Lipopolysaccharide Challenge Induces Long Pentraxin 3 Expression in Mice Independently from Acute Lung Injury△
2015-01-09
1Department of Emergency Medicine,3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Thoracic Surgery, Huashan Hospital, Fudan University, Shanghai 200040, China
Lipopolysaccharide Challenge Induces Long Pentraxin 3 Expression in Mice Independently from Acute Lung Injury△
Gao Zeng1†, Jie Liu1†, Ning Wu2, Cong-wei Jia3, and Shu-bin Guo1*
1Department of Emergency Medicine,3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
2Department of Thoracic Surgery, Huashan Hospital, Fudan University, Shanghai 200040, China
long pentraxin 3; acute lung injury; biomarker; sepsis; lipopolysaccharide
ObjectiveTo determine whether the onset of acute lung injury (ALI) induces the up-regulation of pentraxin 3 (PTX3) expression in mice and whether PTX3 concentration in the biofluid can help recognizing sepsis-induced ALI.
MethodsWild-type C57BL/6 mice (12-14 weeks old) were randomly divided into 3 groups. Mice in the group 1 (n=12) and group 2 (n=12) were instilled with lipopolysaccharide via intratracheal or intraperitoneal routes, respectively. Mice in the group 3 (n=8) were taken as blank controls. Pulmonary morphological and functional alterations were measured to determine the presence of experimental ALI. PTX3 expression in the lung was quantified at both protein and mRNA levels. PTX3 protein concentration in blood and bronchoalveolar lavage fluid was measured to evaluate its ability to diagnose sepsis-induced ALI by computing area under receiver operator characteristic curve (AUROCC).
ResultsALI was commonly confirmed in the group 1 but never in the other groups. PTX3 expression was up-regulated indiscriminately among lipopolysaccharide-challenged mice. PTX3 protein concentration in the biofluid was unable to diagnose sepsis-induced ALI evidenced by its small AUROCC. PTX3 concentration in bronchoalveolar lavage fluid did not correlate with that in serum.
ConclusionsLipopolysaccharide challenges induced PTX3 expression in mice regardless of the presence of ALI. PTX3 may act as an indicator of inflammatory response instead of organ injury per se.
Chin Med Sci J 2015; 30(1):7-17
CUTE lung injury/acute respiratory distress syndrome (ALI/ARDS) may occur as a consequence of critical illness of diverse etiologies, including direct injury to lung and indirect mechanisms, such as sepsis.1 The mortality rate associated with ARDS has declined from 90% twenty years ago to about 40% at present. However, it is still one of the major causes of acute respiratory failure with high morbidity and mortality in critically ill patients.2 With the improved understanding of the pathogenesis of ALI/ARDS, extensive investigations have revealed several molecular mechanisms that offer diagnostic opportunities for ALI/ARDS.3 Many candidates, such as surfactant protein-D, von Willebrand factor, have been assessed for their diagnostic or prognostic capability for the syndrome, but no single biomarker or biomarker panels proved suitable for routine clinical use.4 Long pentraxin 3 (PTX3) as a member of the pentraxin superfamily is characterized by its cyclic multimeric structure and considered as a non-redundant component of the humoral arm of innate immunity.5 Unlike short pentraxins including C-reactive protein and serum amyloid protein, which are mainly produced in the liver, PTX3 is rapidly produced and released by several cell types including macrophages, dendritic cells, fibroblasts, endothelial cells and epithelial cells in response to inflammatory signaling.6 PTX3 expression in alveolar epithelial cells is activated by tumor necrosis factor-α through JNK signaling pathway.7 PTX3 has been suggested as one of the inflammatory mediators related to lung injury and to play a ‶local″ role in host defense and inflammatory lung injury.6, 8 Intratracheal (IT) instillation of lipopolysaccharide (LPS) induced ALI in mice in parallel with increases in pulmonary PTX3 concentration.9 Importantly, PTX3 concentration in bronchoalveolar lavage fluid (BALF) was Aclosely correlated with the severity of lung injury, and PTX3 expression in the lung was down-regulated as tissue injury was attenuated by an anti-coagulant therapy.9 Another study on ‶two hit″ models of ALI reported that high-volume ventilation, either alone or in combination with LPS or hemorrhage/shock challenges, enhanced pulmonary PTX3 expression which was asssociated with the severity of lung injury.10 PTX3 overproduction led to lower mortality rates in endotoxic shock or polymicrobial sepsis among transgenic mice.11 On the other hand, PTX3 deficiency exacerbated tissue injury, inflammatory response, and cell apoptosis in the lung of ptx3 knockout mice when subjected to LPS challenges.12 A study on septic patients showed plasma PTX3 concentration was highly correlated with the severity and unfavorable outcomes of the disease.13 Another study on ARDS patients reported blood PTX3 concentration reflected the extent of respiratory dysfunction and systemic organ failure.14Based on these findings which supported PTX3 acting as a functional role in the pathogenesis of sepsis-induced ALI and as the marker of lung injury, we hypothesized that PTX3 expression should be up-regulated by the onset and progression of sepsis-induced ALI and PTX3 tests could help diagnose sepsis-induced ALI in mice.
In this study, we aimed to develop ALI models which met the diagnostic criteria of experimental ALI proposed by the American Thoracic Society and non-ALI models which underwent similar or even higher levels of systemic inflammation than ALI models did. PTX3 expression was detected locally and systemically at both protein and mRNA levels. We sought to determine whether PTX3 expression in mice was up-regulated with the onset of ALI and whether PTX3 concentration in the biofluid could help recognizing sepsis-induced ALI.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice (12-14 weeks old) of specific pathogen free were purchased from Vitalriver (Beijing, China). They were fed with a standard laboratory diet and water ad libitum and acclimatized for at least one week in the controlled environment of 22°C-26°C, 40%-60% relative humidity, and 12-hour dark/light cycles at Peking Union Medical College Hospital (PUMCH). Those who weighing 30±2 g seem alert with normal exploratory and feeding behavior were qualified for experimentation. The experimental protocol of this study was approved by the Animal Care and Use Committee of PUMCH and in accordance with National Institute of Health guidelines for care and use of laboratory animals.
Development of experimental sepsis and ALI
LPS (Escherichia coli serotype 055:B5, L4524, Sigma-Aldrich Corp., Mo, USA) was dissolved in normal saline at the final concentration of 1.5 mg/ml. Sodium pentobarbital (30-40 mg/kg) was injected via the intraperitoneal (IP) route for anesthesia. Anesthetized mice were allowed to recover in a 100% oxygen chamber. The qualified animals were designated by lottery into 3 groups. Mice in the group 1 (n=12) and group 3 (n=8) were subjected to tracheotomy under anesthesia with a 22-gauge intravenous catheter inserted into the trachea and individually instilled via the IT route with 150 µg LPS and 100 µl normal saline, respectively. Mice in the group 2 (n=12) were injected individually with 300 µg LPS via the IP route. The dosage ofLPS used in the group 1 (about 5 mg/kg) and group 2 (about 10 mg/kg) was decided based on the following considerations. Firstly, the same type and dosage of LPS had been reported to induce ALI in mice within 24 hours by IT instillation.9,12Secondly, systemic inflammatory response to LPS challenges is generally dose-dependent in animals and humans. Thirdly, in our laboratory, IP injection of LPS with dosage ranging between 5 and 15 mg/kg usually caused piloerection, huddling, and lethargy (the macroscopic manifestations) in mice with minimal abnormalities of gas exchange and mild changes of alveolar-capillary barrier function. Only the mice subjected to IT instillation of normal saline were used as blank controls because we hoped to eliminate the confounding effect of trauma-associated inflammatory response and tracheotomy normally brought about more surgical trauma than IP injection did.
At 2, 4, 8 hours during experimentation, each animal received 1.5 ml pre-warmed (37°C) normal saline via the IP route for volume resuscitation. Experimental sepsis is defined by the occurrence of the macroscopic manifestations shortly after LPS insults and increased levels of inflammatory mediators. Experimental ALI is confirmed by the 4 categories of evidence listed in Table 1, and for the confirmation of ALI in 1 condition, at least 3 categories of the evidence are required.15
Collection of blood and tissue samples
At 6 hours after treatment, 0.1 ml blood was obtained from each of the mice by retro-orbital venous plexus puncture; at the end of the observations (10 hours after treatment in the group 1 and 24 hours in the other groups), artery blood was collected under anesthesia by abdomen aortic puncture and analyzed immediately by an iSTAT blood gas analyzer and CG4+ cartridges (Abbott Laboratories, Princeton, NJ, USA). All blood samples were allowed to clot overnight at 4°C and centrifuged for 20 minutes at 2000 × g to collect serum which was stored at -80°C for further use.
Following aortic puncture, the mice were killed immediately by cervical dislocation and pulmonary vasculature was perfused with 4 ml PBS containing 5 mmol/L EDTA at the pressure of 5 mm Hg (0.67 kPa). Subsequently, lungs were lavaged triply with a total of 1.8 ml ice cold PBS containing 5 mmol/L EDTA, 28 µg/ml aprotinin, and 1 µg/ml leupeptin. Recovered BALF was temporarily stored at 4°C for further processing within 24 hours.
Finally, after the ligation of the right main stem bronchus, the right side lung was excised with one lobe snap frozen in liquid nitrogen and stored at -80°C until real-time PCR analysis and the others dried in an oven at 60°C for 72 hours to obtain lung wet-to-dry weight ratio (W/D). The left side lung was insufflated with 10% buffered formalin for 5 minutes at 25 cm water pressure and then immersed in 10% buffered formalin for 5-7 days.
Histological and immunohistochemical evaluation
Lung tissue sections (5 µm) were stained with hematoxylin and eosin and evaluated by a pathologist in a blinded manner. The typical histological changes of ALI include 5 items listed in Table 1. A binary approach was adopted to categorize tissue sections as either injured or normal.
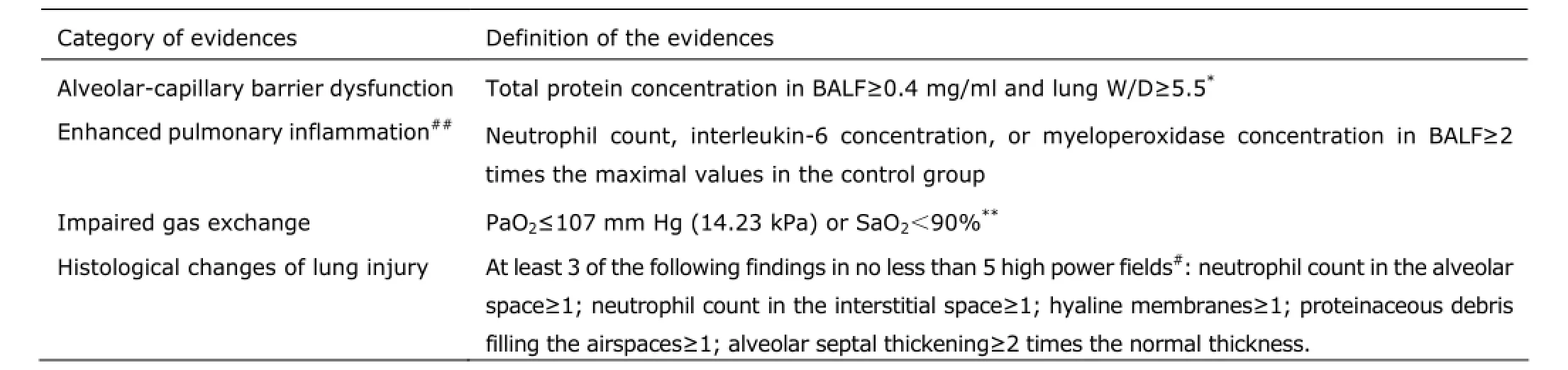
Table 1.Four categories of evidence for the presence of experimental ALI
Immunohistochemical staining was conducted using a Vectastain ABC-AP kit (Vector Laboratories, CA, USA) as described by Okutani et al.10Briefly, tissue sections (4 µm) were incubated in sequence with polyclonal goat IgG against mouse PTX3 (1:200 dilution; R&D Systems, MN, USA) and polyclonal donkey IgG against goat IgG (1:2000 dilution; R&D Systems). Vector red phosphatase alkaline substrate was used as chromogen and Vector methyl green for counterstain. The specificity of the primary antibodies was determined by replacing them with normal goat serum (CoWin Biotech, Beijing, China)
Total protein assay and cell count in BALF
BALF was centrifuged for 20 minutes at 1500 ×g and 4°C to collect supernatant for detection of total protein concentration by the DC protein assay kit (Bio-Rad Laboratories, CA, USA). The cell pellet was resuspended in 0.3 ml PBS for cell counting by the standard hemocytometer technique. A total of 300 karyocytes per slide were counted for differential cell using a Diff-Quick-stained kit (Baxter Diagnostics, IL, USA).
Enzyme-linked immunosorbent assay (ELISA)
Myeloperoxidase (MPO), interleukin (IL)-6, and PTX3 protein in biofluid were quantified by the mouse MPO ELISA kit (Hycult Biotech, the Netherlands), the corresponding Quantikine ELISA kits (R&D Systems), respectively. Each measurement was run in duplicate under the manufacturers’ guidance. The concentrations of the proteins were determined by optical densitometry at 450 nm with an automated plate reader (Bio-Rad Laboratories).
Western blotting
Equal amounts of total protein extracted from homogenized lung tissues by the tissue protein extraction kit (CoWin Biotech) were separated by 10% SDS-PAGE and transferred to PVDF membranes (CoWin Biotech). Subsequently, the membranes were incubated with either anti-PTX3/TSG14 antibodies (R&D Systems) or anti-βactin antibodies (CoWin Biotech) and secondary HRP-conjugated antibodies. The chemiluminescence detection was performed using the cECL Western Blot kit (CoWin Biotech) and quantitative analysis of blotting bands was carried out by densitometer scanning (VersaDoc Imaging System, Bio-Rad Laboratories).
Real-time PCR analysis
Total RNA was extracted from lung tissues and converted into cDNA using TRIzol Reagent and High-Capacity cDNA Reverse Transcription kit (Life Technologies, CA, USA), respectively.
Real-time PCR analysis was performed in triplicates in a total volume of 50 µl for each run using power SYBR green master mix (Life Technologies) on an ABI PRISM 7300 Real-Time PCR System (Applied Biosystems, CA, USA) under the manufacturer’s guidance. The expression of GAPDH was measured in parallel for normalization. Sequences of primer pairs are shown in Table 2. Relative levels of PTX3 expression were calculated by the 2-ΔΔCTmethod.
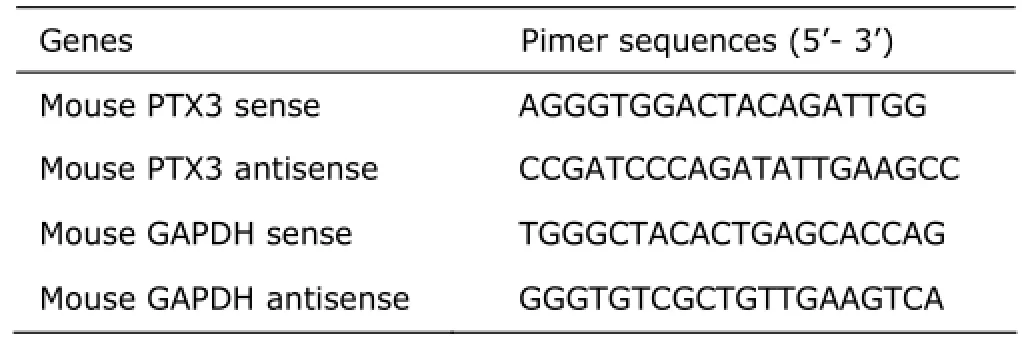
Table 2.Primer sequences for real-time PCR
Observation of 7-day survival
Twenty of the qualified mice were divided into two groups in which ALI and sepsis were developed by the methods used in the group 1 or group 2. The same fluid treatment as described above was repeated on survivals at the first 3 days during 7-day follow-up and no blood or tissue samples were collected. Mice were monitored every 2-8 hours and euthanized by cervical dislocation when moribund.
Statistical analysis
Values were expressed as mean ± standard deviation. Intergroup differences were calculated by analysis of variance test with Tukey’s multiple comparison test. Correlation analysis was conducted by Pearson correlation calculation. The Kaplan-Meier method was used to create survival curves whose difference was analyzed by the log-rank test. A P<0.05 was regarded as significant. Statistical analyses were performed using Prism software 5.0 (GraphPad Inc., USA).
RESULTS
Experimental sepsis and ALI developed in LPS challenged mice
Among the LPS challenged mice in the group 1 and group 2, the macroscopic manifestations became evident by 6 hours post-insult. The blank controls in the group 3 kept alert and behaved normally during the observations.
We have analyzed alveolar-capillary barrier function, pulmonary inflammation as indicator of ALI in micefollowing IT (group 1) or IP (group 2) injection of LPS. To detect alveoli-capillary barrier dysfunction, lung W/D and total protein concentration of BALF were measured, and both showed significant increases in the group 1 (Fig. 1A, B) whose cases all fulfilled the definition of the dysfunction listed in Table 1. However, three cases in the group 2 manifested the dysfunction as well. Mice in the group 1 exhibited the most significant accumulation of neutrophils in the lung (Fig. 1C, D) and the highest level of pulmonary inflammation (Fig. 1E); all the LPS-challenged mice developed systemic inflammatory response evidenced by their boosted serum IL-6 concentration. (Fig. 1F). Given the macroscopic manifestations prevailing in the LPS-challenged mice, we consider they have commonly developed experimental sepsis.
Only two mice in the group 1 manifested normal oxygenation, whereas no hypoxemia cases were present in the other groups. The levels of arterial partial pressure of oxygen (PaO2), oxygen saturation of arterial blood (SaO2) and arterial partial pressure of carbon dioxide (PaCO2) were significantly lower in the group 1 than those in the other groups (Fig. 2A-C).
Typical changes of lung histology in the group 1 included neutrophil accumulation and hemorrhage in the alveolar and interstitial space, alveolar wall thickening, and proteinaceous deposits in the alveolar space (Fig. 3A). In the other groups were commonly seen thin alveolar walls, rare neutrophil accumulation in the lung, and no evident intra-alveolar protein deposition (Fig. 3B, C).
Overall, at least three of the ''main features’ were present in any case in the group 1, while no one in the other groups possessed more than two of the ''main features’’. In view of the definition of experimental ALI mentioned above, we consider that ALI has developed in the group 1 but never in the other groups.
LPS challenges induce the up-regulation of PTX3 expression in mice regardless of the presence of ALIQuantitative analysis of Western blotting results revealed significant increases in PTX3 protein concentration in both the LPS challenged groups (both P<0.01), whose intergroup difference was not statistically significant (Fig. 4A, B). This finding was confirmed at mRNA level by real-time PCR analysis whose results even showed the tendency of higher levels of ptx3 expression in the group 2 than in the group 1 (Fig. 4C).
PTX3 positive staining (pink color) was strong on the alveolar walls of LPS-challenged mice but hardly detected in the blank controls (Fig. 5). No pink color staining was found in the lung when replacing the primary antibody with normal goat serum (data not shown).
PTX3 concentration in serum which was collected at 6 hours post-insult was higher in the group 2 than that in the other groups (P<0.05, Fig. 6A); at the end of the observations PTX3 concentration in serum (Fig. 6B) and BALF (Fig. 6C) indiscriminately increased in the LPS-challenged groups (all P<0.01). Taking into account the fact that the presence of ALI was generally identified in the group 1 but never in the group 2, we conclude that LPS challenges induce the up-regulation of PTX3 expression in mice regardless of the presence of ALI.
PTX3 tests performed poorly in diagnosing ALI among septic mice
When using the ELISA results of PTX3 in biofluid to diagnose ALI among the LPS challenged mice, we found it hard by generating receiver operator characteristic (ROC) curves to draw the line between the cases with and without ALI. Among the PTX3 tests in BALF and serum, the serum PTX3 test of 6 hours post-insult showed the largest diagnostic index (sensitivity plus specificity) of 150% at the cutoff value of 247.4 ng/ml. The serum PTX3 tests of experiment ends proved worthless at identifying ALI versus non-ALI among septic mice evidenced by its area under ROC curve (AUROCC) and P value (Fig. 7A). Similarly poor diagnostic performance was shown in the BALF PTX3 tests of experiment ends (Fig. 7B) and the serum PTX3 tests of 6 hours post-insult (Fig. 7C). Moreover, PTX3 concentration in BALF and in serum did not correlate with each other among septic mice (Fig. 8A).
IT instillation of LPS (about 5 mg/kg) was lethal to mice while IP injection of LPS (about 10 mg/kg) was not in most cases
The LPS challenges led to piloerection, huddling, and lethargy in mice within several hours. For those exposed to IP LPS (about 10 mg/kg), the survival rate of 7 days follow-up was 87.5%, and their macroscopic manifestations tended to alleviate at the second day of the follow-up. For those exposed to IT LPS (about 5 mg/kg), the macroscopic manifestations progressively deteriorated till death, their lethality came up to 100% within 24 hours after treatment (Fig. 8B). By the end of the follow-up, all survivals had been witnessed restoring normal exploratory and feeding behaviors.
DISCUSSION
Organ failure is one of the most ominous complications of sepsis, and one of the first organs to fail is the lung. Indeed, almost half of all patients with severe sepsis will goon to develop ALI/ARDS.18To simulate sepsis-induced ALI in humans, IT administration of LPS is one of the most popular methods used in laboratory studies.19,20However, IP injection of LPS has not been so often used to induce experimental ALI.19,21,22Some studies claimed that LPS caused acute pulmonary damage in mice 24 hours after intranasal or IT administration, whereas IP administration did not lead to a tissue-specific or similar degree of lung injury.23,24Others reported IP administration with LPS dosage at 40 mg/kg induced the presence of most of the‶main features″ of ALI in rats.25,26In our experience, IP administration with LPS dosage ranging between 5 and 15 mg/kg rarely resulted in considerable tissue injury in mouse lung. Even though a larger dose of LPS causes notable lung injury in mice by this method, there is usually more intense systemic inflammation coming around. In this context, we cannot infer that the up-regulation of PTX3 expression (if any) in the cases with larger dosage of LPS results from the onset of ALI rather than the enhancement of systemic inflammation. After all, the intensity of inflammatory response is not the only determinant for the development of ALI.
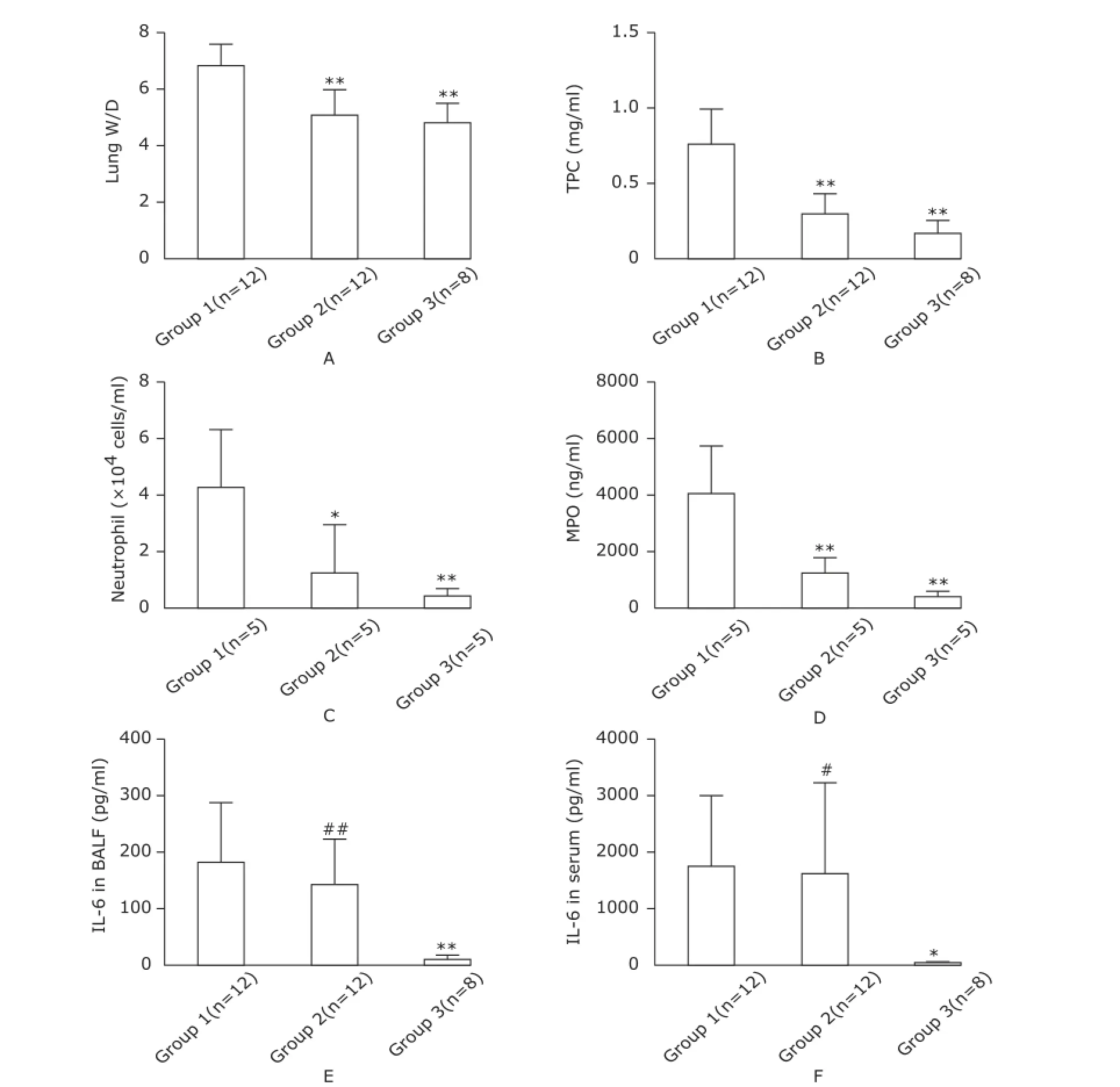
Figure 1.Measurements of alveolar-capillary barrier function and inflammatory response.
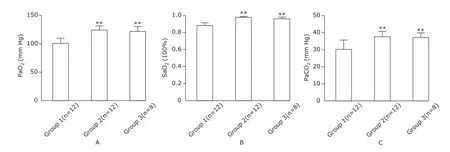
Figure 2.Evaluation of respiratory function.

Figure 3.Typical findings of pulmonary histology in mice (scale bar=100 µm).
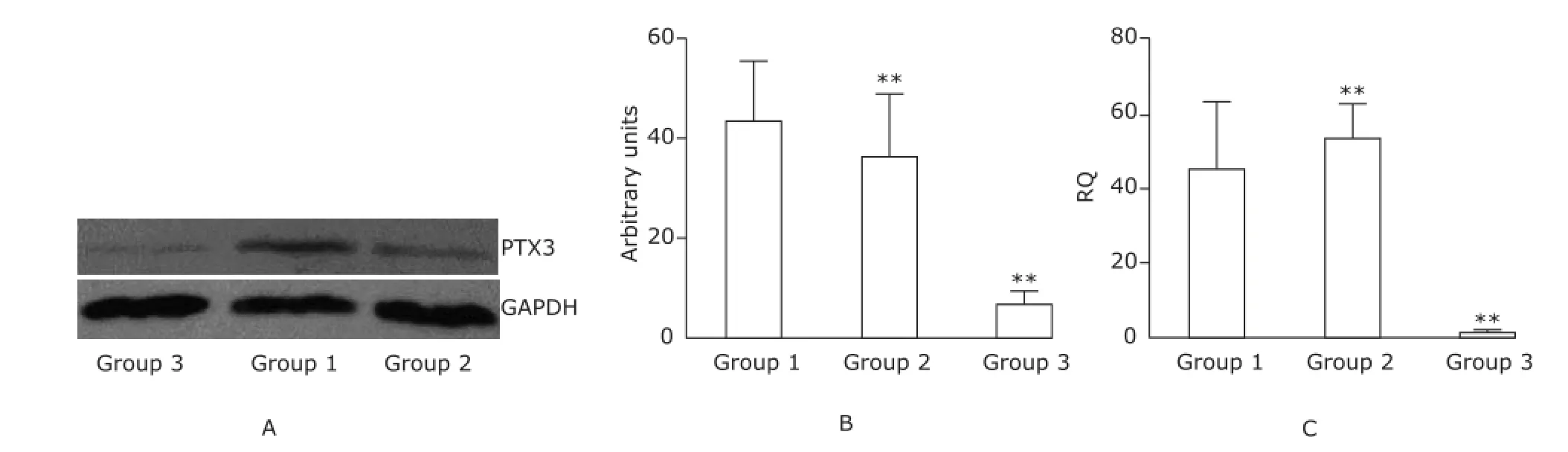
Figure 4. Intergroup comparisons of PTX3 expression level in the lung of mice (n=6).
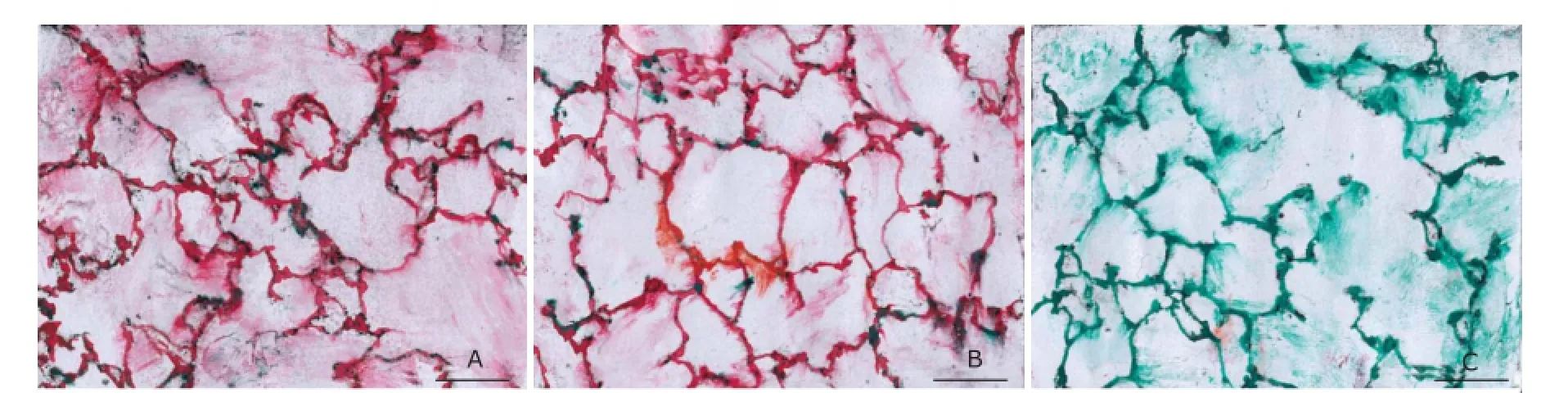
Figure 5.Immunohistochemical staining of PTX3 in the lung of mice (Scale bar=100 µm).

Figure 6.Intergroup comparisons of PTX3 concentration in BALF and serum.

Figure 7.Diagnostic abilities of PTX3 tests.
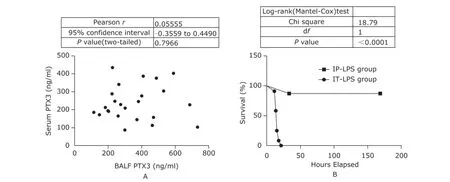
Figure 8.Correlation analysis and survival survey.
For a long time, there had been no universal agreement as to the precise definition of experimental ALI until the American Thoracic Society published an expert consensus on this issue in 2010.15Ideally, an animal model of ALI should capture most of the defining features of human ALI, including rapid onset (hours) after an inciting stimulus, evidence of pulmonary physiological dysfunction (e.g., abnormalities of gas exchange, decreased lung compliance), histological evidence of injury to the lung parenchyma (endothelium, interstitium, epithelium), and evidence of increased permeability of the alveolar-capillary membrane. The question remains as to what constitutes the minimal criteria for the diagnosis of ALI in animal models. This is a question dependent on the experimental design and the specific issues being addressed. In this study, we needed the gold standard to diagnose experimental ALI, and it is prudent to adopt more strict diagnostic criteria to discriminate between ALI and no-ALI cases.
The time for the onset of LPS-induced ALI by IT administration method differed from a couple of hours in some studies9,12up to several days in others.27In our pilot study, this method with LPS dosage at 5 mg/kg caused 100% lethality in mice within 24 hours and the earliest death emerged around 12 hours post-insult. Therefore, 10 hours post-insult can be an appropriate time point to detect the signs of ALI without being too late to sample artery blood. In the current study, IP infusion of LPS (10 kg/mg) caused few deaths with the tendency of recovery occurring at the second day after treatment. So, 24 hours could be enough for them to develop ALI if they would.
Neutrophils are proposed to play an important role in mediating ALI; more neutrophil infiltration in the lung means more chances to develop ALI.28Hypoxemia is not always relevant to lung injury, but measurement of artery blood oxygenation is often very helpful for the assessment of respiratory function. The concurrent decreases in PaO2and PaCO2indicated that hypoxemia in the group 1 impossibly resulted from hypoventilation, but probably from impaired gas exchange in the lung. For the two cases in the group 1 exhibiting normal oxygenation, 10 hours might be too short to develop respiratory dysfunction. The histological evidence of lung injury has been widely accepted as the most relevant defining feature of ALI. To measure histological changes of lung injury, any scoring systems must be approached with caution because of their considerable inter- and intra-observer variations.15A binary approach is the simplest scoring system which is believed to mitigate the variations.
As a soluble pattern recognition receptor, PTX3 functions as the regulator of innate immunity and inflammatory response.29PTX3 deficiency did exacerbate LPS-induced lung injury in PTX3 knockout mice.12However, another study on intestinal ischemia-reperfusion injury reported PTX3 overexpression in the transgenic miceincreased the mortality and inflammatory response with much severe tissue injury in local (gut) and remote (lung) organs.30The overproduction of PTX3 in ‶two hit″ models of ALI was also accompanied by enhanced inflammatory response.10What is the real role of PTX3 in the pathogenesis of ALI remains to be elucidated. In the present study, PTX3 expression was up-regulated indiscriminately among the septic mice with and without ALI, we infer the inducible production of PTX3 in mice may only represent a component of systemic inflammation in response to LPS challenges instead of a direct link to lung injury.
The ideal biomarker should objectively reflect normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention,31and the amount of a biomarker protein in biofluid should reflect a graded, dose-dependent response to damage.32In this regard, the previous findings mentioned above had promised PTX3 the potential as a useful biomarker of ALI. However, one of the basic concerns for organ injury biomarkers is that their concentration in biofluid should keep proportional to their expression at the site of injury in vivo.32In this way, the uncoupled correlation between PTX3 concentration in BALF and that in serum argues against the pontential of PTX3 as a diagnoatic biomarker of lung injury. Indeed, we showed poor diagnostic abilities of PTX3 tests at identifying ALI versus non-ALI no better than flipping a coin.
Pentraxins are evolutionarily conserved from insects to mammals; human PTX3 is highly conserved and shares 82% of the identity and 92% of the similarity in primary sequence with murine PTX3.33,34Blood PTX3 concentration is very low in normal human subjects, which are rapidly and dramatically increased in patients with inflammatory conditions either infectious or non-infectious.13,35-39Perhaps, PTX3 acts as an indicator of inflammatory response just like its cousin C-reactive protein does, it may not reflect organ injury per se.
There are some limitations in this study. Due to difficulty in intermittent sampling in small animals and lack of reliable methodology to quantify experimental lung injury, we did not study whether the amount of PTX3 in biofluid reflected a graded, dose-dependent lung injury. Also, we did not know the time-course changes of PTX3 in the pathological progression of sepsis-induced ALI. Moreover, the fact that only one pro-inflammatory cytokines (IL-6) was examined in this study gave an incomplete picture of systemic inflammation and innate immunity in host when confronted with injurious stimulus.
In conclusion, LPS challenges induced PTX3 expression in mice regardless of the presence of ALI. PTX3 probably acts as an indicator of inflammatory response instead of organ injury per se.
ACKNOWLEDGEMENT
We thank Wen Lee, Rui-min Lee, and Jing-fang Sun for their technical assistance in the animal experimentation.
REFERENCES
1. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334-49.
2. Luh SP, Chiang CH. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): The mechanism, present strategies and future perspectives of therapies. J Zhejiang Univ Sci B 2007; 8:60-9.
3. Janz DR, Ware LB. Biomarkers of ALI/ARDS: Pathogenesis, discovery, and relevance to clinical trials. Semin Respir Crit Care Med 2013; 34:537-48.
4. Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res 2012; 159:205-17.
5. Agrawal A, Singh PP, Bottazzi B, et al. Pattern recognition by pentraxins. Adv Exp Med Biol 2009; 653:98-116.
6. He X, Han B, Liu M. Long pentraxin 3 in pulmonary infection and acute lung injury. Am J Physiol Lung Cell Mol Physiol 2007; 292:L1039-49.
7. Han B, Mura M, Andrade CF, et al. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol 2005; 175:8303-11.
8. dos Santos CC, Han B, Andrade CF, et al. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNFalpha, LPS, and cyclic stretch. Physiol Genomics 2004; 19:331-42.
9. He X, Han B, Bai X, et al. PTX3 as a potential biomarker of acute lung injury: Supporting evidence from animal experimentation. Intensive Care Med 2010; 36:356-64.
10. Okutani D, Han B, Mura M, et al. High-volume ventilation induces pentraxin 3 expression in multiple acute lung injury models in rats. Am J Physiol Lung Cell Mol Physiol 2007; 292:L144-53.
11. Dias AA, Goodman AR, Dos Santos JL, et al. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol 2001; 69:928-36.
12. Han B, Haitsma JJ, Zhang Y, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med 2011; 37:334-42.
13. Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med 2001; 29:1404-7.
14. Mauri T, Coppadoro A, Bellani G, et al. Pentraxin 3 inacute respiratory distress syndrome: An early marker of severity. Crit Care Med 2008; 36:2302-8.
15. Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011; 44:725-38.
16. Remick DG, Bolgos GR, Siddiqui J, et al. Six at six:Interleukin-6 measured 6 hours after the initiation of sepsis predicts mortality over 3 days. Shock 2002; 17:463-7.
17. Osuchowski MF, Connett J, Welch K, et al. Stratification is the key: Inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med 2009; 37:1567-73.
18. Sevransky JE, Levy MM, Marini JJ. Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: An evidence-based review. Crit Care Med 2004; 32:S548-53.
19. Chen H, Bai C, Wang X. The value of the lipopolysaccharideinduced acute lung injury model in respiratory medicine. Expert Rev Resp Med 2010; 4:773-83.
20. Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol 2012; 91:590-601.
21. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 295:L379-99.
22. Sadowitz B, Roy S, Gatto LA, et al. Lung injury induced by sepsis: Lessons learned from large animal models and future directions for treatment. Expert Rev Anti Infect Ther 2011; 9:1169-78.
23. Szarka RJ, Wang N, Gordon L, et al. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods 1997; 202:49-57.
24. van Helden HP, Kuijpers WC, Steenvoorden D, et al. Intratracheal aerosolization of endotoxin (LPS) in the rat:A comprehensive animal model to study adult (acute) respiratory distress syndrome. Exp Lung Res 1997; 23:297-316.
25. Li QF, Zhu YS, Jiang H, et al. Isoflurane preconditioning ameliorates endotoxin-induced acute lung injury and mortality in rats. Anesth Analg 2009; 109:1591-7.
26. Bashenko Y, Ilan N, Krausz MM, et al. Heparanase pretreatment attenuates endotoxin-induced acute lung injury in rats. Shock 2007; 28:207-12.
27. Nakajima T, Suarez CJ, Lin KW, et al. T cell pathways involving CTLA4 contribute to a model of acute lung injury. J Immunol 2010; 184:5835-41.
28. Perl M, Lomas-Neira J, Venet F, et al. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med 2011; 5:115-26.
29. Baruah P, Propato A, Dumitriu IE, et al. The pattern recognition receptor PTX3 is recruited at the synapse between dying and dendritic cells, and edits the cross-presentation of self, viral, and tumor antigens. Blood 2006; 107:151-8.
30. Souza DG, Soares AC, Pinho V, et al. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol 2002; 160:1755-65.
31. Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69:89-95.
32. Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 2011; 17:216-22.
33. Garlanda C, Bottazzi B, Bastone A, et al. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 2005; 23:337-66.
34. Breviario F, d′Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem 1992; 267:22190-7.
35. Azzurri A, Sow OY, Amedei A, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect 2005; 7:1-8.
36. Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004; 110:2349-54.
37. Mairuhu AT, Peri G, Setiati TE, et al. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol 2005; 76:547-52.
38. Bevelacqua V, Libra M, Mazzarino MC, et al. Long pentraxin 3: A marker of inflammation in untreated psoriatic patients. Int J Mol Med 2006; 18:415-23.
39. Inoue K, Sugiyama A, Reid PC, et al. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol 2007; 27:161-7.
for publication September 2, 2014.
△Partly supported by a grant from Jie-shou Li Academician Gut Barrier Research Fund.†These authors contributed equally to this work.
. E-mail: Guosb@pumch.cn
杂志排行
Chinese Medical Sciences Journal的其它文章
- Use of Cataract Surgery in Urban Beijing: a Post Screening Follow-up of the Elderly with Visual Impairment due to Age-related Cataract
- Reliability of a Novel Cobb Protractor for Measuring the Cobb Angle of Radiograph in Scoliosis
- Role of Removing Stasis and Reducing Heat Formula in Clearance of Proximal Ureteral Calculi after Ureteroscopic Ho:YAG Laser Lithotripsy: A Prospective Randomized Study
- Redo Coronary Artery Bypass Grafting: On-Pump and Off-Pump Coronary Artery Bypass Grafting Revascularization Techniques
- MRI Evaluation of Lateral Geniculate Body in Normal Aging Brain Using Quantitative Susceptibility Mapping.△
- Total Glycosides of Ranunculus Japonius Prevent Hypertrophy in Cardiomyocytes via Alleviating Chronic Ca2+Overload
