A score model for predicting post-liver transplantation survival in HBV cirrhosis-related hepatocellular carcinoma recipients: a single center 5-year experience
2015-02-07
Hangzhou, China
A score model for predicting post-liver transplantation survival in HBV cirrhosis-related hepatocellular carcinoma recipients: a single center 5-year experience
Li-Ying Wang, Shu-Sen Zheng, Xiao Xu, Wei-Lin Wang, Jian Wu, Min Zhang, Yan Shen, Sheng Yan, Hai-Yang Xie, Xin-Hua Chen, Tian-An Jiang and Fen Chen
Hangzhou, China
BACKGROUND:The prognostic prediction of liver transplantation (LT) guides the donor organ allocation. However, there is currently no satisfactory model to predict the recipients' outcome, especially for the patients with HBV cirrhosis-related hepatocellular carcinoma (HCC). The present study was to develop a quantitative assessment model for predicting the post-LT survival in HBV-related HCC patients.
METHODS:Two hundred and thirty-eight LT recipients at the Liver Transplant Center, First Affliated Hospital, Zhejiang University School of Medicine between 2008 and 2013 were included in this study. Their post-LT prognosis was recorded and multiple risk factors were analyzed using univariate and multivariate analyses in Cox regression.
RESULTS:The score model was as follows: 0.114×(Child-Pugh score)-0.002×(positive HBV DNA detection time)+0.647× (number of tumor nodules)+0.055×(max diameter of tumor nodules)+0.231×lnAFP+0.437×(tumor differentiation grade).The receiver operating characteristic curve analysis showed that the area under the curve of the scoring model for predicting the post-LT survival was 0.887. The cut-off value was 1.27, which was associated with a sensitivity of 72.5% and a specifcity of 90.7%, respectively.
CONCLUSION:The quantitative score model for predicting post-LT survival proved to be sensitive and specifc.
(Hepatobiliary Pancreat Dis Int 2015;14:43-49)
HBV cirrhosis;hepatocellular carcinoma; liver transplantation; Hangzhou criteria; post-LT survival
Introduction
Several criterion such as Milan criteria and Hangzhou criteria are used to select recipients to prolong the survival time.[4-9]Many scientists searched the risk factors of survival after liver transplantation in patients with HBV-related HCC. They found that tumor morphologic features, AFP level, histological differentiation and HCC recurrence could be used to predict post-LT survival.[4,10-12]Itis well known that patients with AFP >400 ng/mL had poor prognosis.[11]However, the ideal cut-off line of AFP value has not been defned. Furthermore, the detectable AFP mRNA in blood indicated that the tumor is poorly differentiated.[13,14]However, no quantitative model for predicting post-LT survival has been established. We therefore tried to analyze the risk factors and to derive a mathematical model to predict post-LT survival.
Methods
Ethics statement
Ethical approval was obtained from the Committee of Ethics in Biomedical Research of Zhejiang University. The current regulations of the Chinese Government and theDeclaration of Helsinkiwere strictly followed for each organ donation and transplant performed at our center. Written informed consent from each donor and recipient were obtained.
Patients
From 2008 to 2013, 238 HCC patients with HBV-related cirrhosis received LT at the Liver Transplant Center, First Affliated Hospital, Zhejiang University School of Medicine. All recipients received a post-LT HBV prophylaxis of anti-viral nucleoside analogues and hepatitis B immunoglobulin. They were followed up with liver image changes and AFP levels. The clinical and pathological results of all recipients were retrospectively analyzed, and the post-LT prognosis of all patients was recorded. The baseline demographics and clinical characteristics of the liver transplant recipients are shown in Table 1.
Study design
All data were obtained from the clinical records of recipients. In order to determine which variables were independent predictors of survival and to develop the fnal post-LT survival prediction model, 15 potential predictors were screened. The following predictors were selected based on previous studies and clinical experience: gender, age, body mass index (BMI), pre-LT diabetes, waiting time for transplant, pre-LT intervention (yes or no), MELD scores, Child-Pugh scores, number of tumor nodules, max diameter of tumor nodules, total diameter of tumor nodules, microvascular invasion (yes or no), preoperative AFP levels, tumor differentiation grades, positive HBV DNA detection time (days between LT and positive HBV DNA detection).
The Cox proportional hazard regression models was used to identify the association of factors with prognosis. First, a univariate analysis was used to evaluate the poten-tial value of risk factors. Then, candidate variables (Pvalue <0.10) were entered into a multivariate analysis. A prognostic model was established from the multivariate Cox proportional hazards analysis: [(regression coeffcients β1)×(variable 1)+(regression coeffcients β2)×(variable 2)+(regression coeffcients β3)×(variable 3)+...]. We also calculated the area under the receiver-operator curve (AUC) and the sensitivity and specifcity of an optimal cut-off point for the model score.
成都东郊龙泉驿片区原主要为坡地场地,地形起伏较大,原建筑物(构筑物)主要集中于较为平坦地区。随着成都市城市发展,东扩进程的推进,大量山区土地得到使用,但因地形起伏大,建筑场地使用过程中不可避免的涉及到场地大挖大填工作,建(构)筑物建造前需对场地边坡进行稳定性评价及相应的治理设计。边坡问题作为拟建物地基基础设计前的主要问题摆在了工程勘察设计人员面前。
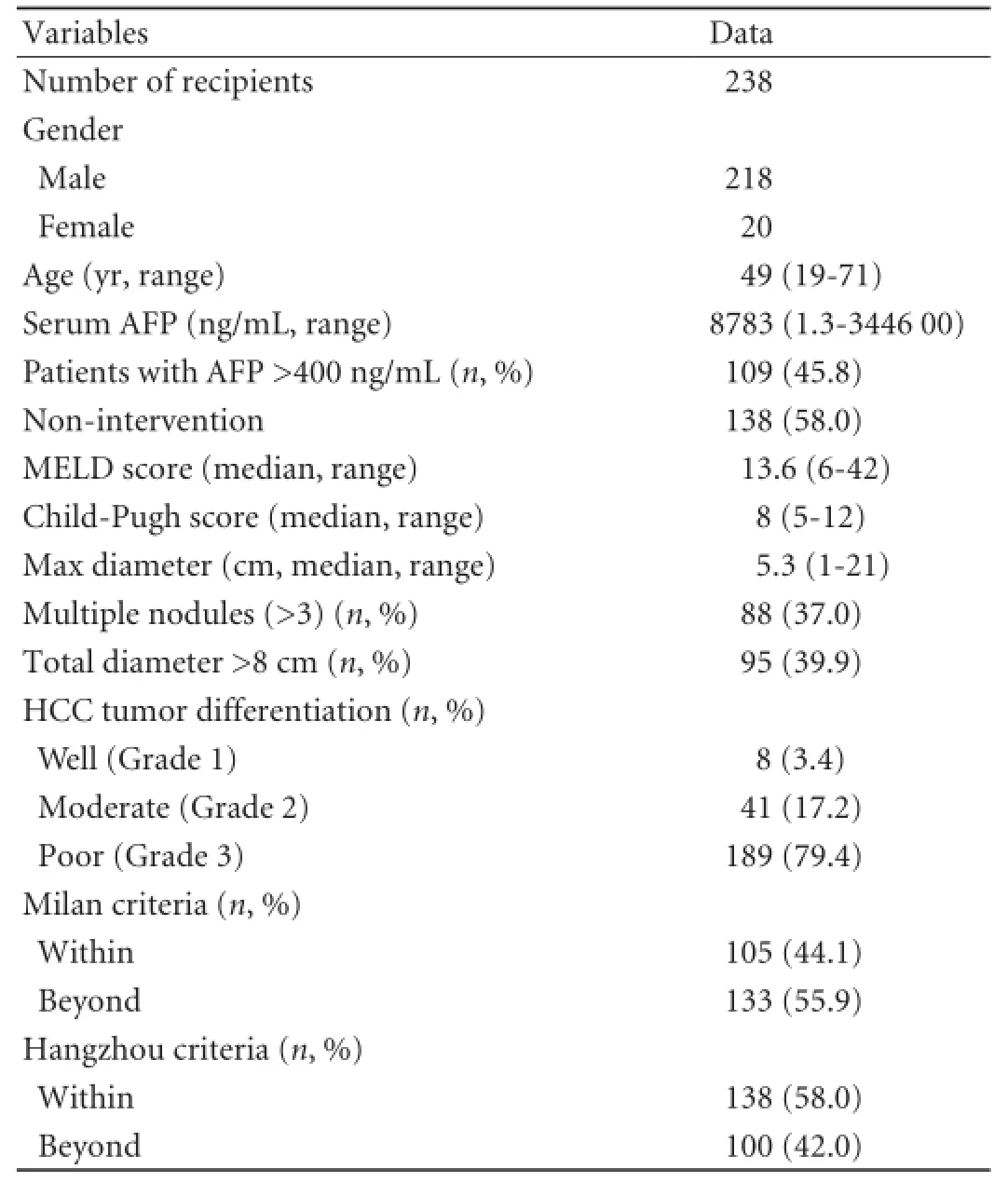
Table 1.Recipient characteristics
Statistical analysis
Survival curves were estimated using the Kaplan-Meier method, and differences were evaluated by the log-rank test. The statistically signifcant variables from the univariate analysis were also evaluated using the Cox regression analysis to determine the risks for post-LT survival. When the multivariate analysis was applicable or continuous covariates were involved, the Cox proportional hazards regression model was used to determine the effect of covariates, and a stepwise model selection tool was used to construct the fnal multivariate model. Hazard ratios (HRs) and 95% CIs were estimated using the Cox proportional hazards regression model. AP<0.05 was considered statistically signifcant. All data were analyzed using SPSS software version 16.0.
Results
Survival
With a mean follow-up of 983 days, the 1-year survival rate of recipients within the Hangzhou criteria was signifcantly higher than that of recipients beyond the Hangzhou criteria (86.0% vs 64.0%,P<0.001). Also, the 3-year survival rate of recipients within the Hangzhou criteria was higher than that of recipients beyond the Hangzhou criteria (77.2% vs 30.9%,P<0.001). The results were similar for the 5-year survival rate (within the Hangzhou criteria 71.8% vs beyond the Hangzhou criteria 21.4%,P<0.001). These results are shown in Fig. 1.
Risk factors for post-LT survival
The univariate analysis showed that 10 variables were signifcant risk factors for survival of the recipients: positive HBV DNA detection time, waiting time for transplant, MELD scores, Child-Pugh classifcation, number of tumor nodules, maximum diameter of tumor nodules, total diameter of tumor nodules, microvascular invasion (yes or no), preoperative AFP levels, and tumor differentiation grades. All of the predictors that were tested for inclusion were hazard ratio, 95% CI, andPvalues from univariate analyses (Table 2). All of the identifed survival risk factors were then analyzed by multivariate Cox regression. The analysis was a composite derived from 10 imputed data sets in the fnal model, and variables included in the fnal model are shown in Table 3. The positive HBV DNA detection time, Child-Pugh score, number of tumor nodules, max diameter of tumor nodules, preoperative AFP levels, and tumor differentiation grades were signifcant predictors for survival in HBV cirrhosis-related HCC recipients. The variables used, the associated parameter estimates (regression coeffcients or weights), andPvalues from the fnal model are shown in Table 3. Other variables were analyzed but not included in the fnal model-building process. The prognostic index from the model was the sum of the variables, each multiplied by its weight. A positive weight attached to a prognostic factor indicated higher mortality, and a nega-tive weight indicated a lower mortality. These parameters were the same for the prognostic index. The post-LT survival could be calculated by the following survival score equation: 0.114×(Child-Pugh score)-0.002 ×(positive HBV DNA detection time)+0.647×(number of tumor nodules)+0.055×(max diameter of tumor nodules)+0.231× lnAFP+0.437×(tumor differentiation grade). The receiver operating characteristic (ROC) curve was constructed for the scoring model (Fig. 2) and the AUC was calculated. The ROC curve analysis showed that the AUC was 0.887 (95% CI 0.845-0.930,P<0.001). The cut-off score was 1.27 with a sensitivity of 72.5% and a specifcity of 90.7%. The 1-, 3-, and 5-year survival rates of recipients with survival scores less than 1.27 were 94.6%, 86.1%, and 77.1%, respectively. Only 19.6% (27/138) of the Hangzhou criteria recipients' survival scores were over 1.27 (Fig. 3). Patients with survival score of less than 1.27 had more favorable outcomes. Overall survival for all recipients and for recipients within the Hangzhou criteria are shown in Fig. 3.
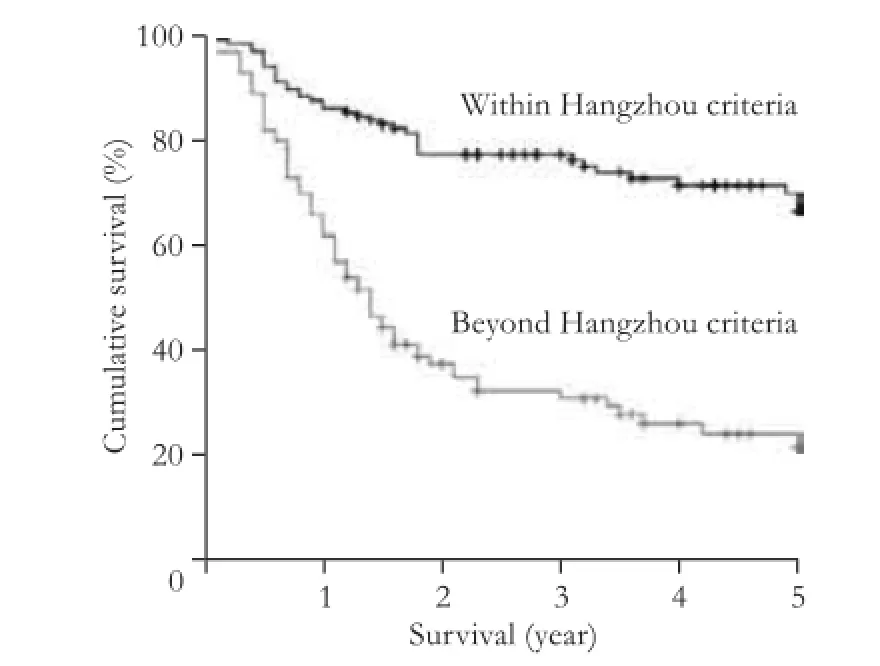
Fig. 1.Survival of recipients within Hangzhou criteria and beyond Hangzhou criteria.
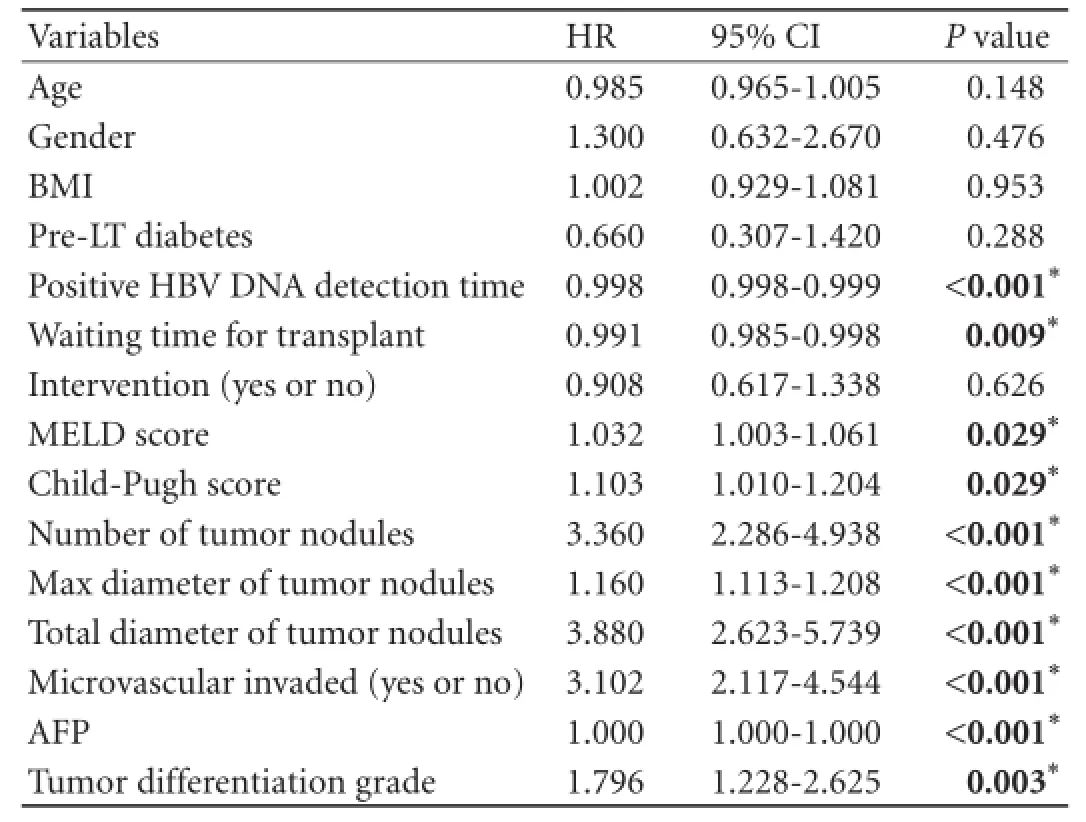
Table 2.Univariate analysis of variables related to post-LT survival
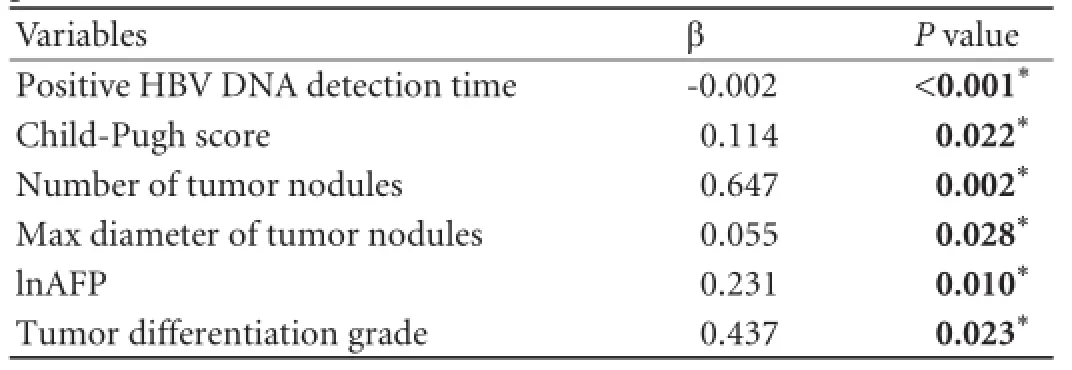
Table 3.Independent variables in the Cox regression analysis for post-LT survival
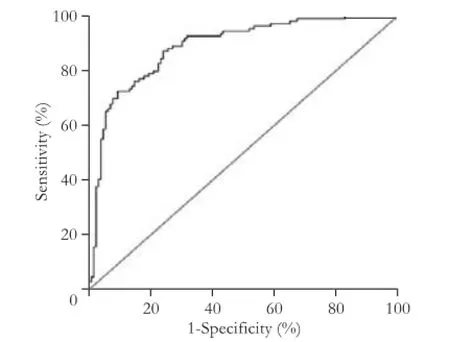
Fig. 2.The ROC curves of survival score in assessing the post-LT survival. AUC was 0.887 (95% CI 0.845-0.930,P<0.001). The cutoff point of survival score was 1.27 with sensitivity of 72.5% and specifcity of 90.7%.
Selection of cut-off value of AFP values
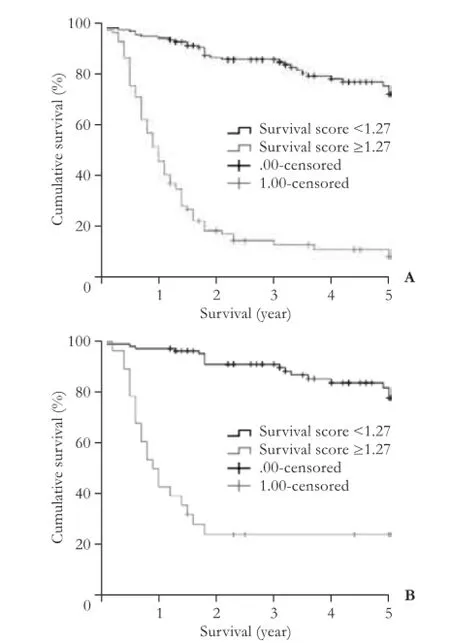
Fig. 3.Survival rates of recipients with survival scores below the cut-off line of 1.27 were signifcantly higher than survival scores over 1.27.A: all recipients (P<0.001);B: recipients within Hangzhou criteria (P<0.001).
To investigate the predictive value of preoperative AFP levels in tumor differentiation, a scatter diagram was constructed (Fig. 4A). Preoperative AFP levels were related to tumor differentiation in recipients with diameters of <5 cm sono-nodule. Because preoperative AFP is a signifcant prognostic factor for recipients' post-LT recurrence and post-LT survival, ROC curve analysis was performed on the predictive value of preoperative AFP in post-LT prognosis (Fig. 4B, C). The ROC curve analysis showed that the AUC was 0.710 and 0.731 for preoperative AFP in predicting post-LT recurrence andpost-LT survival, and an preoperative AFP level of 325.3 ng/mL was the cut-off value. Recipients with AFP levels above 325.3 ng/mL had high post-LT recurrence and poor survival rates. AUC,Pvalues and 95% CI were collected (Table 4).
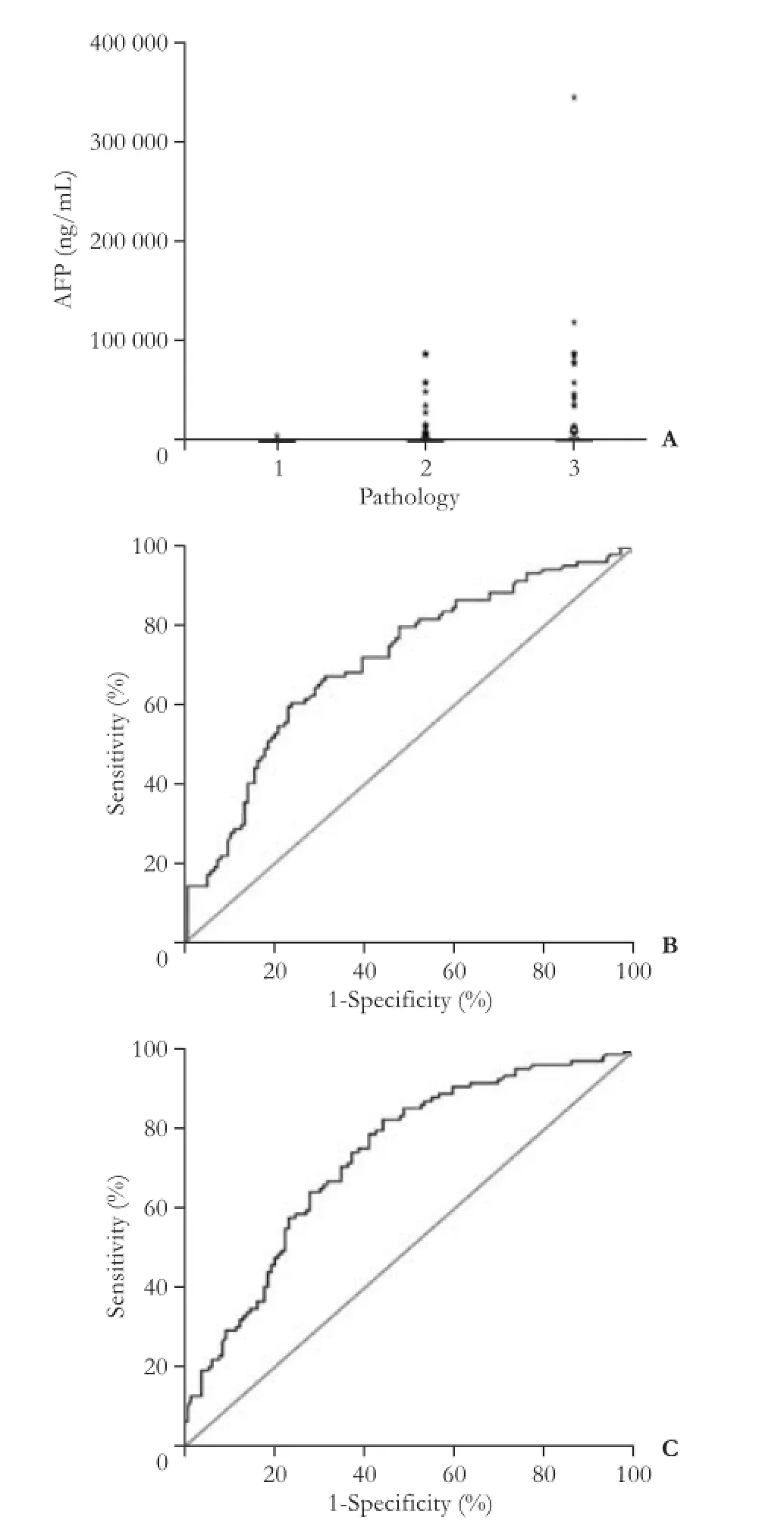
Fig. 4.A: Scatter diagram of preoperative AFP values in different tumor differentiation groups (1=well differentiation; 2=moderate differentiation; 3=poor differentiation). The ROC curves of AFP predictive value regarding (B) post-LT recurrence and (C) post-LT survival.
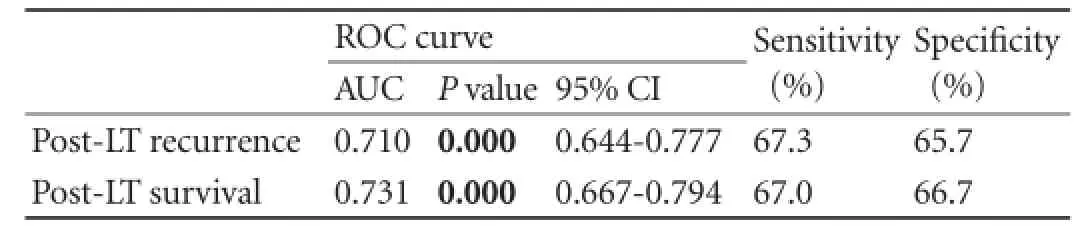
Table 4.Preoperative AFP cut-off value (325.3 ng/mL) for post-LT prognosis
Discussion
In Asia, liver transplantation offers a feasible therapy for HBV cirrhosis-related HCC. The Milan, UCSF, Barcelona, and “up-to-seven“ criterion are all used to predict patients' survival in patients with HCC after LT. We proposed our candidate selection criteria as the Hangzhou criteria for patients with HCC.[4]Our criteria are to identify recipients that do not meet the Milan criteria but have favorable post-LT outcomes. In this study, the 1-, 3-, and 5-year survival rates of HBV cirrhosis-related HCC recipients within the Hangzhou criteria were 86.0%, 77.2%, 71.8%, which are comparable to other published studies adapted either the Hangzhou or Milan criteria.[7,15-17]These rates were all signifcantly higher than those that did not meet the Hangzhou criteria. As recipients beyond the Milan criteria increase over time, the number of such transplants also increases. Gao et al[6]reported that the Milan criteria should be the preferred criteria for LT. However, the consensus is that the Milan criteria are too restrictive, and LTs can be performed safely in other carefully selected recipients.[18,19]In the present study, we demonstrated that survival rates in patients who met the Hangzhou criteria were similar to those who met the Milan criteria. The Hangzhou criteria only moderately expand the Milan criteria and could be accepted as the preferred criteria for LT.
During the past decade, studies[20,21]found that some indicators could predict post-LT survival. In this study, we selected variables based on a comprehensive assessment of previously reported clinical prognostic factors and added other new variables in order to derive a mathematical model for predicting post-LT survival in a more rational and effective manner. In this study, multiple factors for post-LT survival of HBV cirrhosis-related HCC recipients were chosen based on both tumor morphologic features (tumor size, tumor number, and max diameter of tumor nodules) and on tumor biology factors (preoperative AFP levels, tumor differentiation, and positive HBV DNA detection time). All these factors have been extensively studied in an effort to reduce post-LT recurrence and achieve better survival in order to use donor organs reasonably and effectively. The positive HBV DNA detection time, Child-Pugh score, number of tumor nodules, max diameter of tumor nodules, preoperative AFP levels, and tumor differentiation were signifcantly correlated with post-LT survival in HBV cirrhosisrelated HCC recipients.
Positive HBV DNA detection time is a new predictive and characteristic risk factor for HBV cirrhosis-related HCC recipients' post-LT prognoses. The introduction of this new risk factor in the survival score formula was based on its characteristics related to liver injury and the recurrence of HCC. The recurrence of HBV also affects graft loss and death, consequently affecting post-LT recipients' survival.[21-26]
Tumor differentiation associated with post-LT survival and recurrence of HCC has been recently reported. The post-LT survival rates have been reported to be higher in recipients with well-differentiated tumors than in those with poor-differentiated tumors.[4,11,27-29]Our results showed that tumor differentiation based on tumor biology is a remarkable risk factor in prognosis. After analyzing the correlation between AFP levels and tumor differentiation, the results indicated that preoperative AFP levels are correlated with tumor differentiation for recipients with tumor diameter <5 cm sono-nodule.
The diagnostic accuracy of the survival score equation was quantifed by the receiver operating characteristic area under the curve (ROC AUC). AUC reached a high level of 0.887 (95% CI 0.845-0.930,P<0.001). The post-LT survival could be quantitatively assessed by the survival score equation and the cut-off score of 1.27. The sensitivity and specifcity were 72.5% and 90.7%, respectively. Such high specifcity indicates high true-negative rate and low false-positive rate. Hence, if the survival score is below 1.27, there is a high probability that the recipient will have a good post-LT survival rate. Conversely, when the survival score is above 1.27, the recipient is unlikely to have a good post-LT survival rate.
The 1-, 3-, and 5-year survival rates of recipients with survival scores of less than 1.27 were 94.6%, 86.1%, and 77.1%, respectively. The equation produces a prognostic score that is an objective and sensitive tool for predicting post-LT survival of recipients and could encourage more rational and effective LT recipient selections. Additionally, the survival score equation helps assess the patients who beneft most from liver transplantation. The scoring model for predicting post-LT survival was specifc and sensitive as a quantitative predictive model for LT recipients. The percentage of Hangzhou criteria recipients whose survival score was more than 1.27 was only 19.6%,indicating that the Hangzhou criteria can be the safe and preferred criteria for LT.
Preoperative AFP levels were signifcantly associated with post-LT recurrence and survival. This observation was similar to several previous studies.[4,30,31]Several different cut-off values for AFP levels (100, 210, 400, and 1000 ng/mL) have been proposed.[30-44]The AFP cut-off value of 400 ng/mL has been widely adopted for predicting HCC recurrence.[4,30,32-35]A similar AFP cut-off value of 455 ng/mL has also been reported as a predictive parameter for post-LT survival.[36]Other studies[43-44]reported a much higher cut-off value that reaches 1000 ng/ mL or greater. In this study, an AFP level of 325.3 ng/ mL was the optimal cut-off value for predicting post-LT recurrence and survival in HBV cirrhosis-related HCC recipients.
In conclusion, HBV cirrhosis-related HCC recipients within the Hangzhou criteria have favorable survival rates after LT. The quantitative predictive scoring model proposed in our investigation was sensitive and specifc for predicting post-LT survival in LT recipients.
Acknowledgements:We would like to thank Dr. Bill Thompson (Old Dominion University, USA) for proofreading this manuscript.
Contributors:ZSS proposed the study. WLY wrote the frst draft. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Funding:This study was supported by grants from National S&T Major Project (2012ZX10002017), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81121002) and the National Natural Science Foundation of China (81200331).
Ethical approval:This study was approved by the Committee of Ethics in Biomedical Research of Zhejiang University.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 WHO (2013) Hepatitis B. Fact sheet N°204. Accessed in August 15, 2014. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
2 Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127: S5-S16.
3 Yuen MF, Hou JL, Chutaputti A; Asia Pacifc Working Party on Prevention of Hepatocellular Carcinoma. Hepatocellular carcinoma in the Asia pacifc region. J Gastroenterol Hepatol 2009;24:346-353.
4 Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-1732.
5 Chen J, Xu X, Wu J, Ling Q, Wang K, Wang W, et al. The stratifying value of Hangzhou criteria in liver transplantation for hepatocellular carcinoma. PLoS One 2014;9:e93128.
6 Gao T, Xia Q, Qiu de K, Feng YY, Chi JC, Wang SY, et al. Comparison of survival and tumor recurrence rates in patients undergoing liver transplantation for hepatitis B-related hepatocellular carcinoma using Milan, Shanghai Fudan and Hangzhou criteria. J Dig Dis 2013;14:552-558.
7 Lei JY, Wang WT, Yan LN. Hangzhou criteria for liver transplantation in hepatocellular carcinoma: a single-center experience. Eur J Gastroenterol Hepatol 2014;26:200-204.
8 Audet M, Panaro F, Piardi T, Wolf P. Are the Hangzhou criteria adaptable to hepatocellular carcinoma patients for liver transplantation in Western countries? Liver Transpl 2009;15:822-826.
9 Hu Z, Zhou J, Li Z, Xiang J, Qian Z, Wu J, et al. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: retrospective study of the Milan and Hangzhou criteria. PLoS One 2014;9:e87222.
10 Han SH, Reddy KR, Keeffe EB, Soldevila-Pico C, Gish R, Chung RT, et al. Clinical outcomes of liver transplantation for HBV-related hepatocellular carcinoma: data from the NIH HBV OLT study. Clin Transplant 2011;25:E152-162.
11 Li WX, Li Z, Gao PJ, Gao J, Zhu JY. Histological differentiation predicts post-liver transplantation survival time. Clin Res Hepatol Gastroenterol 2014;38:201-208.
12 Del Gaudio M, Grazi GL, Principe A, Ravaioli M, Ercolani G, Cescon M, et al. Infuence of prognostic factors on the outcome of liver transplantation for hepatocellular carcinoma on cirrhosis: a univariate and multivariate analysis. Hepatogastroenterology 2004;51:510-514.
13 Cillo U, Vitale A, Navaglia F, Basso D, Montin U, Bassanello M, et al. Role of blood AFP mRNA and tumor grade in the preoperative prognostic evaluation of patients with hepatocellular carcinoma. World J Gastroenterol 2005;11:6920-6925.
14 Cillo U, Navaglia F, Vitale A, Molari A, Basso D, Bassanello M, et al. Clinical signifcance of alpha-fetoprotein mRNA in blood of patients with hepatocellular carcinoma. Clin Chim Acta 2004;347:129-138.
15 Jiang L, Liao A, Wen T, Yan L, Li B, Yang J. Living donor liver transplantation or resection for Child-Pugh A hepatocellular carcinoma patients with multiple nodules meeting the Milan criteria. Transpl Int 2014;27:562-569.
16 Lei J, Wang W, Yan L. Downstaging advanced hepatocellular carcinoma to the Milan criteria may provide a comparable outcome to conventional Milan criteria. J Gastrointest Surg 2013;17:1440-1446.
17 Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, et al. Milan Criteria and UCSF Criteria: A Preliminary Comparative Study of Liver Transplantation Outcomes in the United States. Int J Hepatol 2012;2012:253517.
18 Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001-1007.
19 Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2008;14:1107-1115.
20 Yaprak O, Akyildiz M, Dayangac M, Demirbas BT, Guler N, Dogusoy GB, et al. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012;11:256-261.
21 Zou WL, Zang YJ, Chen XG, Shen ZY. Risk factors for fatal re-currence of hepatocellular carcinoma and their role in selecting candidates for liver transplantation. Hepatobiliary Pancreat Dis Int 2008;7:145-251.
22 Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008 Jun;134(7):1890-1899; quiz 2155.
23 Kiyici M, Yilmaz M, Akyildiz M, Arikan C, Aydin U, Sigirli D, et al. Association between hepatitis B and hepatocellular carcinoma recurrence in patients undergoing liver transplantation. ransplant Proc 2008;40:1511-1517.
24 Campsen J, Zimmerman M, Trotter J, Hong J, Freise C, Brown R, et al. Liver transplantation for hepatitis B liver disease and concomitant hepatocellular carcinoma in the United States With hepatitis B immunoglobulin and nucleoside/nucleotide analogues. Liver Transpl 2013;19:1020-1029.
25 Nath DS, Kalis A, Nelson S, Payne WD, Lake JR, Humar A. Hepatitis B prophylaxis post-liver transplant without maintenance hepatitis B immunoglobulin therapy. Clin Transplant 2006;20:206-210.
26 Neff GW, Kemmer N, Kaiser TE, Zacharias VC, Alonzo M, Thomas M, et al. Combination therapy in liver transplant recipients with hepatitis B virus without hepatitis B immune globulin. Dig Dis Sci 2007;52:2497-2500.
27 Decaens T, Roudot-Thoraval F, Badran H, Wolf P, Durand F, Adam R, et al. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int 2011;31:792-801.
28 De Carlis L, Giacomoni A, Lauterio A, Slim A, Sammartino C, Pirotta V, et al. Liver transplantation for hepatocellular cancer: should the current indication criteria be changed? Transpl Int 2003;16:115-122.
29 DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-172.
30 Wong LL, Naugler WE, Schwartz J, Scott DL, Bhattacharya R, Reyes J, et al. Impact of locoregional therapy and alphafetoprotein on outcomes in transplantation for liver cancer: a UNOS Region 6 pooled analysis. Clin Transplant 2013;27: E72-79.
31 Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery 2007;141:598-609.
32 Merli M, Nicolini G, Gentili F, Novelli G, Iappelli M, Casciaro G, et al. Predictive factors of outcome after liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Transplant Proc 2005;37:2535-2540.
33 Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientifc Registry of Transplant Recipients database. Hepatology 2009;49:832-838.
34 Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis 2007;25:313-319.
35 Xu X, Ke QH, Shao ZX, Wu J, Chen J, Zhou L, et al. The value of serum alpha-fetoprotein in predicting tumor recurrence after liver transplantation for hepatocellular carcinoma. Dig Dis Sci 2009;54:385-388.
36 Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008;134: 1342-1351.
37 Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-1092.
38 Ciccarelli O, Lai Q, Goffette P, Finet P, De Reyck C, Roggen F, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refned selection criteria. Transpl Int 2012;25:867-875.
39 Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, et al. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant 2012;26: E125-131.
40 Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-994. e3; quiz e14-5.
41 Lai Q, Avolio AW, Manzia TM, Agnes S, Tisone G, Berloco PB, et al. Role of alpha-fetoprotein in selection of patients with hepatocellular carcinoma waiting for liver transplantation: must we reconsider it? Int J Biol Markers 2011;26:153-159.
42 Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129-137.
43 Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alphafetoprotein level >1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-951.
44 Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim GS, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma. Transplant Proc 2014;46:726-729.
Received October 19, 2014
Accepted after revision December 15, 2014
AuthorAffliations:Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affliated Hospital, Zhejiang University School of Medicine (Wang LY, Zheng SS, Xu X, Wang WL, Wu J, Zhang M, Shen Y, Yan S, Chen XH, Jiang TA and Chen F); Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Health, Zhejiang Province (Zheng SS, Xu X, Wang WL, Wu J, Zhang M, Shen Y, Yan S and Xie HY), Hangzhou 310003, China
Shu-Sen Zheng, MD, PhD, FACS, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Health, Department of Hepatobiliary and Pancreatic Surgery, First Affliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: +86-571-87236601; Fax: +86-571-87236628; Email: shusenzheng@zju. edu.cn)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60335-6
Published online January 29, 2015.
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- p38 MAPK inhibition alleviates experimental acute pancreatitis in mice
- Development of hybrid-type modifed chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells
- Liver, biliary and pancreatic injuries in pancreaticobiliary maljunction model in cats
- Predictors of incidental gallbladder cancer in elderly patients
- Primary non-Hodgkin's lymphoma of the liver: sonographic and CT fndings
- Enlarged pancreas: not always a cancer
