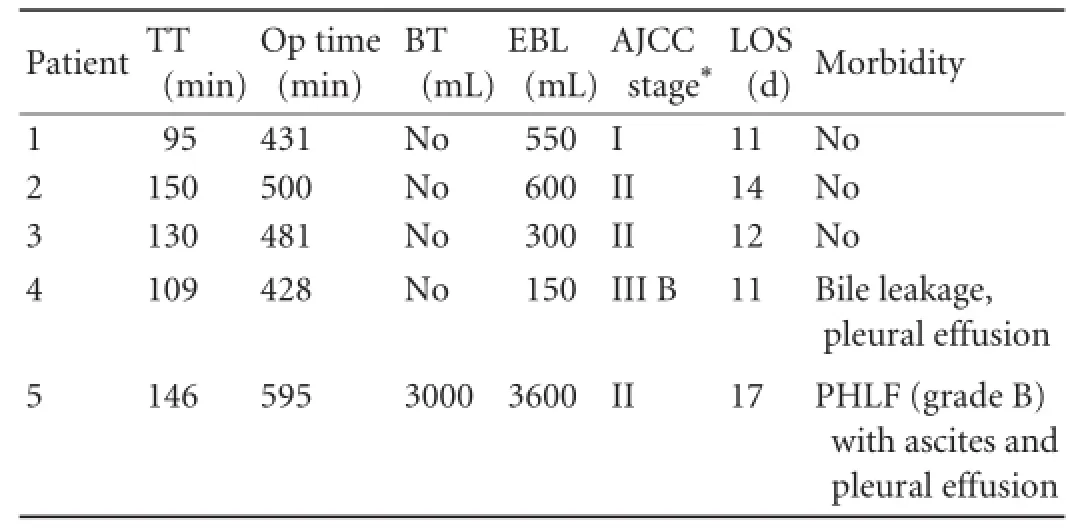
Table 2.Perioperative outcomes and follow-up
Discussion
Since the report by Makuuchi et al,[8]few studies on segments 4, 5, 7 and 8 resection have been published as case reports.[8,16]To our knowledge, the present case series is the frst one describing this procedure with segment 6 outfow reconstruction. The procedure was named extended central bisegmentectomy according to Tanaka et al.[16]This name does not correspond well with the nomenclature for liver resections created by the scientifc committee of the Hepato-Pancreatico-Biliary Association. Also resection of right anterior and left medial segments 4, 5 and 8 is not referred to the Brisbane terminology.[17]Based on the predominant use of “central hepatectomy“ referring to resection of segments 4, 5 and 8[18-20]and the reserved acceptance of the term “extended“by the Brisbane terminology,[17]we propose the name“extended central hepatectomy“ for the procedure.
A balance between conserving functional liver mass and obtaining adequate oncologic margins is challenging in resection of HCC in cirrhotic liver.[21]Extended hemihepatectomy is considered to be the frst curative option for the treatment of centrally located liver tumors.[1,2]However, extended hepatectomies carry more risk of PHLF because they usually result in small FRLV.[5,6]This risk is increased when the liver is impaired with cirrhosis.[3,4]Extended central hepatectomy has the advantage of segment 6 preservation in patients with central hepatic tumors necessitating RHV division, this preserves about 15%-20% of the FRLV.[22]In this study, mean standardized FRLV after extended right hepatectomy on preoperative CT volumetry for segments 2 and 3 was 28%, whereas on segment 6 preservation it was 45%. Preserving segment 6 increased the mean FRLV by 17%. The cut off value of standardized FRLV for prediction of postoperative hepatic dysfunction ranged between ≤ 20% and ≤30%, but this was applied to livers with normal non-tumorous parenchyma.[14,23]Three of our patients had histologically proven chronic hepatitis and one had cirrhosis. Although one patient would have FRLV of 31.6% if extended right hepatectomy was performed, cirrhosis was suspected because of preoperative liver functions and CT fndings and it was confrmed histologically after operation. So far, no cut-off values for the FRLV in cirrhosis are available, and further studies are needed to determine the safe limit of extended resection in the liver affected by fbrosis or hepatitis.
On the other hand, the mean resection margin was 3 mm. Central hepatic resections are usually associated with narrow resection margins,[15,18]especially on posterior section preservation.[24]However, the value of resection margin was usually overestimated, as studies showed that resection margin during hepatectomy for HCC has no impact on postoperative recurrence.[15,25]Although 3 of our patients had recurrence, none of them experienced cut-end relapse. Recurrence in those patients may be attributed to other risk factors like microvascular invasion (4 out of 5 cases) and large tumor size (mean 7.4 cm) which were proved to be independent risk factors for tumor recurrence.[12,25,26]
In patients with absence of the thick IRHV and RHV, venous outfow from segment 6 should be reconstructed after resection.[11]Many graft materials were used for hepatic vein reconstruction including the saphenous vein, inferior mesenteric vein and external iliac vein.[27-29]The use of Gore-Tex grafts was also reported. The grafts are easily handled and available in a wide range of lengths and diameters.[21]Also, they have no donor site complications as autologous grafts do.[29]Prosthetic grafts are increasingly used in patients receiving living donor liver transplantation with outfow reconstruction in anterior section drainage without the fear of infection.[30,31]In our series, the 1-month patency rate was 75%. The patient with early graft obstruction developed PHLF and was treated conservatively.
In conclusion, although technically demanding, extended central hepatectomy is an alternative for extended hepatic resection in selected patients attempting to preserve the functional liver parenchyma. Outfow reconstruction of segment 6, if needed, is feasible with a sound patency rate.
Contributors:AMA and CJF drafted the article and revised it critically for important intellectual content. WCC approved the fnal version to be published. All authors contributed to the design, interpretation and the fnal draft of the paper. WCC is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Scudamore CH, Buczkowski AK, Shayan H, Ho SG, Legiehn GM, Chung SW, et al. Mesohepatectomy. Am J Surg 2000;179: 356-360.
2 Mehrabi A, Mood ZA, Roshanaei N, Fonouni H, Müller SA, Schmied BM, et al. Mesohepatectomy as an option for the treatment of central liver tumors. J Am Coll Surg 2008;207: 499-509.
3 Hemming AW, Gallinger S, Greig PD, Cattral MS, Langer B, Taylor BR, et al. The hippurate ratio as an indicator of functional hepatic reserve for resection of hepatocellular carcinoma in cirrhotic patients. J Gastrointest Surg 2001;5:316-321.
4 Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993; 18:79-85.
5 Vauthey JN, Baer HU, Guastella T, Blumgart LH. Comparison of outcome between extended and nonextended liver resections for neoplasms. Surgery 1993;114:968-975.
6 Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a defnition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-724.
7 Couinaud C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver. Presse Med 1954;62:709-712.
8 Makuuchi M, Hasegawa H, Yamazaki S, Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet 1987;164:68-72.
9 Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet 1981;152:43-50.
10 Nakamura S, Sakaguchi S, Kitazawa T, Suzuki S, Koyano K, Muro H. Hepatic vein reconstruction for preserving remnant liver function. Arch Surg 1990;125:1455-1459.
11 Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation 2001;71:812-814.
12 Wang CC, Iyer SG, Low JK, Lin CY, Wang SH, Lu SN, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832-1842.
13 Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995;21:1317-1321.
14 Yigitler C, Farges O, Kianmanesh R, Regimbeau JM, Abdalla EK, Belghiti J. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl 2003;9: S18-25.
15 Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg 2003;238:29-34.
16 Tanaka K, Nishimura A, Takenaka K, Yamada K, Ishibe R, Ogata S, et al. Extended central bisegmentectomy--an en bloc resection of hepatic segments 4, 5, 8 and 7: report of a case. Surg Today 1994;24:170-172.
17 Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 2002;4:99-100.
18 Lee JG, Choi SB, Kim KS, Choi JS, Lee WJ, Kim BR. Central bisectionectomy for centrally located hepatocellular carcinoma. Br J Surg 2008;95:990-995.
19 Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery 2003;133:251-256.
20 Stratopoulos C, Soonawalla Z, Brockmann J, Hoffmann K, Friend PJ. Central hepatectomy: the golden mean for treating central liver tumors? Surg Oncol 2007;16:99-106.
21 Hemming AW, Reed AI, Langham MR, Fujita S, van der WerfWJ, Howard RJ. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg 2002;235:850-858.
22 Stone HH, Long WD, Smith RB 3rd, Haynes CD. Physiologic considerations in major hepatic resections. Am J Surg 1969; 117:78-84.
23 Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, et al. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg 2008;12: 123-128.
24 Shimizu A, Kobayashi A, Yokoyama T, Nakata T, Motoyama H, Kubota K, et al. Hepatectomy preserving drainage veins of the posterior section for liver malignancy invading the right hepatic vein: an alternative to right hepatectomy. Am J Surg 2012;204:717-723.
25 Poon RT, Fan ST, Ng IO, Wong J. Signifcance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg 2000;231:544-551.
26 Abdel-Wahab M, El-Husseiny TS, El Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg 2010;395:625-632.
27 Smyrniotis V, Arkadopoulos N, Kehagias D, Kostopanagiotou G, Scondras C, Kotsis T, et al. Liver resection with repair of major hepatic veins. Am J Surg 2002;183:58-61.
28 Kaneoka Y, Yamaguchi A, Isogai M, Hori A. Hepatic vein reconstruction by external iliac vein graft using vascular clips. World J Surg 2000;24:377-382.
29 Kilic M, Aydin U, Sozbilen M, Ozer I, Tamsel S, Demirpolat G, et al. Comparison between allogenic and autologous vascular conduits in the drainage of anterior sector in right living donor liver transplantation. Transpl Int 2007;20:697-701.
30 Hwang S, Jung DH, Ha TY, Ahn CS, Moon DB, Kim KH, et al. Usability of ringed polytetrafuoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl 2012;18:955-965.
31 Yi NJ, Suh KS, Lee HW, Cho EH, Shin WY, Cho JY, et al. An artifcial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl 2007;13:1159-1167.
Received February 8, 2014
Accepted after revision May 22, 2014
AuthorAffliations:Division of General Surgery, Department of Surgery, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taiwan, China (Ali MA, Chuang JF, Yong CC, Wang CC and Chen CL); Department of Surgery, PingTung Christian Hospital, Taiwan, China (Lin CY)
Chih-Chi Wang, MD, Department of Surgery, Liver Transplant Program, Kaohsiung Chang Gung Memorial Hospital, 123 Ta-Pei Road, Niao-Song, Kaohsiung 833, Taiwan, China (Tel: +886-7-731-7123ext8093; Fax: +886-7-735-4309; Email: ufel4996@ms26.hinet.net)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60302-7
Published online December 30, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- p38 MAPK inhibition alleviates experimental acute pancreatitis in mice
- Development of hybrid-type modifed chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells
- Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
- Predictors of incidental gallbladder cancer in elderly patients
- Primary non-Hodgkin's lymphoma of the liver: sonographic and CT fndings
- Enlarged pancreas: not always a cancer
