介孔Fe/SBA-15非均相芬顿氧化水中难降解染料罗丹明B
2015-01-04胡龙兴许丹丹邹联沛上海大学环境与化学工程学院上海200444
胡龙兴 许丹丹 邹联沛 袁 航 胡 星(上海大学环境与化学工程学院,上海200444)
介孔Fe/SBA-15非均相芬顿氧化水中难降解染料罗丹明B
胡龙兴*许丹丹 邹联沛 袁 航 胡 星
(上海大学环境与化学工程学院,上海200444)
以介孔二氧化硅SBA-15为载体,采用等体积浸渍法制备了Fe/SBA-15.通过X射线衍射(XRD)、N2吸附-脱附、扫描电镜(SEM)、透射电镜(TEM)和X射线光电子能谱(XPS)等技术对其进行了表征,并用于对水溶液中罗丹明B(RhB)的芬顿氧化.表征结果表明了Fe/SBA-15维持了长程有序的介孔结构,孔径和比表面积都有所下降,并呈现棒状体的聚集态,平均直径为0.6 μm.Fe以α-Fe2O3的形态同时存在于介孔孔道内外.在Fe/ SBA-15和H2O2同时存在条件下RhB的去除是吸附和催化氧化降解的协同作用所致,并且与Fe/SBA-15投加量密切相关,但与初始溶液pH几乎无关.当Fe/SBA-15投加量为0.15 g·L-1,RhB初始浓度为10.0 mg·L-1, H2O2/Fe3+摩尔比为2000:1,初始溶液pH为5.4和反应温度为21°C时,RhB去除率达到了93%.Fe/SBA-15的Langmiur单分子层饱和吸附量为99.11 mg·g-1.此外,采用H2O2浸泡方式对使用过的Fe/SBA-15可进行再生,连续6次循环使用后仍可维持80%的RhB去除率,且每次使用后Fe浸出浓度都在0.1 mg·L-1(或者0.6%(质量分数))以下.基于淬灭实验、UV-Vis光谱和气相色谱-质谱(GC-MS)联用仪分析的结果,提出了RhB的去除机理.非均相芬顿催化剂Fe/SBA-15可用于去除像RhB这样的生物难降解有机物.
非均相芬顿氧化;吸附;Fe/SBA-15;罗丹明B;羟基自由基
1 Introduction
Textile dyes and other industrial dyestuffs make up one of the largest groups of non-biodegradable materials that impose serious environmental problems because of their existence in the effluents.A number of techniques have been applied to remove refractory organic pollutants from water,such as adsorption,1,2electrochemical oxidation,3,4ultrasonic degradation,5,6photocatalytic degradation,7,8ozonation,9,10etc.Adsorption is easy to operate with high removal efficiency,but it only transfers the pollutants from liquid phase to solid phase,leading to the secondary pollution.The other techniques require special devices and equipment,resulting in the high capital and operational costs. Fenton reaction(Fe2+/H2O2)has been widely applied in treating organic pollutants in water because it is rapid and inexpensive. However,it has several significant drawbacks such as the acidic pH requirement(pH 2-4),a high Fe dosage generating a large amount of iron sludge,and the limited TOC(total organic carbon) removal.11-13Heterogeneous Fenton oxidation using Fe-based solid materials as catalysts has emerged as a promising alternative to the conventional Fenton process due to the absence of the usual disadvantages and feasibility of the catalyst recycle.
To date,iron-based solid catalysts as promising heterogeneous Fenton catalysts for degradation of organic pollutants have been reported,and can be classified as unsupported iron oxides including Fe2O3,Fe3O4,and zero-valence iron,14-16unsupported iron composites including some non-ferrous metals with variable valence,17-19and supported iron catalysts including iron-immobilized resin,20membrane,21clay,22,23carbon,24-26zeolite,27-29mesostructured silica materials,13,30-45and alumina.46,47
Mesoporous silica SBA-15,one of the mesoporous molecular sieves,has highly ordered structure,large pore size,and high surface area,48,49which favors the loading and dispersion of the catalytic active components which possess the nano effect due to the nano-size domain.Accordingly,it is possible for Fe loaded mesoporous molecular sieves to exhibit high activity and stability in heterogeneous Fenton oxidation of organic pollutants.Melero et al.31investigated a nanocomposite of Fe2O3loaded SBA-15 which acted as an efficient and stable catalyst for wet peroxidation of phenolic aqueous solutions.They also used the SBA-15 loaded Fe2O3and CuO crystallites as the heterogeneous Fenton catalyst, preventing the leaching of Fe species and enhancing the TOC degradation.32Xiang et al.36synthesized a Fe/SBA-15 by different impregnation and co-condensation approaches,and ascertained the most active catalysts in the total phenol oxidation by H2O2in aqueous solution.Shukla et al.37prepared Fe/SBA-15 by impregnation and tested for adsorption and heterogeneous advanced oxidation of 2,4-dichlorophenol(DCP)in aqueous solution in the presence of H2O2.Botas et al.13synthesized various Fe/SBA-15 catalysts for the oxidation of phenol aqueous solution with H2O2. Mayani et al.38evaluated the Fe/SBA-15 for the oxidation of phenols in the presence and absence of H2O2with satisfactory results.
In the previous investigations,phenolic contaminants were usually used as the target substrates,and Fe loaded mesoporous materials were usually regarded as catalysts.Few investigations were concerned with other refractory organic pollutants and the adsorption performance of the catalysts in heterogeneous Fenton oxidation.23,27,29,34,37,43The dye Rhodamine B(RhB)with four N-ethyl groups at either side of the xanthene ring and chromophore has the complex molecular structure and stable property,which make it resistant to biological degradation or photodegradation.This paper deals with the dual functions of the prepared Fe/ SBA-15 as both an adsorbent and a catalyst in the heterogeneous Fenton oxidation of RhB in aqueous solution.The effects of reaction parameters such as Fe/SBA-15 dosage,initial RhB concentration,molar ratio of H2O2/Fe3+,and initial solution pH on RhB removal,and the regeneration and recycling performance of the Fe/SBA-15 are examined,and the removal mechanism of RhB from aqueous solution is proposed on the basis of experimental results.
2 Experimental
2.1 Preparation of SBA-15 and Fe/SBA-15
SBA-15 as a support was prepared according to the procedure described by Zhao et al.48Pluronic P123 triblock copolymer(EO20-PO70-EO20(EO:ethoxy;PO:propoxy),Aldrich)and tetra ethlyl orthosilicate(TEOS,Aldrich)acted as a template agent and a silica source,respectively.The Fe/SBA-15 with the Fe loading of 10%(mass ratio of Fe to SBA-15,simplified as Fe/SBA-15 in this investigation)was prepared by incipient wetness impregnation.A typical synthesis could be described as follows:the precursor Fe(NO3)3·9H2O(AR,SCRC)was dissolved in deionized water at a concentration dependent on the desired Fe loading,then a certain amount of the prepared SBA-15 was dispersed into the above solution forming a suspension,and the suspension was stirred to make Fe easy to impregnate,and the yielded mixture was dried in oven at 60°C overnight to evaporate the solvent,finally followed by the calcination at 750°C for 5 h in air with a heating rate of 10°C·min-1.
2.2 Characterization of prepared SBA-15 and Fe/ SBA-15
The crystalline form of the prepared samples were identified by X-ray diffraction(XRD,Model,Rigaku D/Max-2200X)using a diffraction meter with Cu Kαradiation source of wavelength 0.154056 nm at 40 kV and 40 mA.The small-angle data were collected from 0.5°to 5.0°(2θ)with a scan speed of 0.5(°)·min-1; the wide-angle data were collected from 10°to 80°(2θ)with a scan speed of 5(°)·min-1.N2adsorption-desorption isotherms of the samples were acquired using Micromeritics Tristar 3000 apparatus at 77 K.Pore volumes were determined from the data at a relative pressure(p/p0)of 0.99,the specific surface areas were calculated with BET(Brunner-Emmet-Teller)equation,and pore size distributions were determined by BJH(Barrett-Joyner-Halenda)method according to the adsorption branch of the isotherms.The external morphology of samples was collected on a JSM-6700F scanning electron microscope(SEM).The internal structure of samples was characterized by a JEM-2010F(JEOL) high resolution-transmission electron microscope(HRTEM) with field emission gun at 200 kV.X-ray photoelectron spectroscopy(XPS)measurements were recorded on a ESCALAB 250Xi device.
2.3 Adsorption(adsorption mode)
The adsorption tests were carried out respectively at~21°C in 100 and 1000 mLglass vessels with 50 and 500 mLof RhB(AR,>99%,SCRC)solutions which were magnetically stirred,respectively.The initial solution pH was not adjusted.Only a known amount of Fe/SBA-15 was added into the aqueous solution to start the contact.The vessel was covered to avoid volatilization.At the end of the contact or at fixed intervals,the suspension samples of 7 mL for each were taken with a syringe.The suspensions were separated by centrifugation at 3500 r·min-1for 10 min to obtain the supernatant for analysis.
2.4 Catalytic oxidative degradation(catalytic degradation mode)
The catalytic activity of Fe/SBA-15 was evaluated by the degradation of RhB instead of the adsorption.The catalytic oxidative degradation tests of RhB were carried out respectively at a given temperature in 1000 mL glass vessels with 500 mL of RhB solution for each which was magnetically stirred.The Fe/SBA-15 was first added into the RhB solutions and the suspensions were stirred overnight to achieve the adsorption-desorption equilibrium of RhB.The RhB concentrations at the equilibrium were taken as the initial concentrations(c0)for the RhB catalytic degradation. Then a given amount of H2O2solution(AR,30%(w,mass fraction),SCRC)was added into the vessel to initiate the reaction. The vessel was also covered to avoid volatilization.At fixed intervals,the suspension samples of 7 mL for each were taken with a syringe and quenched with excess methanol to stop the reaction. The following procedures were the same as above.
2.5 Combined adsorption and catalytic oxidative degradation(combined mode)
The tests with the combined mode were carried out respectively at~21°C in 100 and 1000 mL glass vessels with 50 and 500 mL of RhB solutions which were magnetically stirred for 14 h,respectively.The initial solution pH was adjusted by 1.0 mol·L-1NaOH and 1.0 mol·L-1H2SO4solutions using a pH meter(pHS-3C,Shanghai,China).Aknown amount of Fe/SBA-15 was added into the aqueous solution,immediately followed by the addition of a given amount of H2O2solution to start the reaction.The subsequent procedures were the same as above.
2.6 Analytical methods
The RhB concentrations were quantified with a UV-Vis spectrophotometer(UV-5300PC,Shanghai)at 552 nm.The Fe concentration in solution was measured by an inductively coupled plasma(ICP)emission spectrometer(Prodigy).The concentration of H2O2in aqueous solution was determined by the titanium complexing method described by Vassilakis et al.50The UV-Vis spectra were obtained using a UV-Vis spectrophotometer(UV-5300PC,Shanghai)at different reaction time,with the spectrum scanned from 350 to 650 nm.The principal degradation products of RhB were detected by GC-MS,with an Agilent 7890A gas chromatograph equipped with a DB-5 capillary column(30 mm× 0.25 mm×0.18 mm)combined with an Agilent 5975C mass spectroscopy equipment employed.
Generally,the tests were conducted in duplicate and the errors of the experimental results were below 3%.
3 Results and discussion
3.1 Characterization of SBA-15 and Fe/SBA-15
3.1.1 XRD

Fig.1 Small(a)and wide(b)angle XRD patterns of SBA-15 and Fe/SBA-15
The small and wide angle XRD patterns of different samples are shown in Fig.1(a,b),respectively.As seen from the smallangle XRD patterns(Fig.1(a)),SBA-15 and Fe/SBA-15 exhibit a strong diffraction peak and two obvious sub-peaks,corresponding to the diffraction planes(100),(110),and(200),respectively, indicating that the SBA-15 possesses the typical highly ordered hexagonally mesoporous structure and the Fe/SBA-15 maintains the structure of SBA-15.However,the obvious decrease in intensity of the diffraction peaks in Fe/SBA-15 compared with SBA-15 could be observed,which was attributed to the decrease in average electron density contrast and the formation of iron oxides inside the channels of SBA-15.51,52As seen from Fig.1(b),besides one major peak at 24°for SBA-15 and Fe/SBA-15,there also exist other peaks for Fe/SBA-15 which can be ascribed to the existence of α-Fe2O3.Fe2O3is very stable under ambient conditions and is usually the end product of the transformation of other iron oxides.53Fe2O3,Fe3O4,and FeO are the principal Fe species available, and the specific species loaded into the supports tend to be dependent on the preparation methods and conditions.54-56
3.1.2 N2adsorption-desorption
N2adsorption-desorption isotherms and pore size distribution at 77 K of SBA-15 and Fe/SBA-15 are shown in Fig.2.It is evident in Fig.2(a)that Fe/SBA-15 has similar isotherm to SBA-15, exhibiting type IV isotherms with a H1-type hysteresis loop, which was the characteristic of mesoporous materials having cylindrical type mesostructures.For the isotherm of the pure SBA-15,with increased relative pressure,a sharp jump resulting from capillary condensation of N2was observed at p/p0of 0.6-0.8, while the relative pressure with the emergence of the sharp step for Fe/SBA-15 decreased to 0.45-0.75,indicating the reduced pore size due to Fe species introduction into the channel of SBA-15.The lower sharp jump of Fe/SBA-15 than that of SBA-15 could also be explained by the reduced order degree of mesoporous structure due to Fe loading.The profiles of pore size distribution(Fig.2(b))show that the pore sizes of SBA-15 and Fe/SBA-15 are relatively uniform and center at 6-10 nm.The BET specific surface areas and pore parameters of prepared SBA-15 and Fe/SBA-15 are listed in Table 1.The results indicated that loading Fe into SBA-15 reduced the specific surface area,total pore volume,and pore diameter of the support,implying that a fraction of Fe loaded after calcination occupied part of the pore channel of SBA-15.
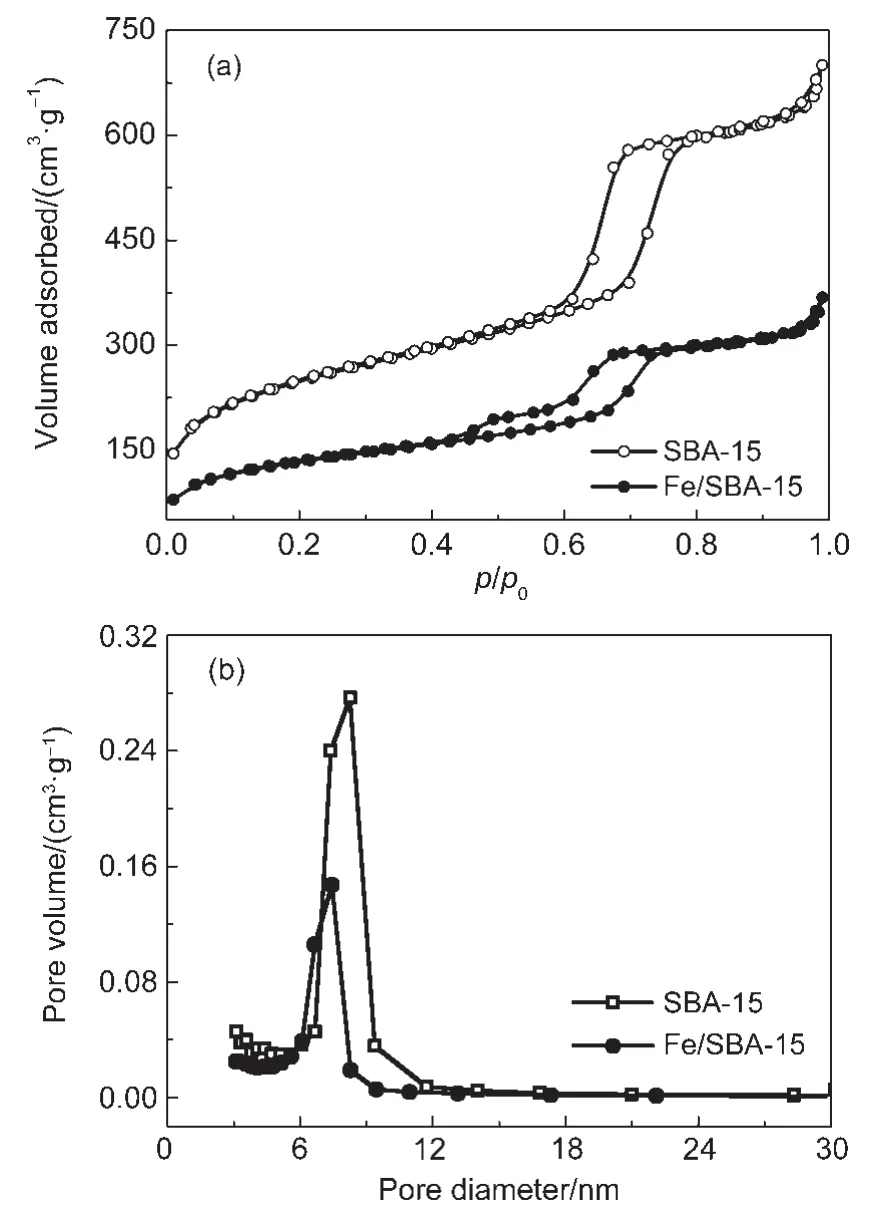
Fig.2 N2adsorption-desorption isotherms(a)and pore size distributions(b)for SBA-15 and Fe/SBA-15
3.1.3 SEM
The external morphologies of the SBA-15 and Fe/SBA-15 characterized by SEM can be observed from Fig.3.It can be found from Fig.3(a)that the SBA-15 presents the aggregates with the diameter greater than 0.7 μm,consisting of rod-like crystallites with the diameter greater than 0.2 μm and axial length greater than 1.3 μm.It can also be found from Fig.3(b)that the Fe/SBA-15 also displays the aggregates with the diameter more than 0.6 μm, consisting of rod-like crystallites,demonstrating that the Fe loading on SBA-15 does not change the original morphology.There are many small particles attached to the surface of the Fe/SBA-15 (Fig.3(b)),which can be ascribed to the sintering of iron oxidesduring high-temperature calcination.56The sintering makes a fraction of Fe2O3supported on the surface forming Fe2O3aggregates with the diameter more than 0.4 μm.

Table 1 Pore parameters of SBA-15 and Fe/SBA-15
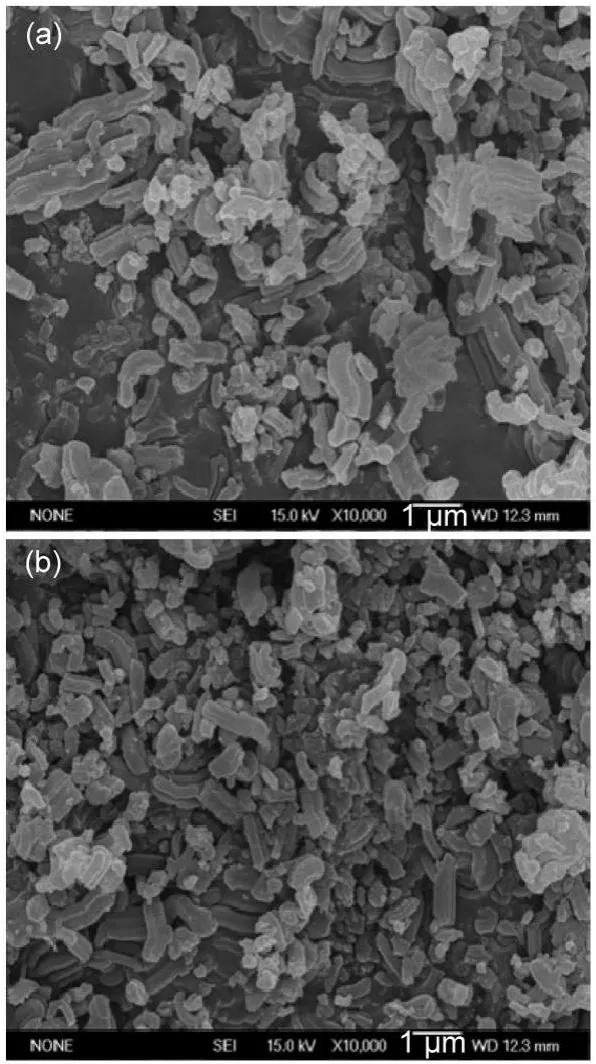
Fig.3 SEM images of SBA-15(a)and Fe/SBA-15(b)
3.1.4 TEM
The TEM images of the prepared SBA-15 and Fe/SBA-15 are presented in Fig.4.Fig.4(a,b)shows the bright-field images of SBA-15 and Fe/SBA-15,respectively.Fig.(c,d)shows the brightand dark-field images of Fe/SBA-15,respectively.The highly ordered hexagonal array of SBA-15 with the pore diameter of approximately 7 nm and uniform channel wall thickness of approximately 4 nm is observed from Fig.4(a).Fig.4(b,c,d)displays the representative internal morphology of Fe/SBA-15,the distribution of the supported iron oxides,and a better dispersion of Fe2O3crystallites over the SBA-15 framework.There exist many small black dots(Fig.4(b))and bright white dots(Fig.4(c, d))in the images,both dots representing the Fe2O3crystallites located in the SBA-15 channels.From TEM image inserted in Fig.4(b),the measured lattice spacings of 0.25 nm correspond to (110)interplanar distance of α-Fe2O3.The growth of the crystallites embedded into the SBA-15 channels was limited by the channel walls,thus leading to the rod-like shape of the crystallites. From Fig.4(c),it can be seen that the crystallites have the diameter of approximately 6 nm,consistent with the pore diameter of SBA-15,and the average length more than 25 nm,attributable to the uncontrolled growth of Fe2O3crystallites along the channels.The shaded parts circled in Fig.4(b,c,d)represent a fraction of the aggregates of Fe2O3crystallites loaded outside the channels of SBA-15.

Fig.4 TEM images of SBA-15(a)and Fe/SBA-15(b,c,d)

Fig.5 XPS analyses of Fe/SBA-15
3.1.5 XPS
In the XPS spectra of Fe/SBA-15 composites shown in Fig.5 (a),strong peaks of Fe 2p,Si 2p,and O 1s are observed.In Fig.5 (b),peaks at 711.58 and 724.58 eV are assigned to Fe 2p3/2and Fe2p1/2binding energies,which is the main characteristic of Fe3+state.57Additionally,O 1s spectrum of Fe/SBA-15 is shown in Fig.5(c).The main binding energy peak is observed at 532.38 eV, which corresponds to O2-in the iron oxide lattice57and Si 2p binding energy was estimated from Fig.5(d)to be about 103.68 eV.
3.2 Adsorption of RhB on Fe/SBA-15
To understand the adsorption characteristics of mesoporous material Fe/SBA-15,the adsorption tests were carried out in 100 mL glass vessels with 50 mL of RhB solution with an initial RhB concentration from 5.0 to 40.0 mg·L-1,Fe/SBA-15 dosage of 0.15 g·L-1,and unadjusted initial pH of 5.4.The contact time and temperature were 14 h and 21°C,respectively.The adsorption isotherm of RhB on Fe/SBA-15 is presented in Fig.6.As observed,RhB adsorption increases with increasing RhB equilibrium concentration.At the equilibrium concentration of 29.47 mg·L-1, the RhB adsorption on Fe/SBA-15 is 86.55 mg·g-1.Two isotherm models,Langmuir(Eq.(1))and Freundlich(Eq.(2))isotherms, were applied to fit the experimental data:

where qeand Cerepresent the equilibrium adsorption capacity of the adsorbent(mg·g-1)and equilibrium concentration of RhB(mg· L-1),respectively,qmaxis monolayer adsorption capacity of the adsorbent(mg·g-1),KLis the Langmuir adsorption constant(L· mg-1),KF(L·mg-1)and n(dimensionless)are the Freundlich constants,related to the adsorption capacity and adsorption intensity,respectively.The parameters of the fitted curves of two isotherms are listed in Table 2.As seen,the fitted curve of Langmuir isotherm(R2=0.9976)is more reasonable than that of Freundlich one(R2=0.9217).qmaxof 99.11 mg·g-1from Langmuir isotherm represents a complete monolayer adsorption capacity of RhB on the Fe/SBA-15 surface.

Fig.6 Adsorption curve of RhB on Fe/SBA-15

Table 2 Parameters of Langmuir and Freundlich isotherms
The substantial characteristic of Langmuir isotherm can be shown as the equilibrium parameter RL(dimensionless),which is defined as follows:1

where C0is the initial adsorbate concentration(mg·L-1),RLis a characteristic value for the adsorption process.It is generally accepted that RL>1 represents an unfavorable adsorption process, with 0<RL<1 favorable,RL=1 linear,and RL=0 irreversible adsorption process.Given KL=0.21 L·mg-1,and initial RhB concentrations at 5.0,10.0,15.0,20.0,25.0,30.0,and 40.0 mg·L-1respectively,all the RLvalues calculated in this investigation ranged between 0 and 1,indicating that the RhB adsorption on SBA-15 is a favorable process and the Fe/SBA-15 may be used as an efficient adsorbent.
As shown in Table 3,58-62the Fe/SBA-15 possesses higher adsorption capacity than fly ash,MIF-B,Fe-Ben,and BiFeO3,etc, but lower capacity than SBA-15.The maximum adsorption capacity of pure SBA-15 for Rhodamine B was determined to be 497.9 mg·g-1.62When Fe was supported on SBA-15,the adsorption capacity of Fe/SBA-15 was reduced since α-Fe2O3was introduced into the SBA-15 channel.The high adsorption capacity provides adequate adsorption sites,and also benefits the subsequent heterogeneous catalytic reaction.
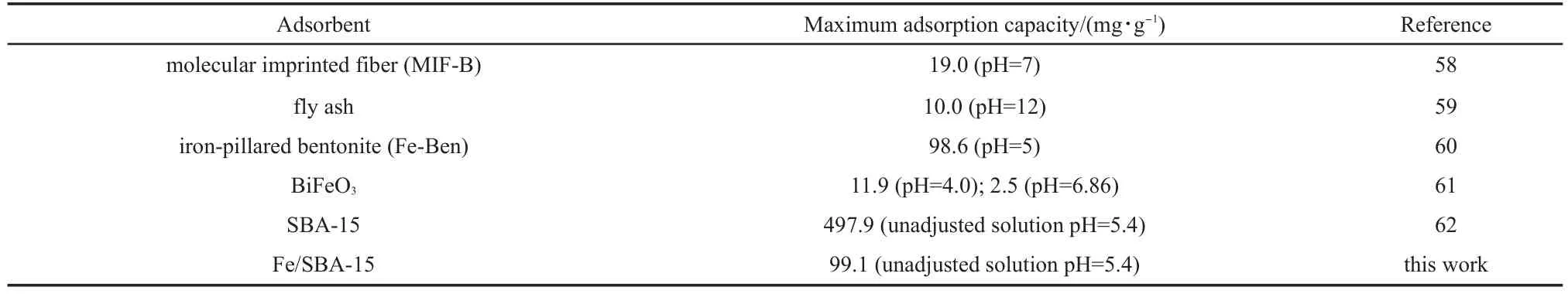
Table 3 Comparison of RhB adsorption on different adsorbents(Langmuir isotherms)
3.3 Effect of various reaction parameters on RhB removal with combined mode
3.3.1 Preliminary test
As a preliminary test,the variation of RhB concentrations in aqueous solution with time under three different operation modes was investigated:adsorption,catalytic degradation,combined adsorption and catalytic degradation,and the results are shown in Fig.7.The corresponding operation conditions are Fe/SBA-15 dosage at 0.15 g·L-1,molar ratio of H2O2/Fe3+at 2000:1,initial RhB concentration at 10.0 mg·L-1,initial solution pH at 5.4 (unadjusted),and reaction temperature at 21°C.
As seen from Fig.7,in the absence of H2O2,the Fe/SBA-15 exhibits the RhB adsorption removal up to 55%,and the ad-sorption is very fast within the initial 60 min resulting in approximately 50%RhB removal and attains a plateau in 120 min (curve a).The Fe/SBA-15 is an efficient catalyst for Fenton oxidation,and the catalytic oxidative degradation of RhB is able to take place at a significant but quite uniform rate,with 84%RhB removal achieved(curve c).In the presence of both Fe/SBA-15 and H2O2in the RhB solution,the change of RhB concentrations with time is different from the above-mentioned two variations, attaining the RhB removal of 93%,and is also not equal to the simple sum of the adsorption and catalytic degradation contributions(curve b),which demonstrates the existence of the synergic effect of adsorption and catalytic degradation for RhB removal.
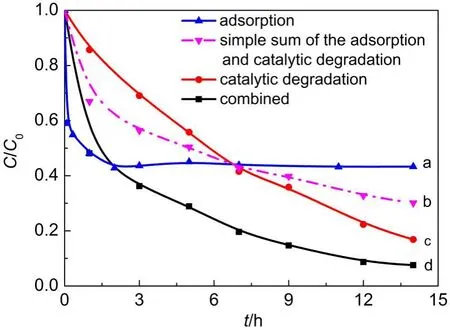
Fig.7 Removal of RhB in aqueous solution under three operational modes
3.3.2 Effect of Fe/SBA-15 dosage
The effect of Fe/SBA-15 dosage on RhB removal in the presence of H2O2is presented in Fig.8.As observed,in the presence of H2O2without Fe/SBA-15,the RhB removal is only 17%,illustrating that H2O2alone fails to oxidize RhB effectively.The increase in Fe/SBA-15 dosage from 0 to 0.15 g·L-1leads to a significant increase of RhB removal to 94%,but the further increase in Fe/SBA-15 dosage from 0.15 to 0.30 g·L-1results in only a slight increase of RhB removal,approaching the upper removal limit.The increased Fe/SBA-15 dosage would increase adsorption sites for reactants and also provide more catalytic active sites for activation of H2O2to generate more hydroxyl radicals,thus leading to a remarkable enhancement of the RhB removal efficiency and removal rate.
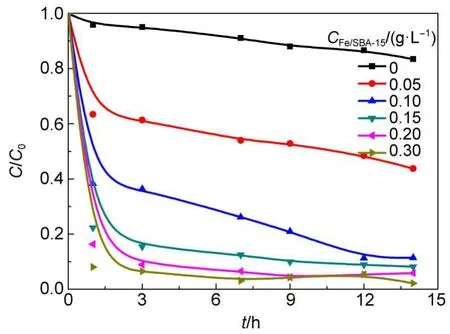
Fig.8 Effect of Fe/SBA-15 dosage on RhB removal
3.3.3 Effect of initial RhB concentration
Fig.9 displays the RhB removal at various initial RhB concentrations.As shown,the lower initial RhB concentrations in aqueous solution with the fixed dosages of Fe/SBA-15 and H2O2favor the RhB removal.The RhB removal in 14 h declines from 92%to 37%as the initial RhB concentration increases from 5.0 to 50.0 mg·L-1.This can be ascribed to the following reasons:(1) The fixed dosage of Fe/SBA-15 and H2O2generates the fixed HO· amount,and with higher RhB initial concentration,the amount of RhB molecules per unit volume is larger,causing the decrease in the percentage of RhB molecule involved into the reaction and the decrease of the removal.(2)The active sites blockage arising from more carbonaceous deposit on the Fe/SBA-15 for higher RhB concentration may lead to the decline in catalytic activity of Fe/ SBA-15.A few investigations have presented the similar conclusion that the higher the dye concentration,the more the removal of the dye is suppressed.15,22
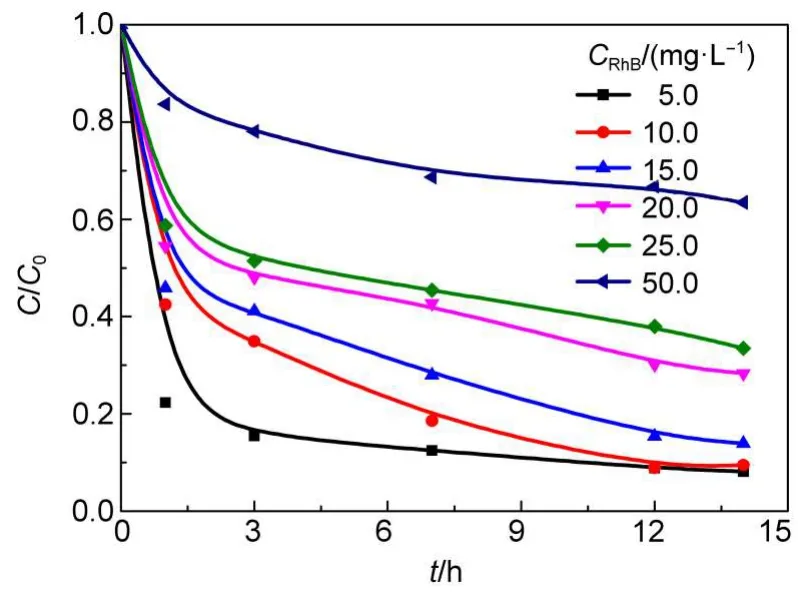
Fig.9 Effect of initial RhB concentration in aqueous solution on RhB removal
3.3.4 Effect of initial solution pH
The effect of initial solution pH on RhB removal in the presence of Fe/SBA-15 and H2O2was investigated to demonstrate the different pH effect between the present heterogeneous Fenton oxidation and conventional homogeneous Fenton reaction.Fig.10 displays the RhB removal at various initial pH ranging from 3 to 9 including the unadjusted initial solution pH of 5.4.As observed, the RhB removal efficiency and rate changes little with initial solution pH.The RhB removal ranged approximately from 82% to 93%at the pH from 3.0 to 9.0,with the maximum removal of 93%at pH 5.4.Accordingly,this heterogeneous Fenton oxidation has the significant advantages over conventional homogeneous Fenton oxidation processes,removing the pH adjustment requirement and promoting the practical applications.The removal of RhB under the combined adsorption and catalytic degradation mode is closely related to the surface properties of Fe/SBA-15 and the distribution of the RhB species,etc.in aqueous solution,all ofwhich are dependent on the solution pH.The combined dependence of the several factors on the pH results in the above effect of initial solution pH.
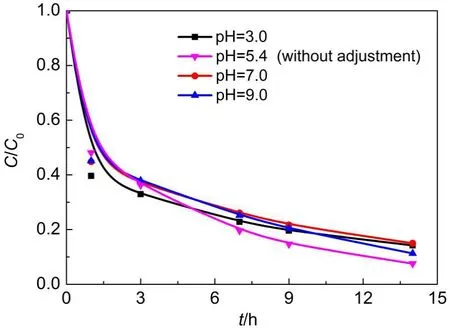
Fig.10 Effect of initial solution pH on RhB removal
3.3.5 Effect of molar ratio of H2O2/Fe3+
Fig.11 presents the effect of initial H2O2concentrations(in terms of molar ratio of H2O2/Fe3+)on RhB removal in the presence of Fe/SBA-15.As observed,RhB removal reaches 55%in the presence of Fe/SBA-15 without any oxidant H2O2,which can be attributed to the RhB adsorption on Fe/SBA-15.The higher the molar ratio of H2O2/Fe3+in the range of 0:1 to 2000:1 at the Fe/ SBA-15 dosage of 0.15 g·L-1,initial RhB concentration of 10.0 mg·L-1,initial solution pH of 3.0,and 21°C,the higher the removal efficiency and rate of the RhB.However,the further improvement of RhB removal was not significant at the H2O2/Fe3+molar ratio ranging from 2000:1 to 2500:1.It is known that for the heterogeneous Fenton reaction more hydroxyl radicals,hence higher RhB removal,are available at higher initial H2O2concentrations.Nevertheless,H2O2can also act as a hydroxyl radical scavenger as described in Eqs.(4)and(5),and can also take place the self-decomposition as described in Eq.(6),so the further increase of the H2O2concentration cannot result in the significant increase of hydroxyl radicals and the enhancement of the RhB removal.


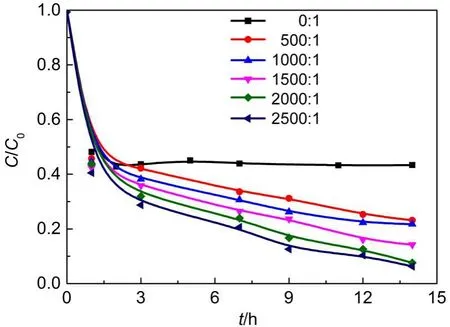
Fig.11 Effect of molar ratio of H2O2/Fe3+on RhB removal
3.4 Reusability of the Fe/SBA-15
In the case of removing organic pollutants in heterogeneous Fenton oxidation system,the reusability of Fenton catalyst tends to be one of the technical advantages.The reusability of catalyst is reflected by the catalytic activity and stability of the catalyst. The catalytic activity of the Fe/SBA-15 can be evaluated by the removal of RhB in aqueous solution in the presence of H2O2and used Fe/SBA-15,while the stability of the Fe/SBA-15 can be estimated by the leaching of Fe.The results are presented in Figs.12 and 13.The H2O2soaking technique was employed to treat the used Fe/SBA-15 for the next run as follows:the used solid Fe/ SBA-15 was collected by suction filtration with 0.22 μm membrane,then soaked in 5 mol·L-1H2O2solution for one night,and finally dried in oven at 80°C for 5 h.The H2O2soaking technique is not only simple in operation,but also inexpensive,and is better than the calcination technique employed by other researchers.32,33The performance difference between the untreated Fe/SBA-15 and the treated one is displayed in the inset of Fig.12,showing asignificant drop in the performance,adsorptive and catalytic,for the used Fe/SBA-15 without any treatment.In the reuse tests of the Fe/SBA-15,several parallel tests were carried out in every run until the last one to ensure that the Fe/SBA-15 amount was sufficient for the next run.As shown in Fig.12,the performance of the used Fe/SBA-15 suffers slight decline in RhB removal during 6 recycled runs,with RhB removal of approximately 90%for the 1st run and approximately 80%for the 6th run.
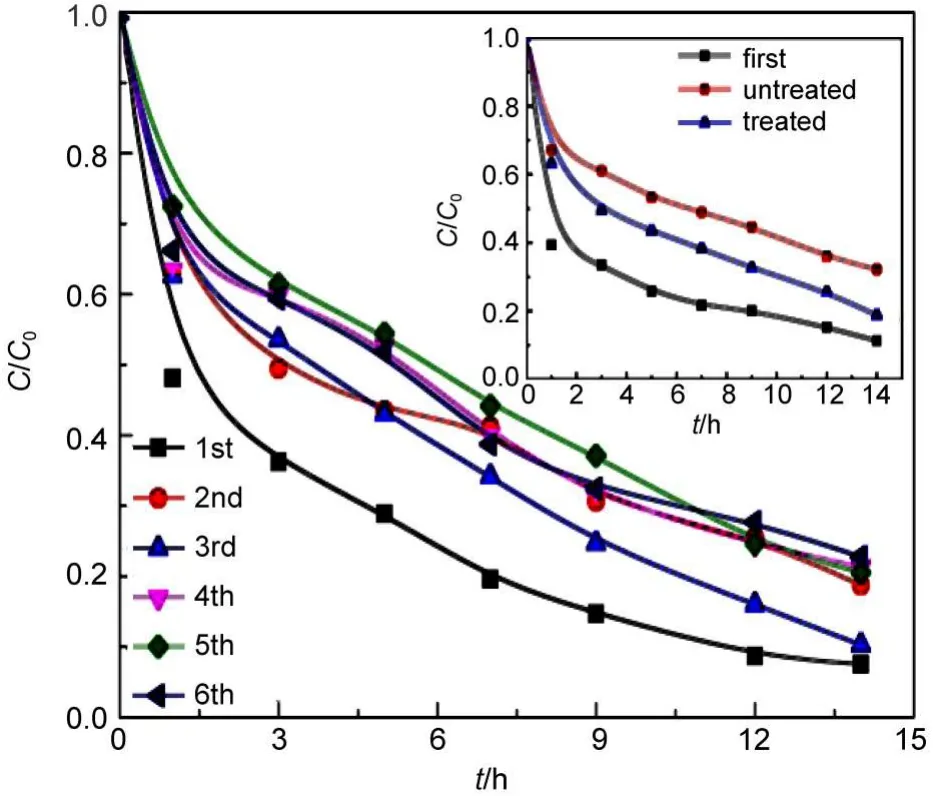
Fig.12 Variation of removal of RhB with time during six recycled runs of Fe/SBA-15

Fig.13 Fe leaching during six recycled runs of Fe/SBA-15
Additionally,the Fe leaching from the heterogeneous catalyst may be also responsible for the decrease in the number of active sites,hence the catalytic activity22.The Fe leaching was investigated and the results are presented in Fig.13.For any one of the six runs,the Fe leaching concentration and leaching percent are less than 0.1 mg·L-1and 0.6%,respectively(Fig.13).Such a low Fe leaching does not pose the contaminated water.The total Fe leaching percent after the six runs was calculated to be approximately 3.2%,which is a lower Fe leaching and sustains the stable catalytic activity of Fe/SBA-15.It was reported that some heterogeneous Fe catalysts were reused in the successive Fenton-like oxidation runs with significant Fe lost and most poorly-bounded iron species lost during the first run32.
3.5 RhB removal mechanism in the presence of Fe/SBA-15 and H2O2
3.5.1 Comparison of homogeneous and heterogeneous Fenton reaction for RhB removal
Fig.14 presents the comparison of RhB removal in the presence of H2O2and heterogeneous Fe/SBA-15 or homogeneous Fe(III). As observed,RhB removal(degradation)in the homogeneous Fe (III)-H2O2system shows similar behavior,with the degradation efficiency increasing with increasing Fe3+concentration and degradation rate keeping relatively slow in the process.The degradation of RhB in 14 h enhanced from 18%to 28%as Fe3+concentration increased from 0.1 to 1.0 mg·L-1,indicating that 1.0 mg·L-1of Fe3+concentration was not high enough to attain the significant decomposition of RhB.RhB removal in the heterogeneous Fe/SBA-15-H2O2-RhB system was much faster than that in the homogeneous Fe(III)-H2O2system with up to 1.0 mg·L-1of Fe3+concentration.For the heterogeneous system,almost complete RhB removal could be achieved in 14 h with Fe leaching less than 0.1 mg·L-1.Based on the comparison,it is believed that the heterogeneous Fe/SBA-15 instead of the Fe3+leached in the solution is responsible for the RhB removal,i.e.,the homogeneous reaction initiated by the leached Fe3+has little effect on the RhB removal.

Fig.14 Comparison of homogeneous and heterogeneous Fenton reactions for RhB removal
3.5.2 Quenching tests
In order to confirm whether the RhB degradation in the presence of Fe/SBA-15 and H2O2involves the hydroxyl radicals(HO·), free radicals quenching tests were conducted with tert-butanol,an efficient hydroxyl radical scavenger with the reaction rate of 3.8× 108-7.6×108mol-1·L·s-1,61and the result is shown in Fig.15.As observed,the removal efficiency of RhB in the absence of tertbutanol in the Fe/SBA-15-H2O2-RhB system is 93%,but the presence of tert-butanol in the system remarkably suppresses the RhB removal efficiency and rate,indicating that a large number of hydroxyl radicals(HO·)are scavenged by tert-butanol.Therefore,the primary approach of the RhB degradation with the couple of Fe/SBA-15 and H2O2is for hydroxyl radicals from the H2O2to attack the targeted RhB.In conclusion,the primary identified reactive radical in the heterogeneous Fenton system is hydroxyl radical,HO·.

Fig.15 Effect of hydroxyl radical scavenger on removal of RhB
3.5.3 UV-Vis spectra of RhB degradation
Fig.16 shows the temporal absorption spectrum changes of RhB degradation in the presence of Fe/SBA-15 and H2O2.It is generally accepted that there exist two competitive processes for RhB degradation:one is N-de-ethylation,and the other is the destruction of the conjugated structure.63,64Based on Fig.15,the following explanations can be made.First,the phenomenon that characteristic absorption peak at 552 nm rapidly decreased and almost disappeared over time manifested the destruction of conjugated xanthene structure,and meanwhile no new band occurrence obviously eliminated the possibility of any complex formation originated from Fe/SBA-15,H2O2,and RhB.This is coincident with the results reported in other literature.4,15,65Second,the maximum absorption band of the solution appeared blue shift resulted from the formation of series of N-de-ethylated interme-diates,indicating that the energy required for electron transition may be elevated and RhB molecular structure has changed in degradation process.Nevertheless,the blue shift of the absorption band observed was insignificant,with its shift numbers asΔλ=13 nm(553-540 nm).Consequently,it can be deduced from the spectral changes that the ring-opening happened simultaneously with N-de-ethylation during the degradation process,and compared with N-de-ethylation process,the destruction of conjugated xanthene structure played a dominant role in our system.
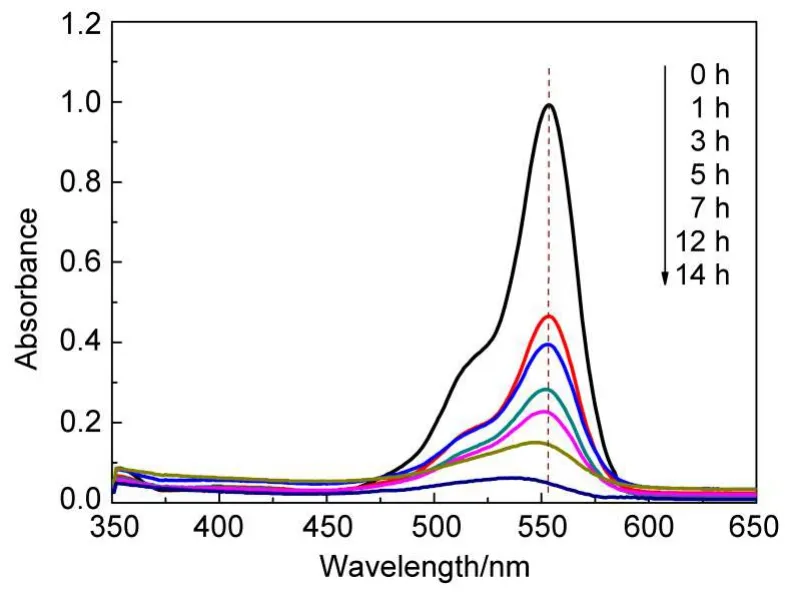
Fig.16 UV-Vis absorption spectra of RhB removal in the presence of Fe/SBA-15 and H2O2
3.5.4 GC-MS analysis of RhB degradation products
The GC-MS analysis of the supernatant phase is very useful for acquiring the information about the degradation products.Before GC-MS analysis,sample derivatization of the resultant solution coupled with the extraction with organic solvent methylene chloride was carried out.The GC-MS analysis results for the samples generated from the Fe/SBA-15-H2O2-RhB system are presented in Table 4.The degradation products can be divided into two groups:the aromatic compounds with different substituent groups,and the organics with relatively lower molecular weights, most of which are organic acid and alcohol.Among the degradation products,the content of glycerol is the highest,which is evidence that RhB suffered the destruction of the conjugated structure and decomposed into simple organics which can be easily converted to CO2and H2O.
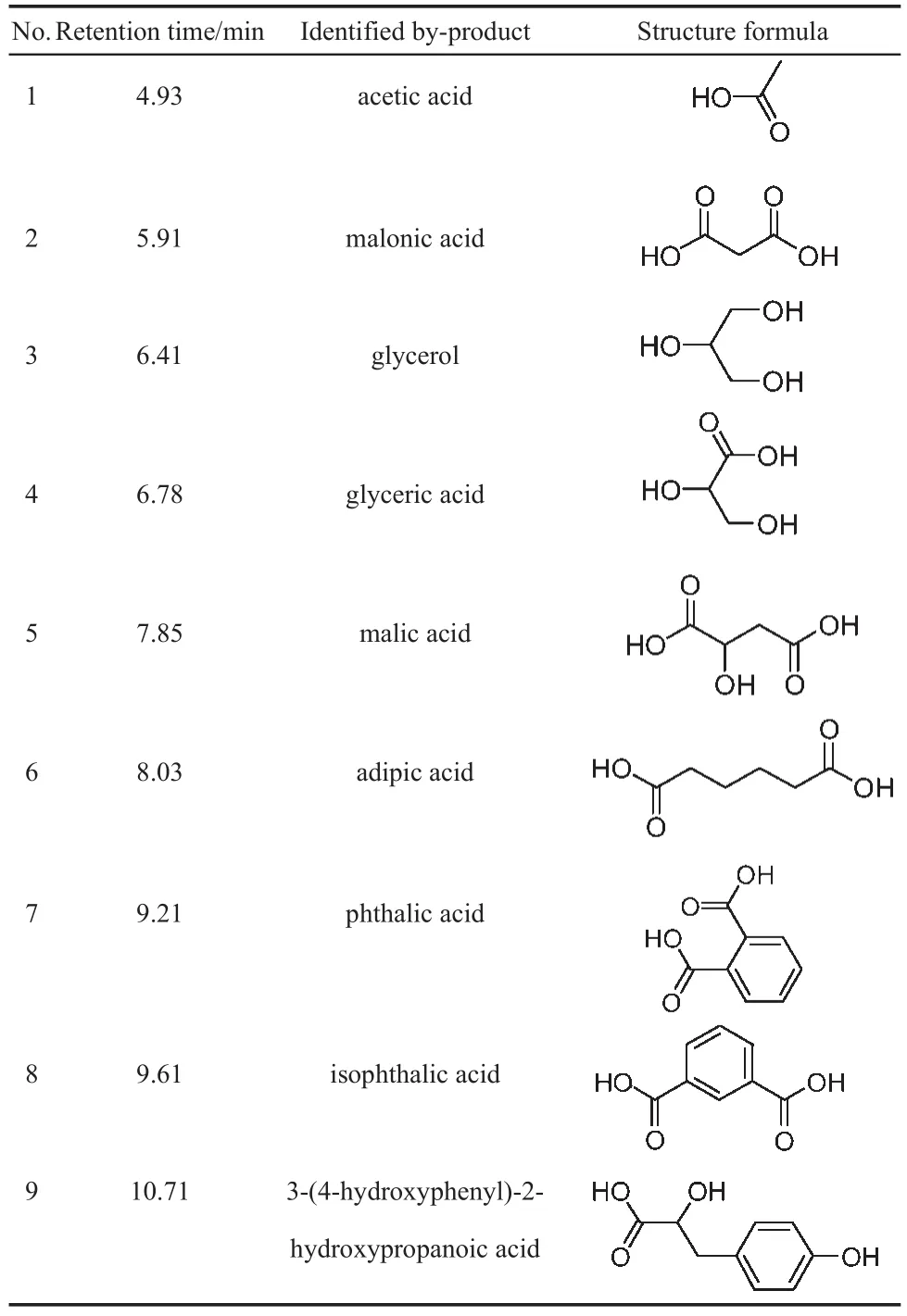
Table 4 Principal by-products of RhB removal detected by GC-MS
3.5.5 Pathway of removing RhB from aqueous solution
In this investigation,XRD characterization showed that the Fe species occurred in the support in the form of α-Fe2O3crystallites, and other experimental results demonstrated that heterogeneous Fe/SBA-15 instead of the Fe3+leached in the solution was responsible for the Fenton oxidation of RhB.It has been reported that heterogeneous Fenton oxidation occurs mainly on the surface of solid catalyst via Fe oxide active sites,which promote the formation of peroxide radicals.37Under acidic and neutral pH conditions for our heterogeneous system,the radical reaction pathway,which is initiated by the formation of a complex between the SiO2-FeIIIand H2O2on the interface,may be proposed as follows:
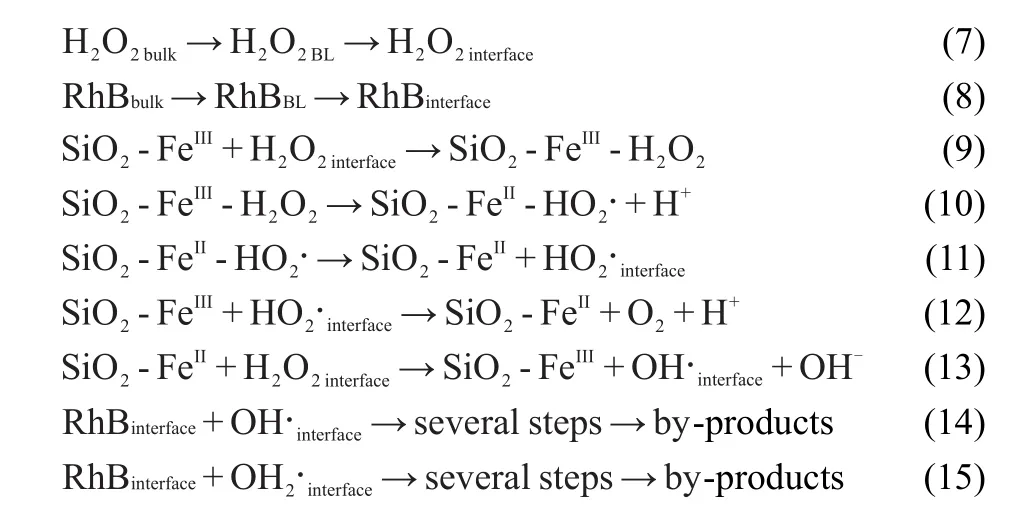
where the H2O2bulkand RhBbulkare H2O2and RhB in bulk solution, respectively,the H2O2BLand RhBBLare H2O2and RhB in boundary layer,respectively,Sinterfaceis the species on the solid-liquid interface,SiO2-FeIIIand SiO2-FeIIare the FeIIIand FeIIspecies anchored to the surface of SBA-15,respectively,and SiO2-FeIII-H2O2and SiO2-FeII-HO2·are the intermediates of the reaction,respectively.
4 Conclusions
The dual-function mesoporous material Fe/SBA-15 can be synthesized by incipient wetness impregnation and applied to remove refractory dye RhB in aqueous solutions in the presence of H2O2.The characterization by XRD,N2adsorption-desorption, SEM,and TEM showed that Fe species occurs both inside and outside the support pores in the form of α-Fe2O3crystallites,theloading of Fe in SBA-15 results in the reduced specific area,pore volume,and pore diameter,and Fe/SBA-15 presents the aggregate of rod-like crystallites with a mean diameter of 0.6 μm.The optimum operation conditions at 21°C for 14 h in Fe/SBA-15-H2O2-RhB system are Fe/SBA-15 dosage at 0.15 g·L-1,molar ratio of H2O2/Fe3+at 2000:1,initial RhB concentration at 10.0 mg·L-1,and initial solution pH at 5.4(unadjusted),with approximately 93% RhB removal achieved under the conditions.The adsorption quantity of Fe/SBA-15 acquired from Langmiur isotherm indicates its strong adsorption capacity,which plays an important role in RhB removal from aqueous solutions.Furthermore,the Fe/ SBA-15 recovered by H2O2soaking technique is capable of maintaining the catalytic performance during six recycled runs, with RhB removal of approximately 80%and Fe leaching less than 0.1 mg·L-1(leaching mass fraction less than 0.6%)for each run.The removal pathway and degradation products of RhB could be determined by UV-Vis absorption spectra and GC-MS analyses.The removal mechanism can be proposed as surface adsorption and Fenton oxidation.
(1) Li,J.J.;Feng J.T.;Yan,W.J.Appl.Polym.Sci.2013,128, 3231.doi:10.1002/app.v128.5
(2) Luo,L.R.;Shen,K.;Xu,Q.Y.;Qin,Z.;Wei,W.;Gondal,M.A. J.Alloy.Compd.2013,558,73.doi:10.1016/j.jallcom. 2013.01.026
(3) Du,L.;Wu,J.;Hu,C.W.Electrochim.Acta 2012,68,69.doi: 10.1016/j.electacta.2012.02.030
(4) Zhao,X.;Zhu,Y.F.Environ.Sci.Technol.2006,40,3367.doi: 10.1021/es052029e
(5) Behnajady,M.A.;Modirshahla,N.;Tabrizi,S.B.;Molanee,S. J.Hazard.Mater.2008,152,381.doi:10.1016/j.jhazmat. 2007.07.019
(6) Zhu,L.;Meng,Z.D.;Park,C.Y.;Ghosh,T.;Oh,W.C. Ultrason.Sonochem.2013,20,478.doi:10.1016/j. ultsonch.2012.08.005
(7) Li,W.J.;Li,D.Z.;Meng,S.G.;Chen,W.;Fu,X.Z.;Shao,Y. Environ.Sci.Technol.2011,45,2987.doi:10.1021/es103041f
(8) Bae,S.T.;Shin,H.;Lee,S.;Kim,D.W.;Jung,H.S.;Hong,K. S.Reac.Kinet.Mech.Cat.2012,106,67.doi:10.1007/s11144-011-0404-2
(9) Bai,C.P.;Xiong,X.F.;Gong,W.Q.;Feng,D.X.;Xian,M.; Ge,Z.X.;Xu,N.Desalination 2011,278,84.doi:10.1016/j. desal.2011.05.009
(10) Machado,E.L.;Dambros,V.S.;Kist,L.T.;Lobo,E.A.A.; Tedesco,S.B.;Moro,C.C.Water Air Soil Pollut.2012,223, 1753.doi:10.1007/s11270-011-0980-9
(11) Anipsitakis,G.P.;Dionysiou,D.D.Environ.Sci.Technol.2003, 37,4790.doi:10.1021/es0263792
(12) Cheng,M.M.;Ma,W.H.;Li,J.;Huang,Y.P.;Zhao,J.C.;Wen, Y.X.;Xu,Y.M.Environ.Sci.Technol.2004,38,1569.doi: 10.1021/es034442x
(13) Botas,J.A.;Melero,J.A.;Martínez,F.;Pariente,M.I.Catal. Today 2010,149,334.doi:10.1016/j.cattod.2009.06.014
(14) Zhang,S.X.;Zhao,X.L.;Niu,H.Y.;Shi,Y.L.;Cai,Y.Q.; Jiang,G.B.J.Hazard.Mater.2009,167,560.doi:10.1016/j. jhazmat.2009.01.024
(15) Hou,M.F.;Liao,L.;Zhang,W.D.;Tang,X.Y.;Wan,H.F.; Yin,G.C.Chemosphere 2011,83,1279.doi:10.1016/j. chemosphere.2011.03.005
(16) Ai,Z.H.;Gao,Z.T.;Zhang,L.Z.;He,W.W.;Yin,J.Y. Environ.Sci.Technol.2013,47,5344.doi:10.1021/es4005202
(17) Xu,L.J.;Wang,J.L.Environ.Sci.Technol.2012,46,10145.
(18) Zhang,Y.Y.;Xiong,Y.;Tang,Y.K.;Wang,Y.H.J.Hazard. Mater.2013,244-245,758.
(19) Liang,X.L.;He,Z.S.;Zhong,Y.H.;Tan,W.;He,H.P.;Yuan, P.;Zhu,J.X.;Zhang,J.Colloids Surf.A 2013,435,28.doi: 10.1016/j.colsurfa.2012.12.038
(20) Liou,R.M.;Chen,S.H.;Hung,M.Y.;Hsu,C.S.;Lai,J.Y. Chemosphere 2005,59,117.doi:10.1016/j. chemosphere.2004.09.080
(21) Parra,S.;Nadtotechenko,V.;Albers,P.;Kiwi,J.J.Phys.Chem. B 2004,108,4439.doi:10.1021/jp031127o
(22) Hassan,H.;Hameed,B.H.Chem.Eng.J.2011,171,912.doi: 10.1016/j.cej.2011.04.040
(23) De Leon,M.A.;Sergio,M.;Bussi,J.Reac.Kinet.Mech.Cat. 2013,110,101.doi:10.1007/s11144-013-0593-y
(24) Martínez,F.;Pariente,M.I.;Botas,J.A.;Melero,J.A.; Rubalcaba,A.J.Chem.Technol.Biotechnol.2012,87,880.doi: 10.1002/jctb.v87.7
(25) Duarte,F.M.;Maldonado-Hódar,F.J.;Madeira,L.M.Appl. Catal.A 2013,458,39.doi:10.1016/j.apcata.2013.03.030
(26) Yao,Y.Y.;Wang,L.;Sun,L.J.;Zhu,S.;Huang,Z.F.;Mao,Y. J.;Lu,W.Y.;Chen,W.X.Chem.Eng.Sci.2013,101,424.doi: 10.1016/j.ces.2013.06.009
(27) Yaman,Y.C.;Gündüz,G.;Dükkanci,M.Color.Technol.2013, 129,69.doi:10.1111/cote.2013.129.issue-1
(28) Sashkina,K.A.;Labko,V.S.;Rudina,N.A.;Parmon,V.N.; Parkhomchuk,E.V.J.Catal.2013,299,44.doi:10.1016/j. jcat.2012.11.028
(29) Kiran,I.;Bektaş,N.;Yatmaz,H.C.;Tekbaş,M.Desalin.Water. Treat.2013,51,5768.doi:10.1080/19443994.2012.759517
(30) Calleja,G.;Melero,J.A.;Martínez,F.;Molina,R.Water Res. 2005,39,1741.doi:10.1016/j.watres.2005.02.013
(31) Melero,J.A.;Calleja,G.;Martínez,F.;Molina,R.;Pariente,M. I.Chem.Eng.J.2007,131,245.doi:10.1016/j.cej.2006.12.007
(32) Melero,J.A.;Calleja,G.;Martínez,F.;Molina,R.Catal. Commun.2006,7,478.doi:10.1016/j.catcom.2006.01.008
(33) Lim,H.;Lee,J.;Jin,S.;Kim,J.;Yoon,J.;Hyeon,T.Chem. Commun.2006,463.
(34) Gokulakrishnan,N.;Pandurangan,A.;Sinha,P.K.J.Chem. Technol.Biot.2007,82,25.
(35) Pham,A.L.T.;Lee,C.;Doyle,F.M.;Sedlak,D.L.Environ. Sci.Technol.2009,43,8930.doi:10.1021/es902296k
(36) Xiang,L.;Royer,S.;Zhang,H.;Tatibouët,J.M.;Barrault,J.; Valange,S.J.Hazard.Mater.2009,172,1175.doi:10.1016/j. jhazmat.2009.07.121
(37) Shukla,P.;Wang,S.B.;Sun,H.Q.;Ang,H.M.;Tadé,M. Chem.Eng.J.2010,164,255.doi:10.1016/j.cej.2010.08.061
(38) Mayani,S.V.;Mayani,S.J.;Kim,S.W.Bull.Korean Chem. Soc.2012,33,3009.doi:10.5012/bkcs.2012.33.9.3009
(39) Satishkumar,G.;Landau,M.V.;Buzaglo,T.;Frimet,L.; Ferentz,M.;Vidruk,R.;Wagner,F.;Gal,Y.;Herskowitz,M. Appl.Catal.B 2013,138-139,276.
(40) Aliyan,H.;Fazaeli,R.;Jalilian,R.Appl.Surf.Sci.2013,276, 147.doi:10.1016/j.apsusc.2013.03.049
(41) Huang,H.Y.;Ji,Y.S.;Qiao,Z.F.;Zhao,C.D.;He,J.G.; Zhang,H.X.J.Autom.Methods Manage.Chem.2010,7.
(42) Wang,H.L.;Tian,H.;Hao,Z.P.J.Environ.Sci.2012,24, 536.doi:10.1016/S1001-0742(11)60800-0
(43) Zhong,X.;Royer,S.;Zhang,H.;Huang,Q.Q.;Xiang,L.J.; Valange,S.;Barrault,J.Sep.Purif.Technol.2011,80,163.doi: 10.1016/j.seppur.2011.04.024
(44) Martínez,F.;Calleja,G.;Melero,J.A.;Molina,R.Appl.Catal. B 2005,60,181.doi:10.1016/j.apcatb.2005.03.004
(45) Lazar.K.;Calleja,G.;Melero,J.A.Stud.Surf.Sci.Catal.2004, 154,805.doi:10.1016/S0167-2991(04)80888-7
(46) Munoz,M.;de Pedro,Z.M.;Casas,J.A.;Rodriguez,J.J.Water Res.2013,47,3070.doi:10.1016/j.watres.2013.03.024
(47) Liu,T.;You,H.Reac.Kinet.Mech.Cat.2013,109,233.doi: 10.1007/s11144-012-0534-1
(48) Zhao,D.Y.;Huo,Q.S.;Feng,J.L.Chmelka,B.F.;Stucky,G. D.J.Am.Chem.Soc.1998,120,6024.doi:10.1021/ja974025i
(49) Jun,S.;Joo,S.H.;Ryoo,R.;Kruk,M.;Jaroniec,M.;Liu,Z.; Ohsuna,T.;Terasaki,O.J.Am.Chem.Soc.2000,122, 10712.doi:10.1021/ja002261e
(50) Vassilakis,C.;Pantidou,A.;Psillakis,E.;Kalogerakis,N.; Mantzavinos,D.Water Res.2004,38,3110.doi:10.1016/j. watres.2004.04.014
(51) Marler,B.;Oberhagemann,U.;Vortmann,S.;Gies,H. Microporous Mat.1996,6,375.doi:10.1016/0927-6513(96) 00016-8
(52) Kim,D.J.;Pal,M.;Seo,W.S.Microporous Mesoporous Mat. 2013,180,32.doi:10.1016/j.micromeso.2013.06.006
(53) Teja,A.S.;Koh,P.Y.Prog.Cryst.Growth Charact.Mater. 2009,55,22.doi:10.1016/j.pcrysgrow.2008.08.003
(54) Wang,X.Q.;Ge,H.L.;Jin,H.X.;Cui,Y.J.Microporous Mesoporous Mat.2005,86,335.doi:10.1016/j. micromeso.2005.07.038
(55) Cornu,C.;Bonardet,J.L.;Casale,S.;Davidson,A.;Abramson, S.;André,G.;Porcher,F.;Grčić,I.;Tomasic,V.;Vujevic,D.; Koprivanac,N.J.Phys.Chem.C 2012,116,3437.doi:10.1021/ jp2038625
(56) Tang,H.D.;Lan,G.J.;Zhong,J.;Liu,H.Z.;Li,Y.J.Nat.Gas Chem.2012,21,275.doi:10.1016/S1003-9953(11)60365-4
(57) Navale,S.T.;Khuspe,G.D.;Chougule,M.A.;Patil,V.B.; Polypyrrole.Org.Electron.2014,15,2159.doi:10.1016/j. orgel.2014.06.019
(58) Xu,X.Z.;Chen,S.X.;Wu,Q.H.J.Colloid Interface Sci.2012, 385,193.doi:10.1016/j.jcis.2012.07.013
(59) Chang,S.H.;Wang,K.S.;Li,H.C.;Wey,M.Y.;Chou,J.D. J.Hazard.Mater.2009,172,1131.doi:10.1016/j. jhazmat.2009.07.106
(60) Hou,M.F.;Ma,C.X.;Zhang,W.D.;Tang,X.Y.;Fan,Y.N.; Wan,H.F.J.Hazard.Mater.2011,186,1118.doi:10.1016/j. jhazmat.2010.11.110
(61) Zhang,J.;Gondal,M.A.;Wei,W.;Zhang,T.;Xu,Q.Y.;Shen, K.J.Alloy.Compd.2012,530,107.doi:10.1016/j. jallcom.2012.03.104
(62) Ma,J.F.;Li,L.Y.;Zou,J.;Kong,Y.;Komarneni,S. Microporous Mesoporous Mat.2014,193,154.
(63) Wu,T.X.;Liu,G.M.;Zhao,J.C.;Hidaka,H.;Serpone,N. J.Phys.Chem.B 1998,102,5845.doi:10.1021/jp980922c
(64) He,Z.;Yang,S.G.;Ju,Y.M.;Sun,C.J.Environ.Sci.2009,21, 268.doi:10.1016/S1001-0742(08)62262-7
(65) Ai,Z.H.;Lu,L.R.;Li,J.P.;Zhang,L.Z.;Qiu,J.R.;Wu,M.H. J.Phys.Chem.C 2007,111,4087.doi:10.1021/jp065559l
Heterogeneous Fenton Oxidation of Refractory Dye Rhodamine B in Aqueous Solution with Mesoporous Fe/SBA-15
HU Long-Xing*XU Dan-Dan ZOU Lian-Pei YUAN Hang HU Xing
(School of Environmental and Chemical Engineering,Shanghai University,Shanghai 200444,P.R.China)
An Fe-loaded mesoporous silica SBA-15,Fe/SBA-15,was prepared by incipient wetness impregnation,characterized by X-ray diffraction(XRD),N2adsorption-desorption,scanning electron microscopy (SEM),transmission electron microscopy(TEM),and X-ray photoelectron spectroscopy(XPS)techniques and used for heterogeneous Fenton oxidation of dye Rhodamine B(RhB)in aqueous solution.The characterization showed that the Fe/SBA-15 retained a mesoporous structure with a long-range ordered arrangement,reduced pore diameter and surface area,and existed as agglomerates of rod-like crystallites with a mean diameter of 0.6 μm.The Fe species occurred both inside and outside the support pores in the form of α-Fe2O3crystallites. The removal of RhB in the presence of Fe/SBA-15 and H2O2was shown to be caused by the synergistic effects of adsorption and catalytic oxidative degradation,and was closely related to Fe/SBA-15 dosage.Removal was almost independent of initial solution pH,with approximately 93%achieved at an Fe/SBA-15 dosage of 0.15 g·L-1,initial RhB concentration of 10.0 mg·L-1,H2O2/Fe3+molar ratio of 2000:1;initial solution pH of 5.4 and 21°C. The Langmuir monolayer adsorption capacity of the Fe/SBA-15 was 99.11 mg·g-1.In addition,Fe/SBA-15 can be easily regenerated by soaking in H2O2then reused for up to six runs,with RhB removal greater than 80% and Fe leaching below 0.1 mg·L-1(or 0.6%(mass fraction))for each run.Aremoval mechanism for RhB by Fe/SBA-15 and H2O2was proposed based on the quenching tests,UV-Vis spectra,and gas chromatography-mass spectrometry(GC-MS)analysis.The heterogeneous Fenton catalyst Fe/SBA-15 can be applied to remove nonbiodegradable organics such as dye RhB.©Editorial office ofActa Physico-Chimica Sinica
Heterogeneous Fenton oxidation;Adsorption;Fe/SBA-15;Rhodamine B; Hydroxyl radical
O643
10.3866/PKU.WHXB201503023www.whxb.pku.edu.cn
Received:November 13,2014;Revised:March 2,2015;Published on Web:March 2,2015.
∗Corresponding author.Email:hulxhhhb@shu.edu.cn;Tel:+86-21-66137771.
The project was supported by the Program for Innovative Research Team in Shanghai University,China(IRT 13078).
上海大学创新研究团队计划项目(IRT 13078)资助
