水热合成制备Al掺杂α-MnO2纳米管及其超级电容器电化学性能
2015-01-04谢华清上海第二工业大学城市建设与环境工程学院上海201209
黎 阳 谢华清 李 靖(上海第二工业大学城市建设与环境工程学院,上海201209)
水热合成制备Al掺杂α-MnO2纳米管及其超级电容器电化学性能
黎 阳*谢华清 李 靖
(上海第二工业大学城市建设与环境工程学院,上海201209)
通过水热法制备了未掺杂α-MnO2和Al掺杂α-MnO2,对产物的形貌、结构和电化学性能进行了研究.扫描电镜(SEM)和高分辨透射电镜(HRTEM)观察表明制备产物呈纳米管形态.紫外-可见光谱分析计算了产物的能带间隙:随着Al的掺杂,α-MnO2的能带间隙值降低.以未掺杂α-MnO2与Al掺杂α-MnO2作为电极材料,通过循环伏安(CV)和恒流充放电测试电极的超级电容器性能.在50 mA·g-1电流密度下,未掺杂α-MnO2与Al掺杂α-MnO2电极的比电容分别达到了204.8和228.8 F·g-1.电化学阻抗谱(EIS)分析表明Al的掺杂降低了α-MnO2在电解液中的阻抗,有利于提高其电化学比电容.增强的比电容及在1000个循环后仍具有良好的容量保持率,使Al掺杂α-MnO2在超级电容器中具有较好的应用前景.
α-MnO2;Al掺杂;纳米管;超级电容器;电化学电容器
©Editorial office ofActa Physico-Chimica Sinica
1 Introduction
Manganese oxide,with its versatile structures,low cost,and environmental friendliness,is believed to have great potential to be applied in wide areas.In recent years,manganese oxides have been intensively studied as an electrode material for supercapacitors.The hot research spots mainly focus on the preparation and properties of nanostructured manganese oxides,the combination of manganese oxides with other components,doping in manganese oxides,etc.
Nanostructured manganese oxides with different morphologies have been studied to explore the effects of nanoscale structures on their properties.Yao et al.1prepared high-quality ultra-long α-MnO2nanowires,with a diameter of about 25 nm and a length of several hundred micrometers,which exhibited a high specific capacitance and a superior cycling stability with only 4.7%loss after 1000 cycles.Lambda-MnO2was synthesized with a developed porous,ordered,and interconnected pore structure used as supercapacitor electrode.The as-synthesized porous lambda-MnO2materials showed a noticeably performance(120 F·g-1)at a high constant current(1A·g-1).2Zhu et al.3prepared hierarchical MnO2nanoflower via a hydrothermal treatment.The hierarchical MnO2nanoflower delivered not only high specific capacitance of 347 F·g-1,but also excellent cycle stability(97.5%capacitance retention after 10000 cycles at a scan rate of 20 mV·s-1).Although nanostructured manganese oxides presented promoted electrochemical performances,fussy preparation process seemed inevitable and further efforts are necessary to obtain elevated specific capacitance.
The composites formed by MnO2and other materials,such as carbon/polymers,also demonstrate attractive electrochemical performances.Jiang et al.4developed a three-dimensional(3D) nanostructure comprised of ternary rGO(reduced graphene oxide)/ CNTs(carbon nanotubes)/MnO2nanocomposites for high-rate supercapacitors.The optimized nanocomposite exhibited a high specific capacitance of 319 F·g-1with enhanced rate capability (222 F·g-1even at 60A·g-1)and good cycling stability in 1 mol· L-1Na2SO4aqueous solution.Azhagan et al.5demonstrated a simple and comparatively low temperature synthesis of functionalized multilayer fullerenes so called carbon nano-onions (CNOs).In situ incorporation of MnO2nanoparticles to the CNOs increased the specific capacitance up to 1207 F·g-1,which was close to the theoretical value of pseudocapacitive MnO2.In Zolfaghari'swork,6carbon black(CB)/MnO2composites were prepared by a sonochemical method.When the mass fraction(w) of MnO2in composite material was 65%,the specific capacitance of CB-MnO2composite calculated from the CV curves was 313 F·g-1,showed higher energy density than pure gamma-MnO2.Yu et al.7synthesized ZnO@MnO2andAl-doped ZnO(AZO)@MnO2hybrid electrodes in core/shell geometries on stainless steel substrates by a scalable low-cost solution route.The excellent capacitive properties indicate that the AZO@MnO2hybrid architecture can serve as a promising electrode material for supercapacitors as well as other electrochemical energy storage/ conversion devices.Furthermore,nano composites of partially reduced graphene oxide-K2Mn4O8presented excellent electrochemical performances as well.8
Doping was an effective way to change the properties of materials.It was revealed in many research works that the electrochemical performances of MnO2can be improved prominently via doping various elements into MnO2lattice.Some elements,including Co,9,10Fe,11Al,12-14V,15Pd,16Sn,17Li,18etc.,were doped into manganese oxides and proved to have positive influences on the properties of manganese oxides.Co-doped birnessite-type MnO2was prepared by Wang et al.,10which showed a very high specific capacitance of 326.4 F·g-1and indicated that cobalt had great effects on the micro-morphology and electrochemical properties of manganese dioxide.Dubal and Lokhande11potentiostatically prepared amorphous and highly porous nanonest like Fe:MnO2thin films.The maximum specific capacitance of 273 F·g-1was achieved for 2%(atomic ratio)Fe:MnO2at 5 mV·s-1scan rate.In Hashem'swork,12Al,Cu,and Mg doped cryptomelane manganese dioxides were prepared by wet-chemical method.The electrical conductivity of doped MnO2increased in comparison with pure MnO2,while Al-doped MnO2exhibited the lower resistivity. Various shapes of hierarchical MnO2(nanorod,nanothorn sphere, sphere)were successfully synthesized using the hydrothermal method with quantitative control ofAl3+in solution.13It was found that Al3+species in the solution acted as a functional doping species into(2×2)tunnels of α-MnO2and also as a catalyst. Malankar et al.18developed a new method to synthesize a uniform round-shaped Li-doped MnO2by ozonation of acidic MnSO4in the presence of Li+ions.The electrochemical properties of Lidoped MnO2were studied by recording discharge profile and the mechanisms were discussed.
α-MnO2,with its special(2×2)tunnel cavity structure,presents attractive activity in lithium ion batteries,19,20supercapacitor,1,21microwave absorption,22,23catalyst,24,25etc.However,the inherent semi-conduct properties of α-MnO2leads to its low electrical conductivity,which is not beneficial to charge transfer in electrochemical process.So,it is necessary to enhance the electrical conductivity of α-MnO2.In this work,Al element was doped into α-MnO2nanotube to obtain uniform solid solution via hydrothermal method.It is expected that the doped Al could improve the electrical conductivity and electrochemical performances of α-MnO2.Moreover,the hollow space in the nanotube structure can provide a way for ions in the electrolyte to pass through,which would enlarge the contact areas between nanotube surface and electrolyte.As a result,more electrochemical active positions were formed and beneficial to redox processes on the nanotube surface.The investigations on the influences of doping on the structure and electrochemical performances of α-MnO2were carried out and discussed.
2 Experimental
Un-doped and Al-doped α-MnO2were prepared via hydrothermal method.All of the reactants and solvents were analytical-grade and were used without further purification.In a typical procedure,4.8 mmol potassium permanganate(99.0%)and aluminum nitrate(99.0%)were dissolved in 40 mL de-ionized water by magnetic stirring.Then 18 mmol concentrated hydrochloric acid(36.0%)was added in the above solution with stirring for 30 min.Finally,the solution was transferred to a Teflon reactor(100 mL volume)and placed in an oven and kept at 130°C for 10 h. After the temperature of the oven decreased to room temperature, the product in the reactor was collected by centrifugation,washed with de-ionized water and dried at 100°C for 24 h,resulting in brown black powder.The mole ratio of Mn andAl was controlled at 98:2 in the present study.The un-doped and doped samples were nominated as PM and AM,respectively.The structure and morphology of as-synthesized samples were examined by scanning electron microscopy(SEM,HITACHI,S-4800,Japan),X-ray diffraction(XRD,D8-ADVANCE,Cu Kα,Germany),ultravioletvisible absorption spectroscopy(UV-2550,SHIMADZU,Japan), and high resolution transmission electron microscopy(HRTEM, FEI,TECNAI G2-F20,USA)
As-prepared powders were fabricated as electrodes to investigate their electrochemical properties.The typical procedure for electrode fabrication can be described as follow:as-synthesized samples,10%(w,mass fraction)conducting agent(acetylene black)and 10%(w)PVDF(poly vinyliene tetrafluoroethylene) latex as binder were mixed in mortar.Then the mixture was pressed into foam nickel mesh dried in air at 120°C for 12 h to remove solvent and finally was pressed under 10 MPa pressure to keep good adherence between electrode material and the nickel mesh current collector.Electrochemical investigations were carried out in three-electrode cell using carbon as counter electrode andAg/AgCl as reference electrode.The electrolyte,0.1 mol· L-1Na2SO4,was used to study the capacitive behavior of as-prepared electrodes.Cyclic voltammetry(CV)studies were performed at a potential range of-0.2-0.8 V(vs Ag/AgCl)at scan rates of 5,10,20,30,50,and 100 mV·s-1on electrochemical workstation(VMP3,BioLogic,France).Galvanostatical chargedischarge test was conducted at current densities of 20,50,80, 100,200,500,and 1000 mA·g-1between-0.2-0.8 V(vs Ag/ AgCl)using computer controlled cycling equipment(LAND, Wuhan China).Electrochemical impedance spectra(EIS)were measured over the frequency-range of 100 kHz-10 mHz at a potentiostatic signal amplitude of 5 mV on same workstation as CV test.
3 Results and discussion
3.1 Structure characterization
XRD patterns of the as-prepared pristine α-MnO2andAl-doped α-MnO2are presented in Fig.1.It is apparent that PM and AM samples all demonstrate single tetragonal phase structures,corresponding to the characteristic peaks of α-MnO2phase(JCPDS 44-0141).No diffraction peaks of other phases or impurities etc. could be observed.The doping of Al causes variations of crystalline lattice of α-MnO2,which are listed in Table 1.The lattice parameters a and b increase from 0.9831 to 0.9866 nm,while lattice parameter c shows negligible change.So,the lattice expansion of Al-doped α-MnO2mainly happens along x and y axes and keeps stable in z axes direction.As a result,the volume ofAM sample cell expands from 0.2764 to 0.2783 nm3,which indicates that Al doping has effect on the structure of α-MnO2.During the formation of α-MnO2,Al ion(Al3+,ionic radius 0.057 nm),has similar ionic radius to Mn ion(Mn4+,ionic radius 0.052 nm),may mostly substitute Mn in MnO6octahedron and some Al ions exist at the center of(2×2)tunnel cavity where K+exists previously. Finally,it can be confirmed from XRD patterns that the dopedAl all exists with the form of solid solution in cryptomelane structure of α-MnO2.
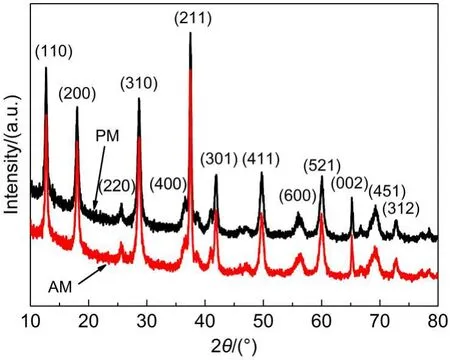
Fig.1 X-ray diffraction patterns of as-prepared α-MnO2(PM)andAl-doped α-MnO2(AM)
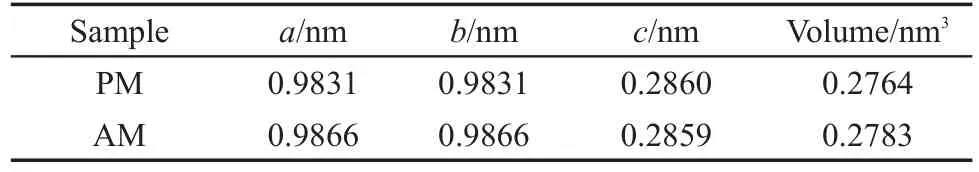
Table 1 Variations of α-MnO2lattice parameters before and afterAl-doping
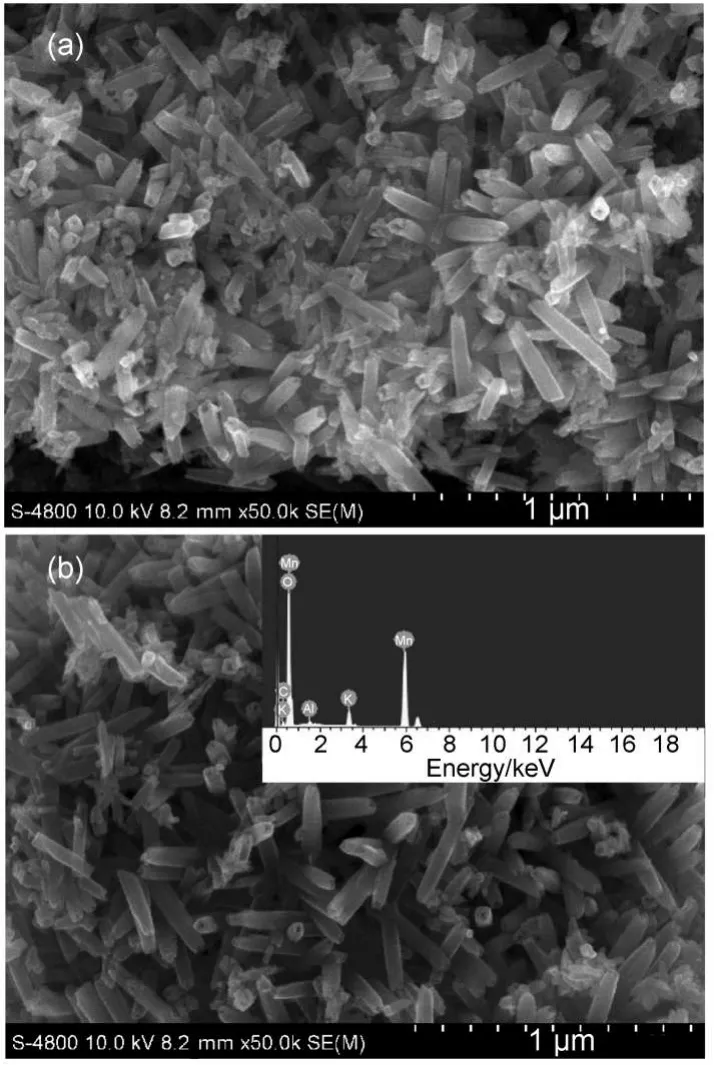
Fig.2 shows the SEM morphologies of un-doped α-MnO2and Al-doped α-MnO2.PM andAM samples all show similar nanotube morphology and distribute homogeneously,which implies that the doping ofAl has no distinct effects on the morphology of α-MnO2. Those nanotubes are scores of nanometers in diameters and several hundreds of nanometers in lengths.It can be clearly observed and estimated that the wall thickness of nanotube is about 20 nm both in Fig.2(a)and Fig.2(b).The growth mechanism of nanotube under hydrothermal conditions can be comprehended in previous study.26The inset in Fig.2(b)shows the compositions of AM sample.The mole ratio of Mn andAl is about 98.1:1.9,which is close to the desired Al content.The details of as-prepared nanotubes are disclosed in high resolution image,as shown in Fig.3.Fig.3(a)apparently demonstrates hollow center structure of the nanotube and the diameters of these nanotubes are about 50-80 nm.The selected area electron diffraction(SAED)pattern in inset reveals the electronic diffraction characteristics of AM sample,indicating the single crystalline structure of individual nanotube.Fig.3(b)presents the zoom out view of selecting area within black circle in Fig.3(a).The lattice line of the sample couldbe distinguished easily.The center region of the nanotube is brighter than that of both side,due to film thickness of the hollow center in nanotube.The lattice-fringe distance is about 0.69 nm, which is corresponding to the(110)d-space of tetragonal α-MnO2(JCPDS file No.44-0141)and such result is in accordance with the XRD results.
Generally,the energy band structures of substances would be altered when heterogeneous atoms are doped,which plays important roles in regulating physical properties of substances.In this study,the influences of doping on the band gaps of as-synthesized α-MnO2nantubes are studied by UV-Vis absorption spectroscopy.In Fig.4,the absorption spectra of α-MnO2nanotubes are presented.The inset demonstrates the Tauc plots calculated from absorption spectrum.By extrapolation of the Tauc plots,the band gaps of PM andAM samples can be determined as 2.14 and 2.05 eV,respectively.In former work,27the band gap values are different,which may have relationship with the grain size,microstructure,and component of various samples.The band gap value decreases upon doping ofAl into α-MnO2lattice,which indicates that the dopant has obvious influence on the band structure of α-MnO2nanotube.
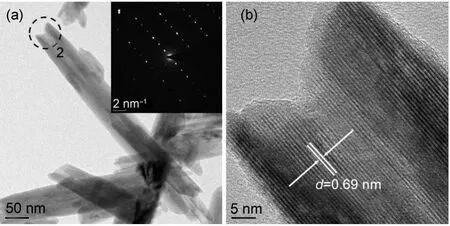
Fig.3 TEM morphology(selected area electron diffraction pattern in inset)of(a)Al-doped α-MnO2and(b)its high resolution image

Fig.4 UV-visible spectra of as-prepared samples (Tauc plot in inset)
3.2 Electrochemical performances
Typical cyclic voltammogram(CV)curves of as-prepared α-MnO2electrodes in 0.1 mol·L-1Na2SO4solution at room temperature are presented in Fig.5.To find appropriate scan potential ranges for CV measurements,PM and AM samples are scanned with several different potential cut-off windows,as indicated in Fig.5(a,b).The profiles of all CV curves present roughly rectangular mirror images,which demonstrate typical capacitive characteristics.When the cut-off window ranges gradually increase,the cathodic and anodic currents all amplify accordingly due to enhanced polarization effects.While the potential cut-off window is located between-0.3 and 0.9 V,the cathodic and anodic currents are apparently larger than those of potential cutoff window between 0.0 and 0.6 V.The self-made three-electrode measuring system maintains stable and no gas bubbles/impurities emerge in solution/electrode surfaces at potential cut-off window of-0.2 and 0.8 V.So,it can be decided that the favorable scan potential cut-off window is between-0.2-0.8 V(vs Ag/AgCl). Various scan rates of 5,10,20,30,50,and 100 mV·s-1are applied in CV investigation to study the capacitive behaviors of PM and AM electrodes between-0.2-0.8 V in Fig.5(c,d).The PM and AM electrodes all exhibit highly electrochemical reversibility between-0.2 and 0.8 V,which can be judged from roughly rectangular mirror shapes in Fig.5(c,d).Obviously,with the increase of scan rates in CV measurements,the CV curves gradually deviate from rectangular shapes and the areas of CV curves enlarge.By comparison of CV patterns of PM andAM samples,the doping ofAl has no distinct effects on the profiles of CV curves.
The capacitance of MnO2is considered to be mainly produced from pseudo-capacitance,which is attributed to reversible redox reaction happened between electrode and electrolyte in terms of intercalation/de-intercalation of H+or Na+.To study the capacitance variations of MnO2before and after doping,galvanostatical charge-discharge measurements are conducted.Fig.6 presents the charge-discharge curves of PM andAM samples.Under 50 mA· g-1current density,the profiles of charge-discharge curves of PMand AM samples display similar variation characteristics,as indicated in Fig.6(a).The IR drops for both samples are displayed in the inset.The potential-time relationships on charge-discharge curves are all approximately linear except the discharge curve become placid at lower potential stage,which implies that both electrodes have regular capacitive behaviors.The specific capacitance(SC)of samples could be calculated with chargedischarge profiles via equation:


Fig.6 Galvanostatical charge-discharge measurements of(a)profiles conducted under 50 mA·g-1current density (zoom in view of pattern in inset)and(b)the variations of specific capacitances upon current density increasing
where C,I,∆t,∆V,and m are the specific capacitance,galvanostatic current,charge-discharge time,potential cut-off window, and mass of electro-active material.When the charge-discharge current densities increase from 20 to 1000 mA·g-1,the SC decreases monotonously both for PM andAM,as shown in Fig.6(b). It can be seen that the SC of AM sample are greater than that of PM sample under all current densities.Upon current density increasing,the discrepancy of SC between PM and AM samples keeps stable,which may be attributed to weaker ion/electrical conductivity of un-doped α-MnO2sample.Poor electrical conductivity is not beneficial to electron exchange between electrode and electrolyte,leading to less pseudo-capacitance faradic reactions and producing smaller capacitances.By calculation from equation(1),when the current density ascends from 20 to 1000 mA·g-1,the SC of PM and AM samples falls from 315.4 and 336.5 F·g-1to 14.4 and 33.6 F·g-1respectively,indicating thatAldoped α-MnO2can maintain superior SC than un-doped sample.For comparison,commercial MnO2(A.R.,Sinapharm Chemical Reagent Co.,Ltd.)is fabricated into electrode and measured.The SC of commercial MnO2is only 124.2 F·g-1under 20 mA·g-1,far less than those of AM and PM samples.The doping is considered to account for the enhancement of SC of doped sample.By doping Al element,the charge transfer ability of α-MnO2is enhanced, which could be convinced in following EIS analysis.
Fig.7(a)shows the electrochemical impedance spectra of doped and un-doped samples.The charge transfer process happened on the electrode interface can be described by the semicircle at high frequencies and the near linear variation at low frequencies represents the ion diffusion process.The semicircles at high frequencies are presented in the inset on the top right corner.The corresponding values of intersection of semicircle and x-coordinate show the charge transfer impedances Rctof samples,which determines the process in terms of redox reactions at the interface between electrode and electrolyte.In this study,the impedance value Rctof un-doped α-MnO2is greater than that of doped samples,which indicates that the charge transfer process is easier to happen on the interface for Al-doped α-MnO2than for un-doped α-MnO2.Furthermore,the simulated equivalent circuit diagram is provided in Fig.7(a),where Rct,Rohm,CPE,and Zware charge charansfer resistance,Ohm resistance,constant phase element, and Warburg resistance,respectively.By analysis of ZsimpWin software,the simulated values of Rctare 8.65 and 10.18 Ω forAM and PM samples respectively,which are in good accordance with the experimental results and indicate that the equivalent circuit diagram can properly simulate the impedance characterizations of the electrodes.
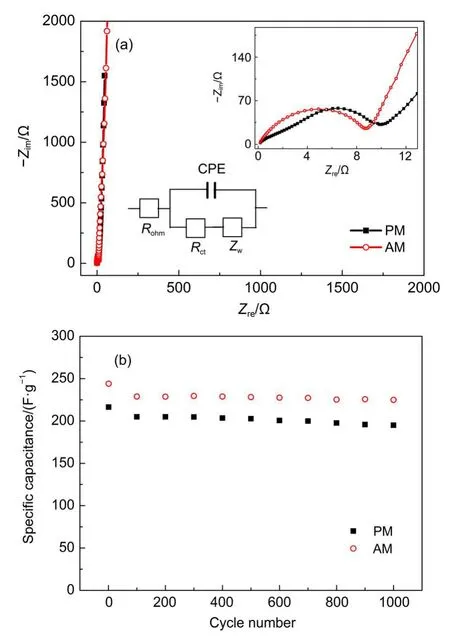
Fig.7 (a)Electrochemical impedance spectra of PM andAM samples(zoom in view of high frequency zone in inset)and (b)cycling performances under a current density of 50 mA·g-1
The cycling performances of un-doped and doped α-MnO2are presented in Fig.7(b).Apparently,both samples demonstrate preferable cycling stabilities.With charge/discharge process going,the decreases of SC are small and the SC discrepancy between doped and un-doped α-MnO2presents little variation,which is different from the results in former literature.28It is considered that doped Al may occupy(2×2)tunnel position or substitute Mn in MnO6octahedron,which could influence the intercalation/deintercalation of H+/Na+upon cycling.In present study,no obvious influences of doping on cycling stability are displayed,which means that un-doped and doped samples all have stable structures upon cycling.As proved in UV-Vis measurement in Fig.4,the band gap of AM decreases in comparison to that of PM,which means that the band structure of MnO2has changed viaAl doping. By further investigation in EIS spectra,doping ofAl into α-MnO2lattices results in lower impedance value and preferable electrochemical capacitance.As a result,the doping is considered to be responsible for improved SC and good cycling performance ofAldoped α-MnO2.
4 Conclusions
Un-doped and Al-doped α-MnO2with nanotube morphology were successfully synthesized via hydrothermal method.Asprepared α-MnO2behaved cryptomelane structures in X-ray diffraction patterns and the variation of lattice parameters was detected in Al-doped α-MnO2.The band gaps of un-doped and doped α-MnO2were determined by ultraviolet-visible absorption spectra,which revealed that the band gap value of α-MnO2decreased after doping.In CV tests,un-doped andAl-doped α-MnO2all displayed good capacitive response.The profiles of CV curves gradually changed with scan rate increasing.The specific capacitances of un-doped and Al-doped α-MnO2were 204.8 and 228.8 F·g-1respectively under 50 mA·g-1current density during charge-discharge process.Both samples exhibited good cycling stability.The superior electrical conductivity ofAl-doped α-MnO2was convinced by EIS measurement,which could account for better specific capacitance of doped sample.Based on the good cycling performance and improved specific capacitance,Al-doped α-MnO2may be used as a potential electrode material for supercapacitors.
(1) Yao,W.;Wang,J.;Li,H.;Lu,Y.J.Power Sources 2014,247, 824.doi:10.1016/j.jpowsour.2013.09.039
(2) Ghimbeu,C.M.;Malak-Polaczyk,A.;Frackowiak,E.;Vix-Guterl,C.J.Appl.Electrochem.2014,44,123.doi:10.1007/ s10800-013-0614-6
(3) Zhu,G.;Deng,L.;Wang,J.;Kang,L.;Liu,Z.H.Colloids Surfaces A 2013,434,42.doi:10.1016/j.colsurfa.2013.05.008
(4) Jiang,H.;Dai,Y.;Hu,Y.;Chen,W.;Li,C.ACS Sustain.Chem. Eng.2014,2,70.doi:10.1021/sc400313y
(5) Azhagan,M.V.K.;Vaishampayan,M.V.;Shelke,M.V. J.Mater.Chem.A 2014,2,2152.doi:10.1039/C3TA14076H
(6) Zolfaghari,A.;Naderi,H.R.;Mortaheb,H.R.J.Electroanal. Chem.2013,697,60.doi:10.1016/j.jelechem.2013.03.012
(7) Yu,M.;Sun,H.;Sun,X.;Lu,F.;Wang,G.;Hu,T.;Qiu,H.; Lian,J.Int.J.Electrochem.Sci.2013,8,2313.
(8) Li,L.;He,Y.Q.;Chu,X.F.;Li,Y.M.;Sun,F.F.;Huang,H.Z. Acta Phys.-Chim.Sin.2013,29,1681.[李 乐,贺蕴秋,储晓菲,李一鸣,孙芳芳,黄河洲.物理化学学报,2013,29,1681.] doi:10.3866/PKU.WHXB201305223
(9) Hashem,A.M.;Abuzeid,H.M.;Mikhailova,D.;Ehrenberg,H.; Mauger,A.;Julien,C.M.J.Mater.Sci.2012,47,2479.doi: 10.1007/s10853-011-6071-x
(10) Wang,G.;Shao,G.;Du,J.;Zhang,Y.;Ma,Z.Mater.Chem. Phys.2013,138,108.doi:10.1016/j.matchemphys.2012.11.024
(11) Dubal,D.P.;Lokhande,C.D.Ceram.Int.2013,39,415.doi: 10.1016/j.ceramint.2012.06.042
(12) Hashem,A.M.;Abuzeid,H.M.;Narayanan,N.;Ehrenberg,H.; Julien,C.M.Mater.Chem.Phys.2011,130,33.doi:10.1016/j. matchemphys.2011.04.074
(13) Ryu,W.H.;Han,D.W.;Kim,W.K.;Kwon,H.S.J.Nanopart. Res.2011,13,4777.doi:10.1007/s11051-011-0448-2
(14) Shanthi,S.;Ravi,S.Int.J.Chem.Tech.Res.2014,6,2066.
(15) Wang,S.;Liu,Q.;Yu,J.;Zeng,J.Int.J.Electrochem.Sci.2012, 7,1242.
(16) Kunkalekar,R.K.;Salker,A.V.React.Kinet.Mech.Catal. 2012,106,395.doi:10.1007/s11144-012-0443-3
(17) Hashem,A.M.;Abdel-Latif,A.M.;Abuzeid,H.M.;Abbas,H. M.;Ehrenberg,H.;Farag,R.S.;Mauger,A.;Julien,C.M. J.Alloy.Compd.2011,509,9669.doi:10.1016/j. jallcom.2011.07.075
(18) Malankar,H.;Umare,S.S.;Singh,K.Mater.Lett.2009,63, 2016.doi:10.1016/j.matlet.2009.06.044
(19) Jung,K.N.;Riaz,A.;Lee,S.B.;Lim,T.H.;Park,S.J.;Song, R.H.;Yoon,S.;Shin,K.H.;Lee,J.W.J.Power Sources 2013, 244,328.doi:10.1016/j.jpowsour.2013.01.028
(20) Zhang,Y.;Liu,H.;Zhu,Z.;Wong,K.W.;Mi,R.;Mei,J.;Lau, W.M.Electrochim.Acta 2013,108,465.doi:10.1016/j. electacta.2013.07.002
(21) Song,Z.;Liu,W.;Zhao,M.;Zhang,Y.;Liu,G.;Yu,C.;Qiu,J. J.Alloy.Compd.2013,560,151.doi:10.1016/j. jallcom.2013.01.117
(22) Wang,G.S.;He,S.;Luo,X.;Wen,B.;Lu,M.M.;Guo,L.;Cao, M.S.RSC Adv.2013,3,18009.doi:10.1039/c3ra42412j
(23) Zhou,M.;Zhang,X.;Wang,L.;Wei,J.;Zhu,K.;Feng,B. J.Nanosci.Nanotechnol.2013,13,904.doi:10.1166/ jnn.2013.5958
(24) Shan,J.;Zhu,Y.;Zhang,S.;Zhu,T.;Rouvimov,S.;Tao,F. J.Phys.Chem.C 2013,117,8329.doi:10.1021/jp4018103
(25) Wu,Y.;Lu,Y.;Song,C.;Ma,Z.;Xing,S.;Gao,Y.Catal.Today 2013,201,32.doi:10.1016/j.cattod.2012.04.032
(26) Umek,P.;Gloter,A.;Pregelj,M.;Dominko,R.;Jagodic,M.; Jaglicic,Z.;Zimina,A.;Brzhezinskaya,M.;Potocnik,A.; Filipic,C.;Levstik,A.;Arcon,D.J.Phys.Chem.C 2009,113, 14798.doi:10.1021/jp9050319
(27) Sakai,N.;Ebina,Y.;Takada,K.;Sasaki,T.J.Phys.Chem.B 2005,109,9651.doi:10.1021/jp0500485
(28) Kang,J.L.;Hirata,A.H.;Kang,L.J.;Zhang,X.M.;Hou,Y.; Chen,L.Y.;Li,C.;Fujita,T.;Atagi,K.;Chen,M.W.Angew. Chem.Int.Edit.2013,52,1664.doi:10.1002/anie.v52.6
Hydrothermal Synthesis of Al-Doped α-MnO2Nanotubes and Their Electrochemical Performance for Supercapacitors
LI Yang*XIE Hua-Qing LI Jing
(School of Urban Development and Environmental Engineering,Shanghai Second Polytechnic University, Shanghai 201209,P.R.China)
α-MnO2and Al-doped α-MnO2were synthesized via a hydrothermal method.The morphologies, structures,and electrochemical performances of as-synthesized un-doped and doped α-MnO2were studied. Scanning electron microscopy(SEM)and high-resolution transmission electron microscopy(HRTEM)show that these un-doped and doped α-MnO2are nanotube shaped.The band gaps of α-MnO2are investigated by ultraviolet-visible absorption spectroscopy,which indicates that the band gap of α-MnO2decreases upon Al doping.The electrochemical performances of un-doped and doped α-MnO2as electrode materials for supercapacitors were measured by cyclic voltammetry(CV)and galvanostatical charge/discharge tests.The specific capacitances of un-doped and Al-doped α-MnO2respectively reach 204.8 and 228.8 F·g-1under a current density of 50 mA·g-1.It was discovered that the electrochemical impedance of Al-doped α-MnO2was decreased byAl doping analyzed using electrochemical impedance spectra(EIS),which provides a beneficial increase to its electrochemical specific capacitance.Enhanced specific capacitance and preferable cycling stability(up to 1000 cycles)forAl-doped α-MnO2mean that these systems are favorable prospects for application in supercapacitors.
α-MnO2;Al doping;Nanotube;Supercapacitor;Electrochemical capacitor
O646;TM911
10.3866/PKU.WHXB201502021www.whxb.pku.edu.cn
Received:September 9,2014;Revised:January 29,2015;Published on Web:February 2,2015.
∗Corresponding author.Email:liyang@sspu.edu.cn;Tel/Fax:+86-21-50216301.
The project was supported by the Key Innovation Foundation of Shanghai Education Commission,China(13ZZ139),Key Discipline Construction (Materials Science)of Shanghai Second Polytechnic University,China(XXKPY1302),and Program for Professor of SpecialAppointment(Eastern Scholar)at Shanghai Institutions of Higher Learning,China.
上海市教委创新重点项目(13ZZ139),上海第二工业大学校级重点培育学科(材料科学)建设(XXKPY1302)及上海市高校“东方学者”岗位计划资助
