Effects of Sunitinib Malate on Growth of Human Bladder Transitional Cell Line T24 In Vitro△
2015-01-09JinWenHanzhongLiZhigangJiandJingJin
Jin Wen, Han-zhong Li*, Zhi-gang Ji, and Jing Jin
1Department of Urology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Pharmacy, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
Effects of Sunitinib Malate on Growth of Human Bladder Transitional Cell Line T24 In Vitro△
Jin Wen1, Han-zhong Li1*, Zhi-gang Ji1, and Jing Jin2
1Department of Urology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Pharmacy, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
sunitinib; bladder cancer; proliferation
ObjectiveTo investigate the growth-inhibitory effect of sunitinib malate on human bladder transitional cell carcinoma (TCC) in vitro.
MethodsHuman bladder TCC cell line T24 was cultured and exposed to graded concentrations of sunitinib malate for 72 hours in vitro to determine the sensitivities to drug. Cell viability was measured by MTT assay. Cell apoptotic morphology was observed by fluorescence microscope following DAPI staining. Band expressions of Fas, Fas ligand, poly (ADP-ribose) polymerase (PARP) and β-actin were analyzed by Western blot. Wound healing process of T24 cells exposed to sunitinib malate was assayed.
ResultsSunitinib malate exerted a concentration-dependent and time-dependent inhibitory effect on the T24 cell lines. Fluorescence microscopy showed that small vacuoles appeared in the nuclei of T24 cells and the vacuoles were bigger with higher drug concentrations. The expressions of Fas ligand and PARP in T24 cells treated with sunitinib malate exhibited a concentration-dependent increase. Moreover sunitinib malate suppressed the wound healing process in a concentration-dependent manner.
ConclusionSunitinib malate exerted marked inhibitory activity against bladder cancer cell line T24.
Chin Med Sci J 2015; 30(1):51-55
R ADICAL cystectomy remains the standard therapy for invasive bladder transitional cell carcinoma (TCC). However, the disease recurs in up to 50% of patients and is potentially lethal despite surgery. Although bladder TCC is relatively sensitive to gemcitabine and cisplatin (GC), the response rates of these regimens are no more than 50%. More effective treatments for metastatic and advanced bladder TCC need to be found.
Sunitinib malate is a multi-targeted receptor tyrosine kinase (RTK) that acts on vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, platelet-derived growth factor (PDGF), stem cell factor receptor (KIT), andFMS-like tyrosine kinase-3 receptor (FLT3). Its antitumor activity has been demonstrated in other cancers, such as renal cell carcinoma, gastrointestinal stromal tumor, non-small-cell lung cancer, and colorectal cancer.1-5In this study, we examined the antitumor effect of sunitinib malate on T24 cell line in vitro.
MATERIALS AND METHODS
Cell culture
Human bladder transitional cell cancer cell line T24 was obtained from the Cell Culture Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The T24 cell line was maintained in DMEM/F12 medium (GIBCO, Grand Island, NY, USA) with 10% heat-inactivated newborn calf serum at 37°C in 5% CO2.
Cell viability measurement by MTT assay
Cells were seeded into 96-well plates at a density of 2×103/well. And 24 hours later, triplicate wells were treated with media mixed test agents (sunitinib malate or cisplatin) at concentrations of 0.625, 1.25, 2.5, 5.0, 10.0, and 20.0 μmol/L respectively. Sunitinib malate and cisplatin were purchased from Pfizer Inc. (USA). After incubation for 72 hours at 37°C in 5% CO2, the drug-containing medium was removed and replaced by 100 μl fresh medium with 0.5 g/L MTT solution. After incubation for 4 hours, the medium with MTT was removed and 150 μl dimethyl sulfoxide was added to each well. Optical density (OD) at a wavelength of 570 nm was determined using a microplate reader (Wellscan MK3, Labsystems Dragon, Finland). Inhibitory rate was calculated according to the formula: inhibitory rate=(ODcontrolgroup-ODexperimental group)/ODcontrol group×100%.
The values of 50% inhibitory concentration (IC50) were measured at the time of 24, 48, and 72 hours respectively after treated with sunitinib malate. The IC50was defined as the concentration that reduced the absorbance of the untreated wells by 50% of vehicle in the MTT assay.
4',6-diamidino-2-phenylindole (DAPI)staining
After incubation with or without sunitinib malate at concentrations of 0.3125, 0.625, 1.25, 2.5, 5.0, and 10.0 μmol/L for 48 hours, T24 cells were stained with DAPI (10 mg/L in PBS) for 30 minutes, followed by fixation with 4% paraformaldehyde for 15 minutes in the dark. Then the cells were washed with PBS. Cell apoptotic morphology was observed by a fluorescence microscope (Olympus BX51, Japan).
Western blot analysis
The protein concentration was measured by the Bio-Rad protein assay. An equal amount of protein was separated using 10% and 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Amersham, Bucks, UK). β-Actin was used as an internal positive control. The primary antibodies included Fas, Fas ligand (FasL), poly (ADP-ribose) polymerase (PARP), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signals were visualized using an enhanced chemiluminescence system (Amersham).
Wound healing assay
T24 cells were seeded at a density of 1×105/well in 24 well plates and cultured for 24 hours. Monolayers were wounded using the tip of a pipette, washed by phosphate buffer saline and further incubated in DMEM/F12 medium with 1% fetal bovine serum in the absence or presence of sunitinib malate of 0.5, 1.5, and 4.5 μmol/L. Images were acquired via a phasecontrast microscope and the wound width was measured.
Statistical analysis
Statistical analysis was performed using the SPSS 19.0 statistical package (SPSS, Chicago, IL, USA). All the data were expressed as mean±standard deviation (SD) and statistically analyzed by paired t-test. P<0.05 was considered as significant.
RESULTS
Antitumor effect of sunitinib malate
Sunitinib malate exerted a concentration-dependent (Fig. 1) and time-dependent (Fig. 2) inhibitory effect on proliferation of T24 cell lines. When drug concentration was more than 5 μmol/L, growth inhibitory rate of the sunitinib malate group was significantly more than the cisplatin group (all P<0.05). IC50of sunitinib malate was decreasing with 72 hours.

Figure 2.Sunitinib malate of 5.0 μmol/L inhibited T24 cell proliferation in a time-dependent manner (n=3).
Apoptosis morphology induced by sunitinib malateAfter 48 hours of exposure to sunitinib malate, T24 cells showed unusual changes in their nuclei. As shown in Fig. 3, at concentrations of 1.25 μmol/L or higher, small vacuoles appeared in the nuclei of T24 cells and the vacuoles were bigger with higher drug concentrations. In comparison, the nuclei of untreated T24 cells and those exposed to lower concentrations of sunitinib malate (0.3125 and 0.625 μmol/L) exhibited normal morphology.
Fas, FasL, PARP expression in T24 cells
As shown in Fig. 4, the expression of FasL in T24 cells treated with sunitinib malate (0, 1.25, 2.5, 5.0, 10.0 μmol/L) for 48 hours exhibited a concentration-dependent increase, but there was practically no change in the expression of Fas. Cleavage of PARP is also an established and reliable apoptosis indicator downstream of caspase activation. As shown in Fig. 4, T24 cells exhibited a concentrationdependent increase of PARP cleavage when exposed to sunitinib malate.
Suppression of wound healing by sunitinib malate in T24 cells
As shown in Fig. 5, sunitinib malate suppressed the wound healing process markedly in a concentration-dependent manner.
DISCUSSION
Sunitinib malate is a small-molecule multi-targeted RTK inhibitor that directly inhibits VEGF receptor, PDGF receptor, KIT, and FLT3. In patients with bladder cancer, the expression level of VEGF mRNA and the serum level of VEGF have been found to be associated with the cancer stage, grade, vascular invasiveness, and metastases6-8. These findings suggest a possible antitumor and anti-angiogenic effect of sunitinib malate in advanced bladder TCC.
In the present study, we explored the antitumor effect of sunitinib malate on T24 cell lines by the MTT assay and analyzed the results compared with the conventional chemotherapeutic agent cisplatin. We found that both sunitinib malate and cisplatin exhibited a significant inhibitory effect against these cancer cells. This finding points to the possibility of clinical application of sunitinib malate for advanced bladder cancer therapy.
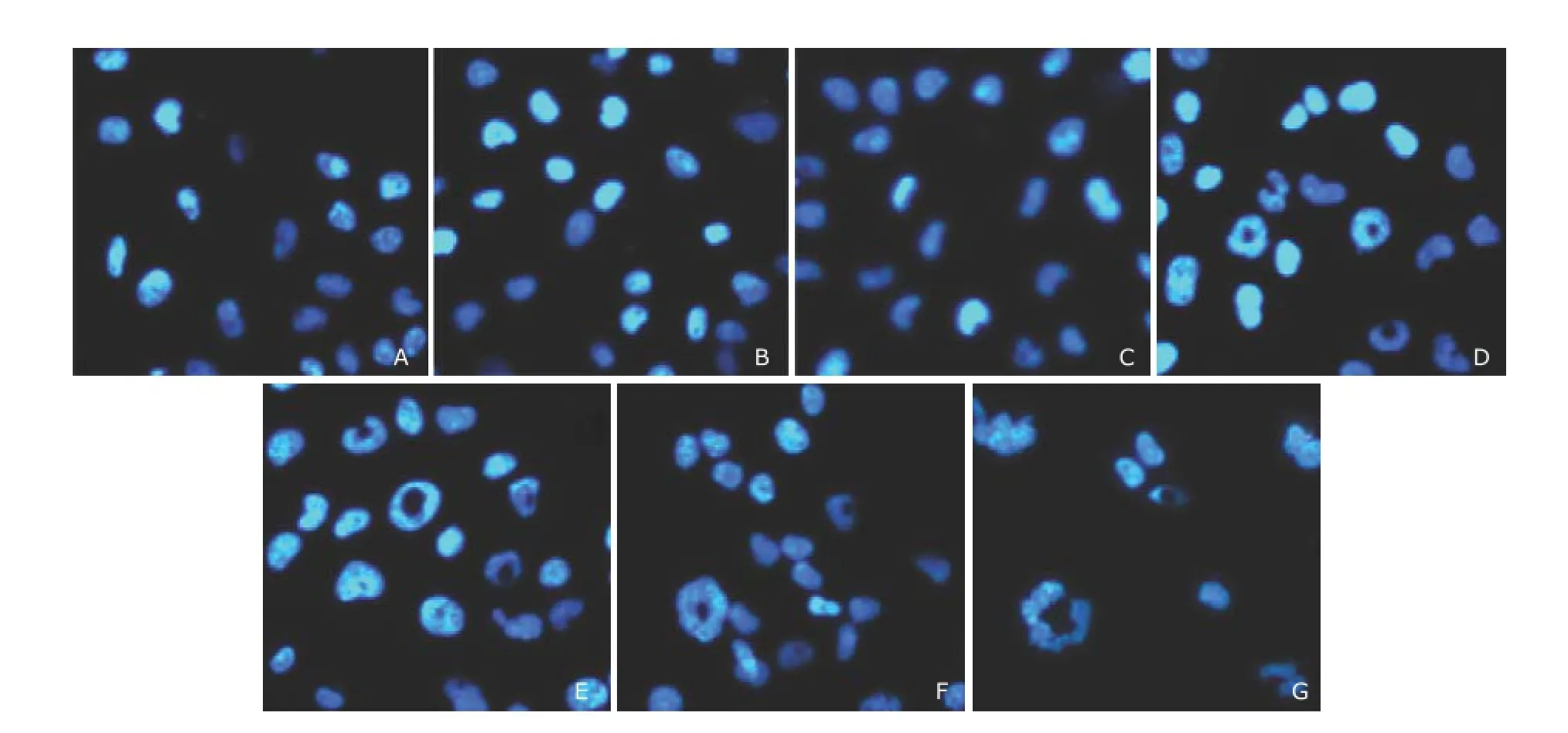
Figure 3.Morphologic changes in T24 cells treated with sunitinib malate. ×200
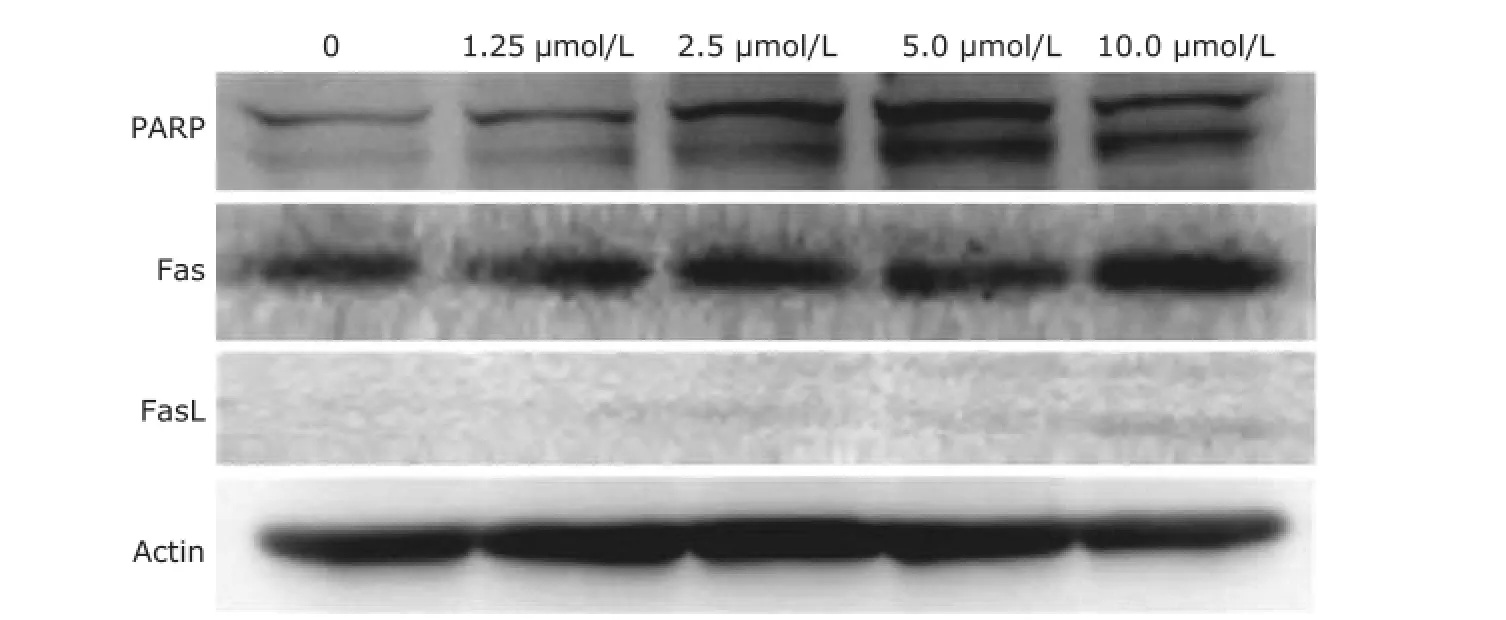
Figure 4.Western blot analysis of protein expression in T24 cells treated with sunitinib malate.

Figure 5.Effect of sunitinib on wound healing. ×200
Although sunitinib malate is a widely used drug with multiple targets, we did not examine its effect on targets such as VEGF receptor, PDGF receptor and KIT etc. in the TCC cell lines utilized in this study. What we were interested in is whether sunitinib malate can induce apoptosis and other characteristic changes in the cells. The morphologic analysis following DAPI staining showed an unusual change of a hole in the nuclei of T24 cells, after exposure to sunitinib malate, which was probably an early apoptosis phenomenon.
The concentration-dependent augmentation of FasL by sunitinib malate noted in our study suggests a possible involvement of the extrinsic apoptosis pathway in the antitumor action of the drug against TCC.
As discussed above, PARP is a nuclear DNA-binding protein that detects DNA strand breaks and functions in base excision repair. However, once PARP is cleaved, it no longer supports the enzymatic DNA repair function, and there is increasing evidence that cleaved PARP may inhibit access to DNA by other repair enzymes. In this study we found that T24 cells exhibited a concentration-dependent increase in PARP cleavage when exposed to sunitinib malate which might partially explain the morphologic findings following DAPI staining.
To gain further insight into the effects of sunitinib malate on other characteristics of TCC, we examined its effect on cell migration and polarity via a wound healing assay and found that it suppressed the wound healing process in a concentration-dependent manner. This finding further suggests its possible usage in the treatment of TCC.
In conclusion, this study demonstrated that sunitinib malate exerted marked inhibitory activity against human bladder TCC cell line T24, and the cell growth inhibitory effect was related to induction of apoptosis. These findings point to the possible clinical application of sunitinib-based therapy for advanced bladder cancer.
REFERENCES
1. Ballas MS, Chachoua A. Rationale for targeting VEGF, FGF, and PDGF for the treatment of NSCLC. Onco Targets Ther 2011; 4:43-58.
2. Petrillo M, Scambia G, Ferrandina G. Novel targets for VEGF-independent anti-angiogenic drugs. Expert Opin Investig Drugs 2012;21:451-72.
3. Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295:2516-24.
4. Tran TA, Kinch L, Peña-Llopis S. Platelet-derived growth factor/vascular endothelial growth factor receptor inactivation by sunitinib results in Tsc1/Tsc2-dependent inhibition of TORC1. Mol Cell Biol 2013; 33:3762-79.
5. Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol 2008; 26:650-6.
6. Wu CL, Ping SY, Yu CP, et al. Tyrosine kinase receptor inhibitor-targeted combined chemotherapy for metastatic bladder cancer. Kaohsiung J Med Sci 2012; 28:194-203.
7. Kim S, Ding W, Zhang L, et al. Clinical response to sunitinib as a multitargeted tyrosine-kinase inhibitor (TKI) in solid cancers: A review of clinical trials. Onco Targets Ther 2014; 12:719-28.
8. Szarvas T, Jäger T, Droste F, et al. Serum levels of angiogenic factors and their prognostic relevance in bladder cancer. Pathol Oncol Res 2009; 15:193-201.
for publication June 18, 2014.
△Supported by the Beijing Natural Science Foundation (7102128).
Tel: 86-10-69156073, E-mail: ljjxmc@163.com
杂志排行
Chinese Medical Sciences Journal的其它文章
- Use of Cataract Surgery in Urban Beijing: a Post Screening Follow-up of the Elderly with Visual Impairment due to Age-related Cataract
- Lipopolysaccharide Challenge Induces Long Pentraxin 3 Expression in Mice Independently from Acute Lung Injury△
- Reliability of a Novel Cobb Protractor for Measuring the Cobb Angle of Radiograph in Scoliosis
- Role of Removing Stasis and Reducing Heat Formula in Clearance of Proximal Ureteral Calculi after Ureteroscopic Ho:YAG Laser Lithotripsy: A Prospective Randomized Study
- Redo Coronary Artery Bypass Grafting: On-Pump and Off-Pump Coronary Artery Bypass Grafting Revascularization Techniques
- MRI Evaluation of Lateral Geniculate Body in Normal Aging Brain Using Quantitative Susceptibility Mapping.△
