Effects of Kupffer cell inactivation on graft survival and liver regeneration after partial liver transplantation in rats
2015-02-07
Beijing, China
Effects of Kupffer cell inactivation on graft survival and liver regeneration after partial liver transplantation in rats
Hang-Yu Luo, Shan-Fang Ma, Ji-Fu Qu and De-Hu Tian
Beijing, China
BACKGROUND:Gadolinium chloride (GdCl3) selectively inactivates Kupffer cells and protects against ischemia/reperfusion and endotoxin injury. However, the effect of Kupffer cell inactivation on liver regeneration after partial liver transplantation (PLTx) is not clear. This study was to investigate the role of GdCl3pretreatment in graft function after PLTx, and to explore the potential mechanism involved in this process.
METHODS:PLTx (30% partial liver transplantation) was performed using Kamada's cuff technique, without hepatic artery reconstruction. Rats were randomly divided into the control low-dose (5 mg/kg) and high-dose (10 mg/kg) GdCl3groups. Liver injury was determined by the plasma levels of alanine aminotransferase and aspartate aminotransferase, liver regeneration by PCNA staining and BrdU uptake, apoptosis by TUNEL assay. IL-6 and p-STAT3 levels were measured by ELISA and Western blotting.
RESULTS:GdCl3depleted Kupffer cells and decreased animal survival rates, but did not signifcantly affect alanine aminotransferase and aspartate aminotransferase (P>0.05). GdCl3pretreatment induced apoptosis and inhibited IL-6 overexpression and STAT3 phosphorylation after PLTx in graft tissues.
CONCLUSION:Kupffer cells may contribute to the liver regeneration after PLTx through inhibition of apoptosis and activation of the IL-6/p-STAT3 signal pathway.
(Hepatobiliary Pancreat Dis Int 2015;14:56-62)
GdCl3; Kupffer cells; partial liver transplantation; liver regeneration; apoptosis
Introduction
Liver transplantation is the only effective therapy for end-stage liver diseases. The number of adultto-adult living donor liver transplants (LDLT) has been increasing annually since the frst successful LDLT in 1994.[1]Although the volume of most transplanted partial liver grafts tends to reach normal with time,[2,3]accumulating evidence has indicated that small grafts may negatively impact the postoperative course.[4,5]It has been suggested that in small-for-size grafts, less than 30% of standard liver volume can be used with careful intraoperative and postoperative management until the grafts regenerate.[6]Therefore, the regeneration of the partial graft is crucial to enable proper biological function, including protein synthesis and toxin (e.g. endotoxin) removal. Kupffer cells (KCs), the largest resident macrophage population in the liver,[6]are involved in liver's response to infection, toxins, ischemia, resection and other stresses.[7]KC activation is important for the optimal regenerative ability of the liver and interleukin-6 (IL-6), produced by activated KC, may play a role in mediating liver regeneration.[7]Gadolinium chloride (GdCl3), a selective KC toxin, has been reported to inhibit phagocytosis of KCs by interfering with calcium-dependent cell surface interactions.[8,9]The effect of KC on liver regeneration is inconclusive. Studies[10,11]reported that depletion of KCs by GdCl3in rat liver alleviated hepatic ischemia/reperfusion injury after experimental liver transplantation. Rai et al[12]reported that depletion of KC by GdCl3enhanced liver regeneration after partial hepatectomy in rats.
Watanabe and coworkers[13]demonstrated that depletion of KC by GdCl3impaired liver regeneration after partial hepatectomy. It is still not clear whether the donor pretreatment with GdCl3is benefcial on graft survival after partial liver transplantation (PLTx).
A variety of molecular mediators and signal transduction networks are involved and contribute to the process of liver regeneration. IL-6, which is mainly released from the parenchymal cells such as KCs, is a critical component of the regenerative response.[14,15]A study[14]demonstrated that IL-6-defcient mice had impaired liver regeneration characterized by liver necrosis and failure. Moreover, the signal transducer and activator of transcription 3 (STAT3), an essential mediator of the IL-6/ gp130 signaling pathway, plays a key role in the regulation of cell growth, differentiation, and survival.[16]It has been shown that up to 40% of the immediate-early genes during liver regeneration are regulated, at least in part, by IL-6, and a signifcant subset of them are also regulated by STAT3.[17]However, the potential involvement of IL-6 and STAT3 during liver regeneration after PLTx has not yet been fully determined.
In the present study, we investigated the survival rate, liver regeneration rate and cell damage in PLTx rats with GdCl3pretreated donor. Our results may help to understand better the mechanism of liver regeneration after PLTx.
Methods
Reagents
Rabbit anti-mouse anti-ED2 polyclonal antibody was purchased from Santa Cruz Biotechnology Co., CA, USA. Mouse anti-proliferating cell nuclear antigen (PCNA) monoclonal antibody was from Transduction Labs, Lexington, KY, USA. Mouse anti-bromodeoxyuridine (BrdU) monoclonal antibody was from BD Biosciences, USA. Mouse anti-Bcl-2 and anti-p-STAT3 primary antibodies were provided by Santa Cruz.
Animals
Male 10-week-old DA rats (RT-1a) (specifc pathogen-free), weighing 190-210 g, were provided by the Animal Experiment Center of Capital Medical University, Beijing. The animals were routinely housed under controlled conditions of lighting (12 hours light/12 hours dark cycle), humidity (50%-60%), and temperature (18-22 ℃) with free access to food and water. Donors and recipients were fasted for 12 hours before operation. All of the animals were treated according to a protocol approved by the Animal Research Committee of Third Military Medical University.
PLTx
Partial hepatectomy was performed as described by Huggins and Anderson.[18]Briefy, the left and median lobes were resected (70% hepatectomy), and liver tissues were perfused with 10 mL of cold Ringer's solution by slow injection into the portal vein. Then, the graft was placed in Ringer's solution on ice for 1 hour. The weight of graft was recorded as “liver weight 1“. PLTx was performed according to the Kamada's cuff technique, without hepatic artery reconstruction.[19]The time of the anhepatic phase was 13-16 minutes. Recipients were sacrifced at indicated time points after transplantation. The enlarged remaining lobes were carefully removed and their weights were recorded as “liver weight 2“. Subtracting “liver weight 1“ from “liver weight 2“ made it possible to calculate the percentage of weight-increase of the liver graft after a specifed period of time.
Experimental design
The animals were randomly divided into 3 groups: control, GdCl3low-dose and GdCl3high-dose groups, with 7 rats in each group. In the control group, donor animals received saline solution (0.5 mL) by intravenous injection through the penile vein at 48- and 24-hour before PLTx. In the GdCl3low-dose and high-dose groups, 5 and 10 mg/kg body weight GdCl3, respectively, dissolved in saline solution were administered prior to PLTx in the same manner.
Determination of liver damage
Arterial blood was withdrawn from the abdominal aorta of rats under anesthesia. Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using an automatic analyzer (7600, Hitachi Ltd., Tokyo, Japan)
Immunohistochemistry
Rats (n=5 from each experimental group) were sacrifced at 3 hours, 1, 3 or 5 days after PLTx. One hour before sacrifce, BrdU was injected intravenously at a dose of 50 mg/kg. A part of the allograft and right lungs were fxed in 10% formalin for hematoxylin and eosin (HE) staining and immunohistochemistry. Liver tissues were sectioned and embedded in paraffn, and KCs were identifed by a specifc antibody against ED2. The liver regeneration was assessed by PCNA staining and BrdU uptake. Immunohistochemistry was performed using the enhanced labeled polymer method with the ENVISION kit (DAKO, Copenhagen, Denmark). Nuclei werestained with HE. The dilutions of primary antibody were used as follows: 1:200 for ED2, 1:500 for PCNA, and 1:200 for BrdU.
Measurement of IL-6
Supernatants were obtained from homogenized liver samples in whole cell lysis buffer containing 50 mmol/L HEPES (pH 7.5), 200 mmol/L NaCl, 0.1% Tween-20, 1 mmol/L EDTA-2Na, 10% glycerol, and 150 mmol/L protease inhibitor. The levels of IL-6 were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Quantikine rat IL-6, R&D Systems, Minneapolis, MN, USA).
Western blotting
Total protein was extracted from the liver tissues using whole cell lysis buffer. A total of 30 μg of the protein samples were loaded on sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, proteins were transferred onto nitrocellulose membranes. After blocking, membranes were incubated with anti-Bcl-2 or anti-p-STAT3 antibodies. Immune complexes were detected by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody and an ECL detection system. The band densities were quantifed using NIH image shareware (Bethesda, MD, USA).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
Cell apoptosis was determined by using anin situapoptosis detection kit Apop Tag (Oncor, Gaithersburg, MD, USA). Briefy, sections prepared as described above were incubated with terminal transferase and digoxigenin-labeled dUTP, followed by incubation with antidigoxigenin monoclonal antibody conjugated to HRP. The slides were subsequently treated with peroxidase substrate and counterstained with methyl green.
Statistical analysis
Data were expressed as mean±SD. Statistical difference was compared by the Tukey-Kramer test. AP<0.05 was considered statistically signifcant.
Results
GdCl3pretreatment depletion of KCs
As shown in Fig. 1, ED2-positive KCs were distributed in the liver lobules of normal rat liver (Fig. 1A). However, the number of ED2-positive KCs was signifcantly reduced after a low-dose of GdCl3pretreatment compared with the control group (P<0.01, Fig. 1B, D). Highdose GdCl3pretreatment completely suppressed KCs growth. There is a signifcant difference between the control, low-dose and high-dose GdCl3groups (P<0.01, Fig. 1C, D).
Rat survival and liver function after PLTx
All control rats survived for two days after PLTx (n=7), whereas the low-dose (n=7) and high-dose (n=7) GdCl3pretreatment decreased the animal survival rates to 71% and 0, respectively. Two rats died and fve survived in the low-dose GdCl3pretreatment group on the second day after surgery, while in the high-dose group all the rats died in two days after surgery. Injection of GdCl3had no effect on serum ALT and AST levels compared with the control group. In all three groups, both serum ALT and AST levels were dramatically increased one day after PLTx, and gradually decreased to normal values on day 5 after the operation (Fig. 2). No statistical differences were found among groups with or without GdCl3pretreatment at any time point.
GdCl3pretreatment suppressed liver regeneration after PLTx
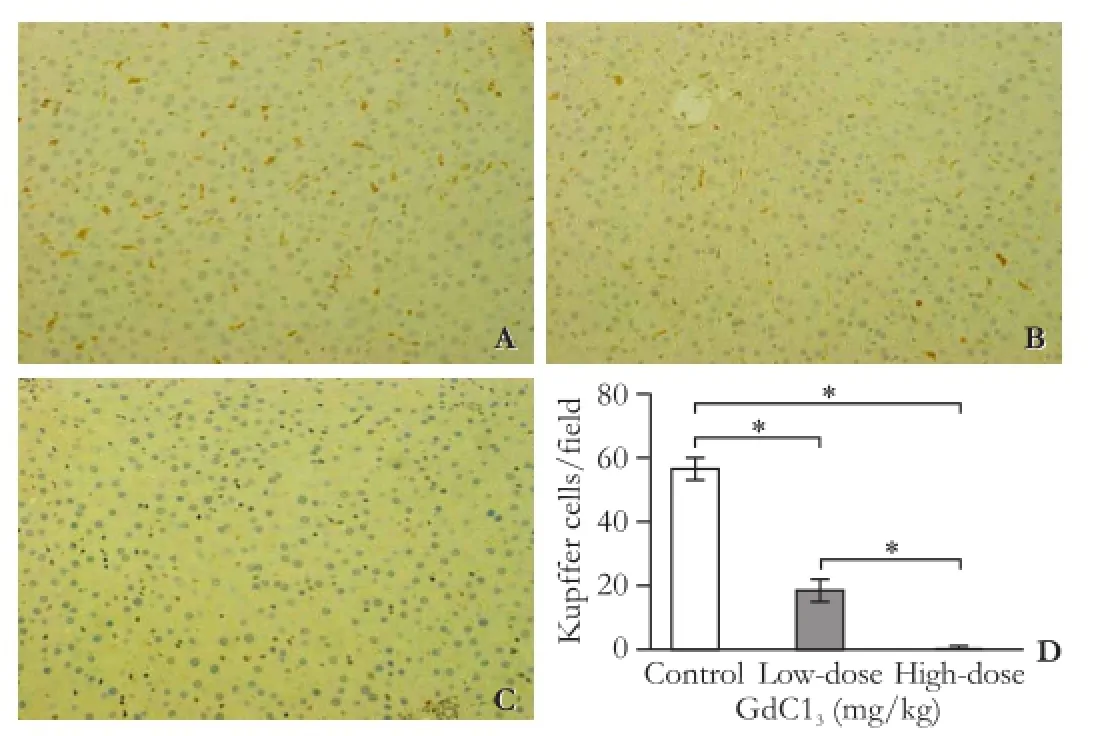
Fig. 1.GdCl3pretreatment may inhibit the proliferation of KCs.A: Liver tissues were obtained from the control group;B: Pretreatment with low-dose GdCl3;C: Pretreatment with high-dose GdCl3;D: The number of ED2-positive cells per feld was quantified in each group. (A-C: original magnification ×200).n=5 for each group (*:P<0.01).
On day 1 after PLTx, signifcantly reduced numbers of BrdU- and PCNA-positive cells were found in the lowdose GdCl3pretreatment group as compared with the control group, suggesting that the GdCl3pretreatment group had suppressed hepatocyte proliferation one day post-PLTx. However, the numbers of BrdU- or PCNA-positive cells in the GdCl3pretreatment group on day 3 post-PLTx showed no signifcant difference compared with the control group (Fig. 3A-D). Furthermore, the recovery of liver weight in the low-dose GdCl3group was signifcantly lower than that in the control group on days 3 and 5 post-PLTx (P<0.05) (Fig. 3E). These observations suggested that GdCl3pretreatment may suppress liver regeneration after PLTx through the inhibition of hepatocyte proliferation in recipients.
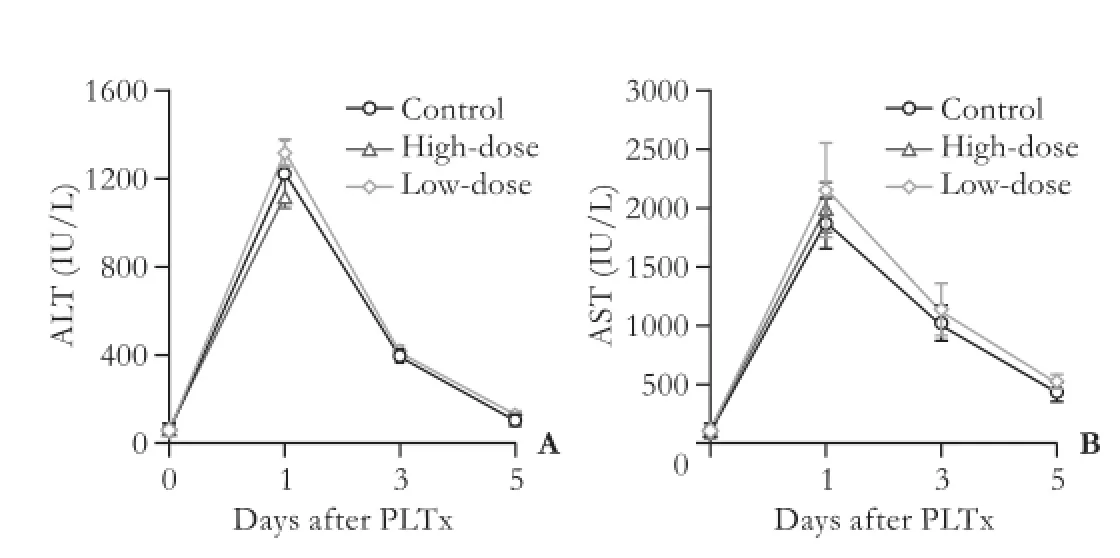
Fig. 2.Serum ALT (A) and AST (B) levels in the control, low-dose GdCl3and high-dose GdCl3pretreatment groups were evaluated on days 0, 1, 3 and 5 after PLTx. Day 0 data refer to recipient levels before transplantation (n=5 for each group).
GdCl3pretreatment induced apoptotic cell death in graft tissues
Western blotting analysis revealed that the levels of Bcl-2 were gradually upregulated with time after PLTx (Fig. 4A, B). On the second day after PLTx, pretreatment with a low-dose of GdCl3signifcantly inhibited Bcl-2 expression compared with the control group (P<0.01). In addition, TUNEL assays showed a signifcantly increased rate of apoptotic cell death in GdCl3-pretreated rat grafts on day 3 post-PLTx (Fig. 4C, D, E).
GdCl3pretreatment prevented the PLTx-induced overexpression of IL-6 and p-STAT3 in graft tissues
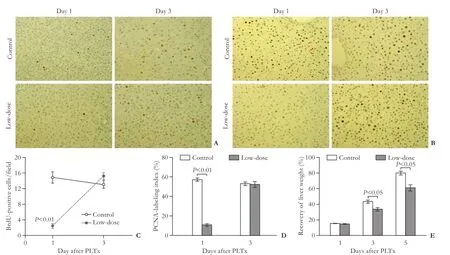
Fig. 3.GdCl3pretreatment suppressed liver regeneration after PLTx. BrdU incorporation (A, original magnifcation × 200) and PCNA staining (B, original magnifcation ×400) in each group were determined in the transplanted liver on days 1 and 3 after PLTx. The number of BrdU- (C) or PCNA-positive (D) cells per feld was counted in each group on days 1 and 3 after PLTx.n=5 for each group.E: The recovery of liver weight was calculated in the untreated or low-dose GdCl3pretreatment groups on days 1, 3 and 5 after PLTx using the following equation: recovery of liver weight=[(actual graft weight—initial graft weight)/whole liver weight]×100%.n=5 for each group.
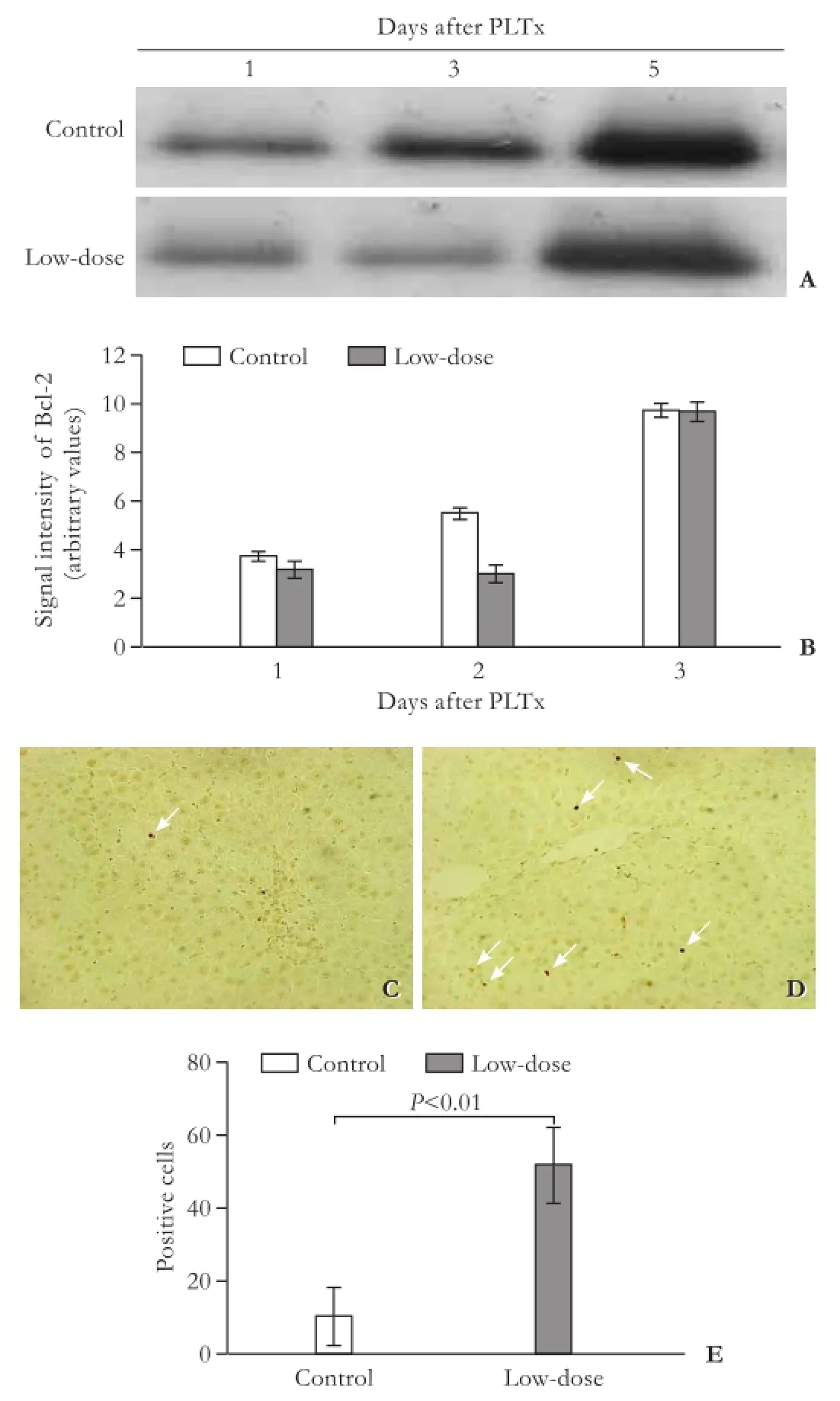
Fig. 4.GdCl3pretreatment induced apoptotic cell death in graft tissues.A: Bcl-2 protein levels were determined by Western blotting analysis in the control and low-dose GdCl3pretreatment groups on days 1, 3 and 5 after PLTx;B: Data were quantified from five independent experiments; TUNEL staining was performed in the control (C) and low-dose GdCl3pretreatment groups (D) on day 3 after PLTx. Arrows indicate the TUNEL-positive apoptotic cells (original magnification ×200);E: The apoptotic cells were counted from 5 microscopic felds for each animal.
As shown in Fig. 5, IL-6 levels were dramatically elevated on days 1 and 3 after PLTx, and gradually decreased to normal values on day 5. Because all the animals in the high-dose GdCl3group died in two days after PLTx operation, we could only determine the IL-6 protein levels in the low-dose GdCl3group. The low-dose GdCl3administration prevented upregulated production of IL-6 on days 1 and 3 after PLTx compared with the control group (P<0.01). Moreover, a low-dose GdCl3administration inhibited the activation of STAT3 on day 1 after PLTx, as decreased expression of p-STAT3 was detected at that time point compared with that in the control group (P<0.01, Fig. 5).
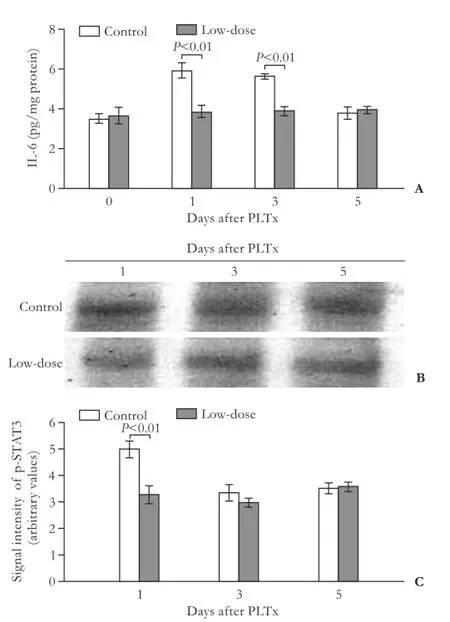
Fig. 5.GdCl3pretreatment prevented the PLTx-induced overexpression of IL-6 and p-STAT3 in graft tissues.A: The levels of IL-6 in graft tissues in the control and low-dose GdCl3pretreatment groups were examined using ELISA assay at indicated time points as described;B: The protein levels of p-STAT3 in the untreated and low-dose GdCl3pretreatment groups on days 1, 3 and 5 after PLTx were examined by Western blotting analysis;C: Data were quantifed from fve independent experiments.
Discussion
In this study, PLTx operation did not lead to animal death, whereas the administration of low-dose GdCl3decreased the survival rate to 71% on 2 days after PLTx. In addition, all the rats that received high-dose GdCl3died in two days after surgery. It seems that deleterious effect of GdCl3pretreatment was not associated with the recovery of liver weight since there was no signifcant difference in liver weight among the different experimental groups on day 1 after PLTx. These observations were in accordance with a previous report showing that in liverspecifc STAT3-defcient mice, cell proliferation was impaired while recovering the liver mass because of increased cell size after hepatectomy.[20]In the current study, GdCl3pretreatment had no infuence on liver damage as no statistical difference was found in serum ALT andAST levels after PLTx. A study[21]has indicated that endotoxins could induce acute respiratory failure. Thus, we hypothesize that respiratory failure may contribute to the high mortality rate because of GdCl3pretreatment. Another possible factor that causes death is the depletion of ATP, and GdCl3pretreatment reduces the hepatic ATP levels after hepatectomy or during the course of ischemia/reperfusion.[13]Nevertheless, the detailed mechanism by which this occurs needs to be further clarifed.
Accumulated evidence indicates that KCs in the liver tissue secrete IL-6, an essential proregenerative factor, and acute-phase inducer in the liver.[14,15]IL-6-defcient mice showed impaired liver regeneration which was characterized by liver necrosis and failure.[14]The rats pretreated with recombinant IL-6 (rIL-6) were subjected to hepatectomy, and ischemia signifcantly showed alleviated injury and promoted regeneration.[22,23]The current data demonstrated that GdCl3pretreatment markedly decreased the levels of IL-6 after PLTx compared with that in the controls, implying that GdCl3impaired liver regeneration by reducing the IL-6 secretion. STAT3 is recognized as a vital transcription factor that is activated downstream of the gp130 receptor, primarily via IL-6 signaling in adult liver.[17]IL-6-activated STAT3 prevents hepatocyte apoptosis, protects against liver injury, and promotes liver regeneration.[16]We found that GdCl3pretreatment signifcantly decreased the protein levels of IL-6 and p-STAT3 on days 1 and 3 after PLTx compared with those in the control group, suggesting that KC inactivation may impair the liver regeneration after PLTx mediated by the IL-6/STAT3 signal pathway. It has been suggested that STAT3 plays a major role in the initiation of liver regeneration.[24]As enhanced hepatocyte proliferation was found immediately (one day) after PLTx, it is possible that the IL-6/STAT3 signal pathway contributes to hepatocyte proliferation during the initiation of liver regeneration. Interestingly, the numbers of BrdU- and PCNA-positive hepatocytes seemed to be equal to those in the control group on day 3 after PLTx in rats pretreated with GdCl3although the IL-6 and Bcl-2 protein levels were lower in the GdCl3pretreated rats compared with the controls at the same time point. The increased proliferation of hepatocyte at this time point in the GdCl3pretreatment groups might be associated with a cytokine independent pathway, such as the transforming growth factor-α (TGF-α)-mediated signal pathway. TGF-α can also be produced by hepatocytes.[25]
In this study, GdCl3pretreatment reduced the expression of Bcl-2 and promoted cell apoptosis on day 3 after PLTx compared with the controls. It has been reported that STAT directly regulates the expression of Bcl-2, and IL-6 stimulates the expression of Bcl-2 by a transcription-dependent mechanism.[26]IL-6 null livers had dramatically reduced Bcl-2 protein levels when compared with normal liver, suggesting that IL-6 may function as a critical anti-apoptotic factor in the liver.[27]Besides the anti-apoptotic effect, Bcl-2 also plays an important role in regulating cell-cycle progression in the early phase of liver regeneration after PLTx.[28]Taken together, these fndings suggest that the Bcl-2-mediated apoptotic signal pathway may interact with the IL-6/STAT3 pathway, and together contribute to the liver regeneration after PLTx.
In conclusion, the current fndings suggest that KCs seem to be important for liver regeneration after PLTx as KC depletion by GdCl3pretreatment signifcantly impaired graft survival following PLTx. Elevated levels of IL-6 from KCs in the liver may activate STAT3 and Bcl-2, thus promoting liver regeneration and reducing apoptosis. The process may be triggered during the early phase of liver regeneration after PLTx. These observations may provide a better understanding of the role of KCs during the liver regeneration after PLTx. However, we could not rule out the infuence of non-arterial ischemia/reperfusion injury which may affect the results. Therefore, further study on the role of KCs in liver regeneration will be carried out by using other animal models.
Contributors:LHY and MSF designed the study. LHY and TDH collected and analyzed the data. LHY and MSF wrote the main body of the article. All authors contributed to the manuscript revision. MSF is the guarantor.
Funding:None.
Ethical approval:The animals used in this study were treated according to a protocol approved by the Animal Research Committee of Third Military Medical University, Chongqing, China.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, et al. Successful living-related partial liver transplantation to an adult patient. Lancet 1994;343:1233-1234.
2 Kawasaki S, Makuuchi M, Ishizone S, Matsunami H, Terada M, Kawarazaki H. Liver regeneration in recipients and donors after transplantation. Lancet 1992;339:580-581.
3 Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Nakazawa Y, et al. Living related liver transplantation in adults. Ann Surg 1998;227:269-274.
4 Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 1999;67:321-327.
5 Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg 2001;192:510-513.
6 Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, et al. Small graft for living donor liver transplantation. Ann Surg 2001;233:575-580.
7 Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int 2006;26:1175-1186.
8 Korolenko TA, Dergunova MA, Alekseenko TV, Zhanaeva SY, Filyushina EE, Filatova TG. Intralysosomal accumulation of gadolinium and lysosomal damage during selective depression of liver macrophages in vivo. Bull Exp Biol Med 2006;142:391-394.
9 Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol 2007;33:318-325.
10 Kinser S, Copple BL, Roth RA, Ganey PE. Enhancement of allyl alcohol hepatotoxicity by endotoxin requires extrahepatic factors. Toxicol Sci 2002;69:470-481.
11 Ganey PE, Barton YW, Kinser S, Sneed RA, Barton CC, Roth RA. Involvement of cyclooxygenase-2 in the potentiation of allyl alcohol-induced liver injury by bacterial lipopolysaccharide. Toxicol Appl Pharmacol 2001;174:113-121.
12 Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol 1996;270:G909-918.
13 Watanabe M, Chijiiwa K, Kameoka N, Yamaguchi K, Kuroki S, Tanaka M. Gadolinium pretreatment decreases survival and impairs liver regeneration after partial hepatectomy under ischemia/reperfusion in rats. Surgery 2000;127:456-463.
14 Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-defcient mice. Science 1996;274: 1379-1383.
15 Tachibana S, Zhang X, Ito K, Ota Y, Cameron AM, Williams GM, Sun Z. Interleukin-6 is required for cell cycle arrest and activation of DNA repair enzymes after partial hepatectomy in mice. Cell Biosci 2014;4:6.
16 Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000;19:2548-2556.
17 Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest 2003;112:978-980.
18 Huggins GM, Anderson RM. Experimental pathology of the liver: I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931;12:186-202.
19 Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979;28:47-50.
20 Haga S, Ogawa W, Inoue H, Terui K, Ogino T, Igarashi R, et al. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specifc STAT3-defcient mice. J Hepatol 2005;43:799-807.
21 Perkowski SZ, Sloane PJ, Spath JA Jr, Elsasser TH, Fisher JK, Gee MH. TNF-alpha and the pathophysiology of endotoxininduced acute respiratory failure in sheep. J Appl Physiol (1985) 1996;80:564-573.
22 Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology 1999;30:469-475.
23 Selzner N, Selzner M, Tian Y, Kadry Z, Clavien PA. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: A TNF-alpha/IL-6-dependent mechanism. Hepatology 2002;36:812-818.
24 Fausto N. Liver regeneration. J Hepatol 2000;32:19-31.
25 Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2-13.
26 Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol 2000;12: 543-549.
27 Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem 2001;276:26605-26613.
28 Takushi Y, Shiraishi M, Nozato E, Toyoda A, Nishimaki T. Expression of anti-apoptotic protein, Bcl-2, in liver regeneration after a partial hepatectomy. J Surg Res 2006;134: 93-101.
Received February 6, 2013
Accepted after revision March 15, 2014
AuthorAffliations:Department of Emergency Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China (Luo HY and Ma SF); Department of Emergency Medicine, Daping Hospital, Third Military Medical University, Chongqing 400042, China (Qu JF); Orthopetics Department, Third Hospital of Hebei Medical University, Shijiazhuang 050051, China (Tian DH)
Shan-Fang Ma, MD, Department of Emergency Medicine, Beijing Ditan Hospital, Capital Medical University, 8 Jingshun East Street, Chaoyang District, Beijing 100015, China (Tel: +86-10-84322120; Fax: +86-10-84322999; Email: enever@live.cn)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60291-5
Published online November 21, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- p38 MAPK inhibition alleviates experimental acute pancreatitis in mice
- Development of hybrid-type modifed chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells
- Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
- Predictors of incidental gallbladder cancer in elderly patients
- Primary non-Hodgkin's lymphoma of the liver: sonographic and CT fndings
- Enlarged pancreas: not always a cancer
