Inhibition of pancreatic stellate cell activity by adipose-derived stem cells
2015-02-06
Wenzhou, China
Inhibition of pancreatic stellate cell activity by adipose-derived stem cells
Fu-Xiang Yu, Long-Feng Su, Chun-Lei Dai, Yang Wang, Yin-Yan Teng, Jun-Hui Fu, Qi-Yu Zhang and Yin-He Tang
Wenzhou, China
BACKGROUND: Pancreatic stellate cells (PSCs) play a critical role in the development of pancreatic fbrosis. In this study we used a novel method to isolate and culture rat PSCs and then investigated the inhibitory effects of adipose-derived stem cells (ADSCs) on activation and proliferation of PSCs.
METHODS: Pancreatic tissue was obtained from Sprague-Dawley rats for PSCs isolation. Transwell cell cultures were adopted for co-culture of ADSCs and PSCs. PSCs proliferation and apoptosis were determined using CCK-8 and fow cytometry, respectively. α-SMA expressions were analyzed using Western blotting. The levels of cytokines [nerve growth factor (NGF), interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1)] in conditioned medium were detected by ELISA. Gene expression (MMP-2, MMP-9 and TIMP-1) was analyzed using qRT-PCR.
RESULTS: This method produced 17.6±6.5×103cells per gram of the body weight with a purity of 90%-95% and a viability of 92%-97%. Co-culture of PSCs with ADSCs signifcantly inhibited PSCs proliferation and induced PSCs apoptosis. Moreover, α-SMA expression was signifcantly reduced in PSCs+ADSCs compared with that in PSC-only cultures, while expression of fbrinolytic proteins (e.g., MMP-2 and MMP-9) was upregulated and anti-fbrinolytic protein (TIMP-1) was downregulated. In addition, NGF expression was up-regulated, but IL-10 and TGF-β1 expressions were down-regulated in the coculture conditioned medium compared with those in the PSC-only culture medium.
CONCLUSIONS: This study provided an easy and reliable technique to isolate PSCs. The data demonstrated the inhibi-tory effects of ADSCs on the activation and proliferation of PSCsin vitro.
(Hepatobiliary Pancreat Dis Int 2015;14:215-221)
pancreatic stellate cells;isolation; mesenchymal stem cells; pancreatic fbrosis; fbrinolytic protein
Introduction
Chronic pancreatitis is characterized by chronic infammation and altered normal structure and functions of the pancreas. The long-standing infammation of the pancreas leads to pancreatic fbrosis as the end-stage of the disease although it may take years to develop.[1]A great number of clinical and experimental studies have confrmed that activated pancreatic stellate cells (PSCs) are the main effector cells in the process of pancreatic fbrosis[1]and therefore, PSCs may play a dominant role in the pathogenesis of pancreatic fbrosis.[2,3]PSCs, also known as myofbroblast-like cells, are able to switch between the quiescent and activated phenotypes, like hepatic stellate cells (HSCs), to participate in tissue repair, and to secrete extracellular matrix. As a result, PSCs have caught attention from researchers, and targeting of these cells could provide novel strategies for anti-pancreatic fbrosis therapy.[2-7]With regards to this, it is an essential need to isolate and obtain purifed and dynamic PSCs with high quality as a key and fundamental basis for the study of pancreatic fbrosis. Indeed, since 1998, there have been continuous studies in the development of improved methods to isolate PSCs from animal and human pancreas.[4,5]However, to date, the isolation of PSCs is still obviously hysteretic compared with the isolation of HSCs, although PSCs are nearly identical to HSCs, with presumably the same origin.[8]Therefore, inthis study, we frst provided an easy and reliable method to isolate and culture rat PSCs. This method combined and improved previous techniques from PSCs separation and HSCs isolation. After that, we explored a potential cell-therapy strategy of anti-pancreatic fbrosis by targeting PSCsin vitro. A previous study[9]demonstrated the immunomodulatory effect of bone marrow-derived mesenchymal stem cells on activated HSCs, since the bone marrow-derived mesenchymal stem cells have been shown to prevent the development of liver fbrosis in a number of pre-clinical studies. We, therefore, determined the inhibitory effects of adipose-derived stem cells (ADSCs) on the regulation of these PSCs activity and proliferation through co-culture with ADSCsin vitro, and our data could provide a hopeful possibility for stem cell transplantation for future treatment of pancreatic fbrosis.
Methods
Animals, materials and reagents
Sprague-Dawley rats weighing 300-400 g were obtained from Wenzhou Medical College Animal Center (Wenzhou, China). Transwell inserts containing semi-permeable membranes with a size of 0.4 μm and plastic six-well culture plates were purchased from Millipore Corporation (Billerica, MA, USA); type IV collagenase and DN-ase I from Sigma (St. Louis, MO, USA); Optiprep ® gradient centrifugation medium from Axis-Shield Company (Oslo, Norway). Trypsin, Hank's balanced salt solution (HBSS), Dulbecco's modifed Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Gibco-Invitrogen (Carlsbad, CA, USA). A rat anti-desmin monoclonal antibody and a mouse anti-α-smooth muscle actin (α-SMA) monoclonal antibody were from Boster Bio-Engineering Co., Ltd. (Beijing, China).
Isolation and cultivation of PSCs and ADSCs
The rats were anesthetized with ether. An abdominal midline incision was made to expose the abdominal aorta. Next, the hilar hepatic artery, splenic artery, left gastric artery, and right gastroepiploic blood vessels were isolated and ligated. After ligation of the abdominal aorta of the upper truncus coeliacus behind the esophagus, the abdominal aorta of the lower truncus coeliacus was intubated. After the blood collection, the portal vein was cut. The pancreas was perfused with 37 ℃ preheated HBSS containing 0.3 mg/ mL type IV collagenase in 5-7 mL/ min. The pancreas became swollen and shiny and easy to be located. After removing all adipose tissues, the pancreas was quickly collected and cut into pieces with a scissor. The fresh pancreatic tissues were then added to 15 mL HBSS containing 1 mg/mL type IV collagenase and 20 μg/mL DNase I and incubated at 37 ℃ for 45 minutes. After that, cell suspension was fltered through 200 mesh fltration followed by 30×g centrifugation for 5 minutes at room temperature. After discarding the sediment, the supernatant was centrifuged at 450×g for 7 minutes, and the sediment was then thoroughly mixed with 6 mL of 15% Optiprep®. Next, 6 mL of 11.5% Optiprep® and 6 mL of HBSS were carefully and sequentially added into the cell solution followed by 1400×g centrifugation for 18 minutes at 4 ℃ and the interface layer of the cells was carefully extracted and resuspended in 5 mL HBSS, followed by 450×g centrifugation for 8 minutes. After discarding the supernatant, the sediment was resuspended in DMEM supplemented with 20% FBS and antibiotics and then cultivated in fasks. Cultured for 3-5 passages, PSCs were prepared for the study of their activation and proliferation. The purity of PSCs was identifed by its morphology. The viability of PSCs was tested by trypan blue staining. Similarly, ADSCs were isolated and purifed from rat celiac fat tissues as described previously.[10]After culturing for 3-5 passages, cell surface markers, such as CD90, CD29 and CD45, were analyzed for characterization of the ADSCs.
Immunocytochemistry
Primary cultured PSCs were immunostained for desmin (Boster, Beijing, China) and α-SMA (Boster, Beijing, China). Primary PSCs were fxed using 2% paraformaldehyde for 10 minutes at 48 ℃. The cell membranes were disrupted with 0.5% Triton X-100 (Sangon Biotech, Shanghai, China) for 10 minutes. The PSCs were incubated with rat monoclonal anti-α-SMA (1:500) and rabbit anti-desmin (1:500) antibodies. Labeled cells were incubated with secondary antibody, an antibody binding analysis was performed using a DAB kit. Finally, the cells were observed and quantifed under a light microscope.
Suppression of PSCs activity and proliferation through co-culture with ADSCs
To suppress PSCs activity and proliferation, we cocultured PSCs with ADSCs in a 6-well plastic culture plate with Transwell inserts. This culture system separated PSCs from ADSCs, but ADSC-conditioned medium could affect PSCs as described previously.[11]In addition, this Transwell co-culture permitted these two cell populations to grow together, but prevented direct cell-tocell contact. In each co-culture well, 3×104cells of both PSCs and ADSCs were seeded in the bottom and top well, respectively. In parallel wells, PSCs replace ADSCs as a negative control (PSCs+PSCs group) and PSCs were cultured without Transwell as a blank control (PSCs group).
The cells were cultured with DMEM containing 10% FBS in a humidifed incubator with 5% CO2at 37 ℃. After 72 hours culture, the dynamic morphological changes of the PSCs were observed under an inverted phase contrast microscope.
Evaluation of ADSCs' effect on PSCs
After 72 hours co-culture with ADSCs, the expression of α-SMA protein, a marker of PSC activation, was analyzed using Western blotting. PSCs proliferation was determined by using a CCK-8 assay, and PSCs apoptosis was assessed by fow cytometry after Annexin V-FITC and propidium iodide staining. Other gene expressions (MMP-2, MMP-9 and TIMP-1) were detected by qRTPCR with gene-specifc primers (Table 1).
Detection of cytokine levels in conditioned cell culture medium
To assess the levels of cytokines in the conditioned cell culture medium, which were secreted by ADSCs and inhibited the PSCs in the bottom well, we took 1 mL of the conditioned cell culture medium after 72 hours co-culture for analysis of various cytokine expressions, such as interleukin-10 (IL-10), tumor growth factor-β1 (TGF-β1), and nerve growth factor (NGF), using ELISA according to the manufacturer's instructions (R&D system, Minneapolis, MN, USA).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA from these cells was isolated using a TRIzol reagent (Invitrogen) according to the manufacturer's protocol, and then was reversely transcribed into cDNA with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). After that, qPCR was performed in a MyIQ real-time PCR system with iQ SYBR Green mix (Bio-Rad) and specifc primers. The qPCR conditions were set for an initial 94 ℃ for 5 minutes and then 40 cycles of 94 ℃ 30 seconds, 56 ℃ 30 seconds, and 72 ℃45 seconds, and a fnal 72 ℃ 10 minutes. The primers for IL-10 were 5'-CGA AGC TTG CCA CCA TGC TTG GCT CAG CAC-3' and 5'-CGT CTA GAT CAA TTT TTC ATT TTG AGT G-3', which amplifed a 505bp product; NGF primers were 5'-GAT CGG CGT ACA GGC AGA AC-3' and 5'-GGC TCG GCA CTT GGT CTC AA-3', which produce a 559bp band; TGF-β1 primers were 5'-GAG GCG GTG CTC GCT TTG T-3' and 5'-CGG GTG ACT TCT TTG GCG TAG-3' to produce a 107bp band; and GAPDH primers were 5'-GAG GAC CAG GTT GTC TCC TG-3' and 5'-GGA TGG AAT TGT GAG GGA GA-3', which amplify a 215bp product.
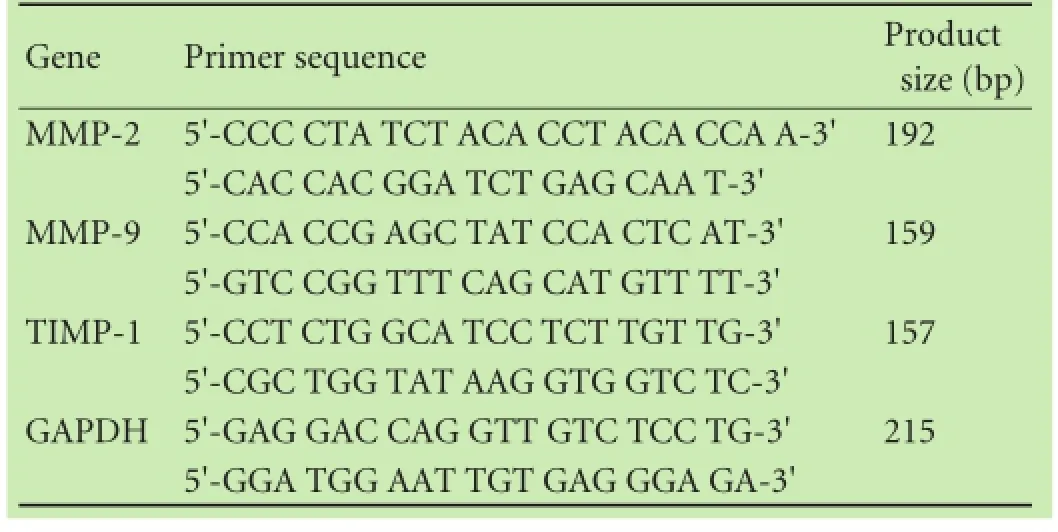
Table 1.Primers used for real-time qRT-PCR
Statistical analysis
All experimental data were represented as mean± standard deviation (SD) for ANOVA analysis in each group with SPSS 16.0 software (SPSS, Chicago, IL, USA). AP<0.05 was considered statistically signifcant.
Results
Isolation and characterization of PSCs
The PSCs localized in the surrounding area of pancreatic lobules and acini around the base of the neighboring glandular cells accounted for approximately 4% of total pancreatic cells. In this study, we frst immunostained α-SMA and desmin proteins, markers of PSCs, in normal rat pancreatic tissues and found their expression in the cytoplasm of PSCs (data not shown). We established an easy and reliable technique to isolate PSCs from rat pancreas. Our data showed that this technique was able to produce 17.6±6.5×103cells per gram of the body weight with a purity of 90%-95% and a viability of 92%-97%. After 48 hour-culture with DMEM, most of the PSCs were adherent to the fask in the oblate shape with granular lipid droplets in the cytoplasm (Fig. 1A) and emitted green fuorescence (Fig. 1C) under a laser excitation microscope. Some cells began to stretch with polygonal pseudopodia (Fig. 1A). After approximately 5-day culture, the cells began to show a typical star-shape with reduced cytoplasmic lipid droplet particles; the highly proliferating cells showed relatively large cell morphology and focal growth (Fig. 1B and D). Moreover, immunohistochemical staining showed that the expression of α-SMA and desmin proteins gradually increased in the cells with extended culture time (Table 2).
Isolation and characteristics of ADSCs
Subsequently, we isolated and cultured ADSCs according to a previously described technique.[10]We obtained approximately 1-2×106ADSCs per 2 mg of ratadipose tissue. The viable fraction of freshly isolated ADSCs was more than 95%, as defned by trypan blue staining. ADSCs adhered to the cell culture fask between 4 and 6 hours and developed a spin phenotype after 48 hours. These ADSCs grew fast after 5-6 days and were passaged when reached about 80% confuence (Fig. 2A). After that, we performed fow cytometry analyses of ADSCs markers in these isolated ADSCs and the data showed high expression of CD29 and CD90 but low expression of CD45 in the isolated ADSCs (Fig. 2B), which was consistent with the previous study.[12]
Inhibitory effect of ADSCs on activation and proliferation of PSCs
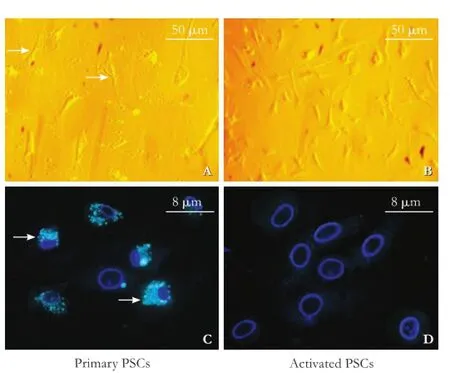
Fig. 1.Characterization of primary PSCs isolated from the rat pancreas.A: The primary PSCs (arrows).B: Morphology of activated PSCs under an inverted phase contrast microscope (original magnifcation ×100).C: Data on fuorescence microscopy. Numbers of small cytoplasmic lipid droplets (arrows) are shown under a fuorescence microscope (original magnifcation ×800; The nuclei were counterstained with DAPI).D: Data on fuorescence microscopy. After 5-day culture, the highly proliferated cells showed a larger size and reduced cytoplasmic lipid droplets particles.
The expression of α-SMA protein was signifcantly lower in the ADSCs+PSCs group after 72-hour coculture than that in the PSCs+PSCs group (Fig. 3). PSCs proliferation was signifcantly inhibited in co-culture of PSCs with ADSCs (P<0.05; Fig. 4A) compared with the control cells. The apoptosis rate of PSCs was higher after co-culture with ADSCs compared with the PSCs+PSCs group or PSCs group (P<0.05; Fig. 4B). In the meantime, we also isolated bone marrow-derived stem cells (MDSCs) and then performed the above experiments using this co-culture assay. As indicated, the inhibitory effect of ADSCs on PSCs activation and proliferation was stronger than that of MDSCs. However, there were no signifcant changes in cell morphology (data not shown).
Cytokine and collagen production in co-culture of PSCs with ADSCs
The expression of fbrinolytic mRNA (e.g. MMP-2and MMP-9) was up-regulated while anti-fbrinolytic mRNA, TIMP-1, was down-regulated in the ADSCs+ PSCs group compared with the PSCs-only or PSCs+PSCs groups (P<0.05, Fig. 5A). The expression of collagen I and III was relatively lower (P<0.05) in the conditionedco-culture medium than that in the PSC-only culture medium (Table 3). Furthermore, we also found that NGF was relatively higher (P<0.05), whereas the expression of IL-10 and TGF-β1 levels was relatively lower (P<0.05,P<0.01, respectively) in the conditioned co-culture medium than that in the PSCs-only culture medium (Table 3). The data indicate that these cytokines played a role in the inhibition of PSCs, which in turn participate in chronic pancreatitis.[13-15]In addition, we also made a qRT-PCR analysis of the gene expression to investigate whether the expression of these genes is regulated at the transcriptional level. Our data showed that the level of NGF mRNA was high in these three types of cell culture, whereas IL-10 and TGF-β1 were down-regulated (P<0.05; Fig. 5B) in PSCs (ADSCs+PSCs) compared with PSCsonly cells, suggesting that ADSCs regulated the expression of these genes in PSCs.
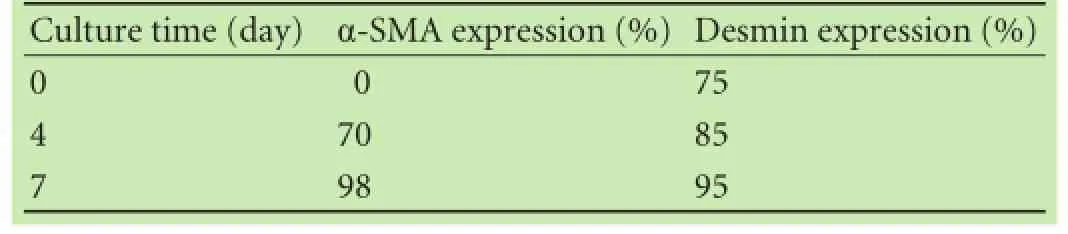
Table 2.Immunohistochemical data of α-SMA and desmin expression in cultured PSCs
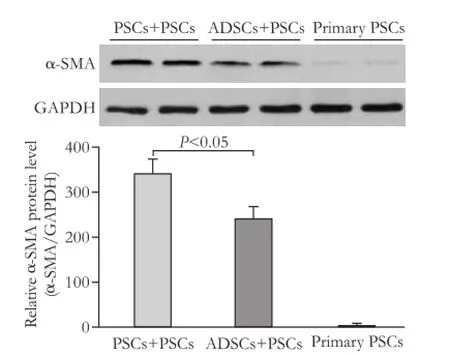
Fig. 3.Western blotting analysis of α-SMA protein level in PSCs co-cultured with PSCs or ADSCs (P<0.05). Representative blots were shown from three independent experiments with similar results. GAPDH served as a loading control.
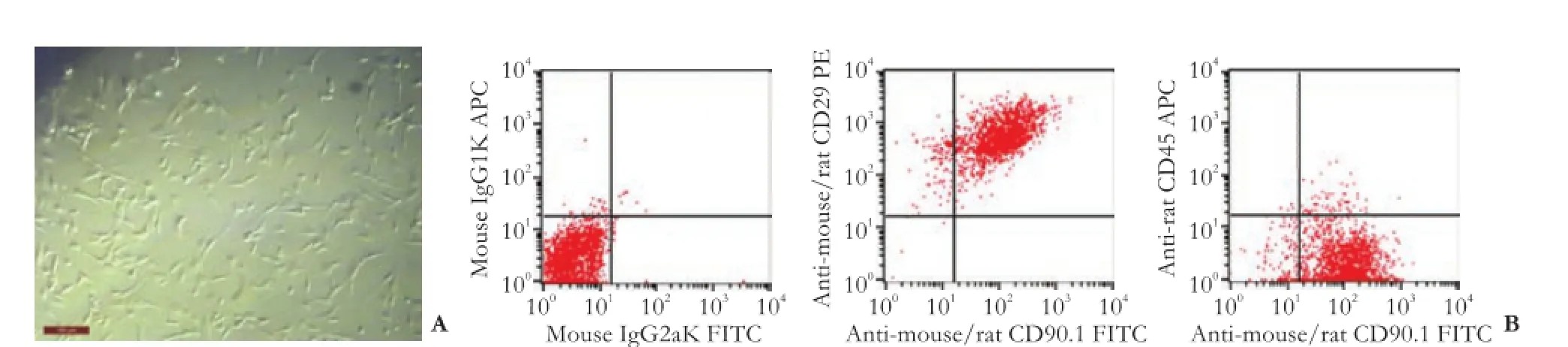
Fig. 2.Characterization of ADSCs.A: The phenotype of ADSCs isolated from the rat adipose tissue on the frst day (original magnifcation ×200).B: Expression of CD90, CD29 and CD45 in the isolated ADSCs (primary ADSCs CD90+: 93.3%; CD29+: 99.7%; CD45−: 96.1%).
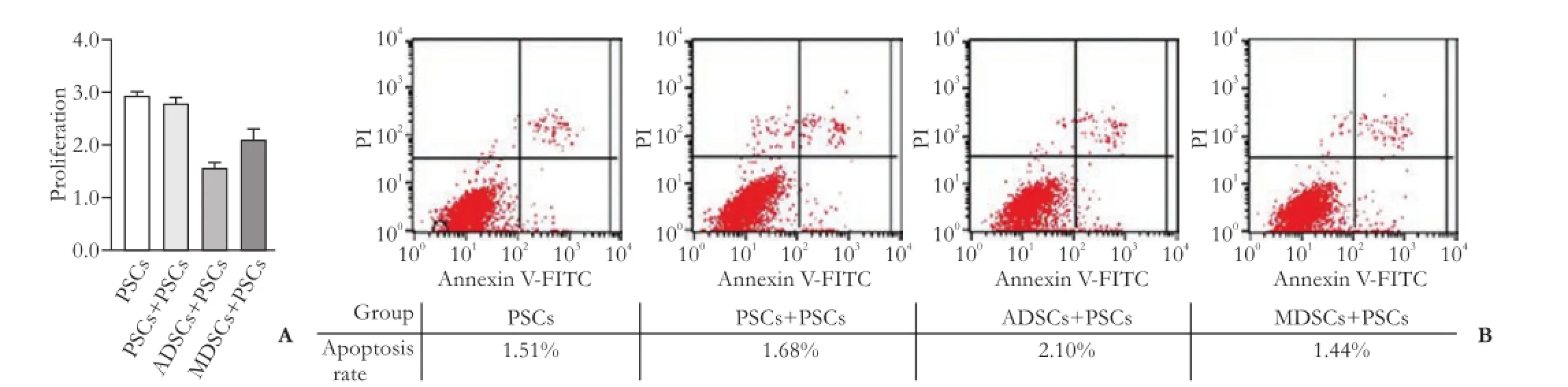
Fig. 4.Inhibition of PSCs proliferation and induction of PSCs apoptosis after co-culturing ADSCs with PSCs.A: The CCK-8 assay. After co-culturing PSCs with PSCs or ADSCs for 72 hours, proliferation of PSCs was examined using the CCK-8 assay. The proliferation of PSCs was signifcantly inhibited compared to PSCs co-cultured with or without PSCs cultured alone (1.573±0.161 vs 2.695±0.150 vs 2.934±0.078,P<0.05). The proliferation of PSCs after co-culture with MDSCs was also examined as another control.B: Flow cytometry cell apoptosis assay. Apoptosis of PSCs was detected by Annexin V-PI double staining and fow cytometry analysis. The apoptosis rate of PSCs was signifcantly higher in the ADSCs+PSCs group than in the PSCs+PSCs group or PSCs group. The apoptosis rate of PSCs after co-culture with MDSCs was also examined as another control (2.172±0.107 vs 1.536±0.133 vs 1.479±0.105,P<0.05). All data were expressed as mean±SD derived from 3 independent experiments performed in triplicate.
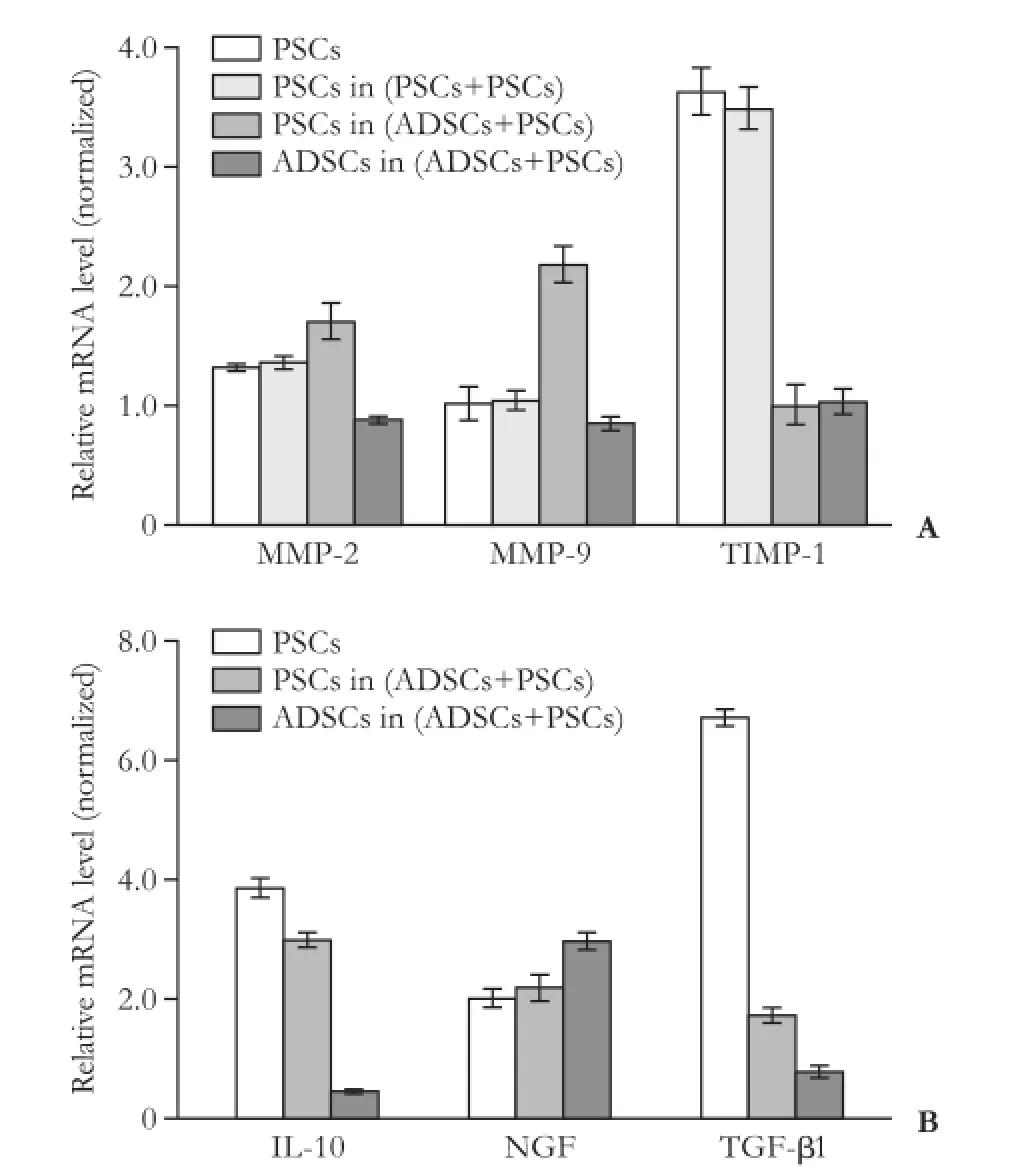
Fig. 5.Regulation of cytokine expression in PSCs after co-cultured ADCSs and PSCs.A: qRT-PCR. After 72 hours co-culture with ADSCs, qRT-PCR data showed that expression of fibrinolytic proteins (e.g. MMP-2 and MMP-9) was upregulated, whereas antifbrinolytic protein, TIMP-1, was downregulated in ADSCs+PSCs compared with the PSCs-only or PSCs+PSCs cells (P<0.05).B: qRT-PCR analysis of gene expression in these co-cultured cells.
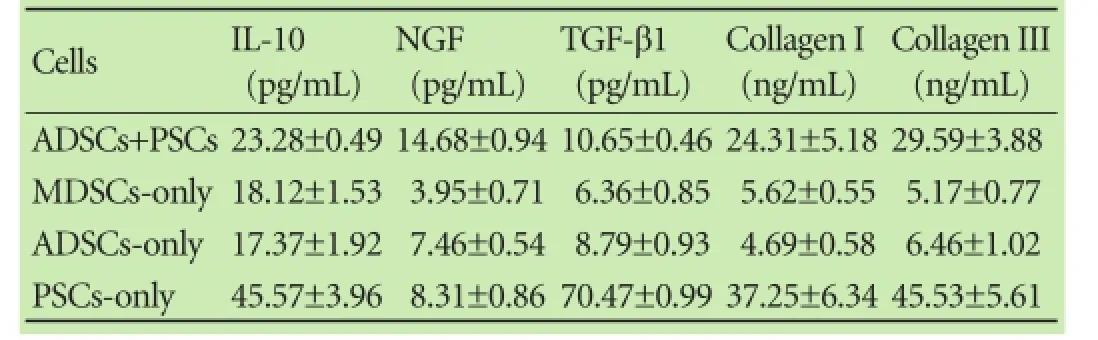
Table 3.Levels of cytokine proteins and collagen in the conditioned cell co-cultured media
Discussion
We improved the method to isolate PSCs from Sprague-Dawley rats. We demonstrated the inhibitory effects of ADSCs on activation and proliferation of PSCsin vitro. Co-culture of PSCs with ADSCs signifcantly inhibitedPSCs proliferation and promoted PSCs apoptosis, while the expression of α-SMA protein was signifcantly lower in PSCs+ADSCs than that in PSCs-only cultures. In contrast, the expression of fbrinolytic proteins (e.g. MMP-2 and MMP-9) and NGF was up- regulated, but anti-fbrinolytic protein (such as TIMP-1) and IL-10 and TGF-β1 proteins were down-regulated in PSCs after co-culture with ADSCs. This study described a useful method to isolate PSCs for study of PSCs in pancreatic fbrosis and demonstrated an inhibitory effect of ADSCs on the activation and proliferation of PSCsin vitro.
The current PSC isolation method was based on our previous experiences in primary HSCs isolation and culture techniques with modifcation of the phase separation procedure, especially by using Optiprep ® gradient separation medium[5]and optimization in the perfusion and cell purifcation details. The current method also simplifed the steps and ensured the purity of PSC isolation, and provided stability and high yield of PSCs by reducing the amount of digestive enzymes. The major advantages of this separation method include: i) Optiprep® gradient separation medium is easy to be prepared and the concentration is precisely controlled during the dilution compared with other density gradient materials (such as Nycodenz and Percoll). Optiprep® was the latest generation of such a reagent in cell separation, which reduces cell damage and cell aggregation with better separation effciency. ii) A high selective infusion step was conducted in this technique. Specifcally, the perfusion through the truncus coeliacus by intubating the abdominal aorta revealed a high selectivity. The pancreatic duct of rats is small and diffcult in intubation. Thus, it resulted in low effciency and poor pertinence. Shinji and colleagues[16]have overcome this disadvantage by the intubation of the thoracic aorta. However, such infusion perfused not only the pancreatic but also other gastrointestinal cells, which caused high consumption and low utilization of digestive enzymes. On this basis, a highly selective perfusion through the truncus coeliacus by intubating the abdominal aorta was conducted in this study. This method not only cleaned the blood cells in the pancreas, but also led most of the perfusate into the pancreas, with a high digestive enzyme utilization rate. The perfusion of post-clipping portal vein made the pancreas swollen that clearly distinguished with the surrounding adipose tissue and easy to locate. In addition, the pancreatic tissue of rats was not only less than liver tissue in quantity, but also with a lower density in structures. By fully shredding tissue with an ophthalmic scissor, the increased surface interacting with digestive enzymes would make cells discrete more easily. The addition of collagenase into the perfusate played a role of pre-digesting.
After that, we performed further experiments by using these newly isolated PSCs. Previous studies[17-19]showed that MDSCs inhibited liver fbrosis by suppression of HSC activation. PSCs and HSCs are speculated to originate from the same progenitor.[8]Thus, we explored the potential effects of ADSCs on PSCs based on the evidence that the biological properties of ADSCs are similar to MDSCs.[20]In normal pancreas, PSCs do not exist as myofbroblast-like cells, and are able to secrete a number of MMPs and TIMPs, which can degrade extracellular matrix. However, when they are activated, these PSCs will increase production and secretion of MMPs and TIMPs differentially, resulting in change in the ratio of MMPs and TIMPs, and in turn change in collagen deposition and formation of fbrosis.[21]Indeed, we showed that ADSC-PSC co-culture was able to affect the activation and proliferation of PSCs by regulation of these gene expressions.
The Transwell insert system delivered a great coculture environment that both types of cells shared cell culture medium but did not have direct contact. The conditioned medium from ADSCs could affect the proliferation and activation of PSCs. Our data indicated that ADSCs were able to inhibit PSC activation and proliferation through indirect contact. However, to date, the antifbrosis mechanism of ADSCs has not yet been clear and there are many factors speculated in the literature.[22-24]For example, the stem cells could differentiate into functional parenchymal cells and promote organ regeneration. In addition, stem cells could regulate the activity of PSCs by regulating the secretion of anti-infammatory and anti-fbrotic mediators such as IL-10, NGF, TGF-β1 and other cytokines. As reported in the literature, IL-10 can inhibit infammation and reduce production of infammatory mediators and matrix fbers,[13]NGF can promote mesenchymal cells to apoptosis but inhibit production of fbrogenic factor, and TGF-β1 can promote organ fbrosis.[25]TGF-β1 can modulate a variety of fbrosis-related gene expressions, which not only promote extracellular matrix synthesis but also inhibit its degradation. TGF-β1 also plays an important role in HSCs activation.[26,27]Our results showed that the PSCs activation was lower in ADSCs+PSCs, which may be due to the modulatory effect of TGF-β1 on IL-10. Thus, our current study showed that ADSCs were able to suppress PSC activation and proliferation, and induce their apoptosis, suggesting that ADSCs could be further developed as a potentially novel strategy in future control of pancreatic fbrosis.
Contributors:YFX and TYH designed the study. YFX performedexperiments and wrote the manuscript. SLF, DCL and WY carried out the experiments. FJH, ZQY and TYH performed statistical analysis. All authors read and approved the fnal manuscript. TYH is the guarantor.
Funding:This study was supported in part by a grant from Zhejiang Province Key Surgery Projects (Zhejiang High-Tech 2008-255).
Ethical approval:The study was approved by the Animal Care and Use Committee of Wenzhou Medical College.
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Madro A, Celiński K, Słomka M. The role of pancreatic stellate cells and cytokines in the development of chronic pancreatitis. Med Sci Monit 2004;10:RA166-170.
2 Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fbrosis. Am J Pathol 1999;155: 1087-1095.
3 Bachem MG, Zhou Z, Zhou S, Siech M. Role of stellate cells in pancreatic fbrogenesis associated with acute and chronic pancreatitis. J Gastroenterol Hepatol 2006;21:S92-96.
4 Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identifcation, isolation, and culture. Gut 1998;43: 128-133.
5 Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, et al. Identifcation, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421-432.
6 Keith RG. Defnition and classifcation of chronic pancreatitis. World J Surg 2003;27:1172-1174.
7 Jesnowski R, Fürst D, Ringel J, Chen Y, Schrödel A, Kleeff J, et al. Immortalization of pancreatic stellate cells as anin vitromodel of pancreatic fbrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest 2005;85:1276-1291.
8 Buchholz M, Kestler HA, Holzmann K, Ellenrieder V, Schneiderhan W, Siech M, et al. Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specifc variations of a common transcriptional phenotype. J Mol Med (Berl) 2005;83:795-805.
9 Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun 2007;363:247-252.
10 Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327:449-462.
11 Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death bothin vitroandin vivo. PLoS One 2009;4:e6278.
12 Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineagein vitroandin vivo. Biochem Biophys Res Commun 2005;328:258-264.
13 Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, et al. Pancreatic stellate cells respond to infammatory cytokines: potential role in chronic pancreatitis. Gut 2002;50:535-541.
14 Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, et al. Expression of transforming growth factorbeta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 2002;160:1787-1798.
15 Haas SL, Fitzner B, Jaster R, Wiercinska E, Gaitantzi H, Jesnowski R, et al. Transforming growth factor-beta induces nerve growth factor expression in pancreatic stellate cells by activation of the ALK-5 pathway. Growth Factors 2009;27: 289-299.
16 Shinji T, Ujike K, Ochi K, Kusano N, Kikui T, Matsumura N, et al. Establishment of a novel collagenase perfusion method to isolate rat pancreatic stellate cells and investigation of their gene expression of TGF-beta1, type I collagen, and CTGF in primary culture or freshly isolated cells. Acta Med Okayama 2002;56:211-218.
17 Kania G, Blyszczuk P, Jochheim A, Ott M, Wobus AM. Generation of glycogen- and albumin-producing hepatocyte-like cells from embryonic stem cells. Biol Chem 2004;385:943- 953.
18 Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol 2006;44:742-748.
19 Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, et al. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun 2001;288:156-164.
20 Shafee A, Seyedjafari E, Soleimani M, Ahmadbeigi N, Dinarvand P, Ghaemi N. A comparison between osteogenic differentiation of human unrestricted somatic stem cells and mesenchymal stem cells from bone marrow and adipose tissue. Biotechnol Lett 2011;33:1257-1264.
21 Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 2003;52:275-282.
22 Takeda M, Yamamoto M, Isoda K, Higashiyama S, Hirose M, Ohgushi H, et al. Availability of bone marrow stromal cells in three-dimensional coculture with hepatocytes and transplantation into liver-damaged mice. J Biosci Bioeng 2005;100:77-81.
23 Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecifc mitogenic stimuli. Blood 2002;99:3838-3843.
24 Kim WH, Matsumoto K, Bessho K, Nakamura T. Growth inhibition and apoptosis in liver myofbroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am J Pathol 2005;166:1017-1028.
25 Aquino JB, Bolontrade MF, García MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fbrosis and hepatocellular carcinoma. Gene Ther 2010;17:692-708.
26 Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fbrosis. Front Biosci 2002;7:d793-807.
27 Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activationin vivo. J Hepatol 1999;30:77-87.
Received July 24, 2013
Accepted after revision April 8, 2014
AuthorAffliations:Department of Hepatobiliary and Pancreatic Surgery, First Affliated Hospital, Wenzhou Medical College, Wenzhou 325000, China (Yu FX, Su LF, Dai CL, Wang Y, Teng YY, Fu JH, Zhang QY and Tang YH)
Yin-He Tang, MD, Department of Hepatobiliary and Pancreatic Surgery, First Affliated Hospital, Wenzhou Medical College, Wenzhou 325000, China (Tel: +86-577-88069639; Fax: +86-577-88069703; Email: tangyinhe@sina.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60283-6
Published online July 17, 2014.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Meetings and Courses
- Current strategies for preventing the recurrence of hepatocellular carcinoma after livertransplantation
- Glypican-3 as a specifc biomarker for hepatocellular carcinoma
- Mutations in thep16gene in DMBA-induced pancreatic intraepithelial neoplasia and pancreatic cancer in rats
- Contrast-enhanced ultrasound in diagnosis of gallbladder adenoma
- Pentoxifylline enhances the protective effects of hypertonic saline solution on liver ischemia reperfusion injury through inhibition of oxidative stress
