氧化煅前针状焦制备超级电容器用纳米多孔炭
2015-01-01王九洲王立群陈明鸣王成扬
王九洲,王立群,陈明鸣,王成扬,张 翠,何 琲
(1.天津大学化工学院,教育部绿色合成与转化重点实验室,天津 300072)
1 Introduction
Electric double-layer capacitors (EDLCs)or supercapacitors,are electrochemical energy storage devices that provide high power density for electric vehicles,backup power systems,and electronic components.Much effort[1-5]has been made to develop appropriate electrode materials,which were key materials to the electrochemical performance of supercapacitors.
Porous carbon materials are widely used material for EDLC electrodes due to their highly developed porous structure and stable electrochemical properties.And pore structure is crucial to the formation of electric double-layers.Some researches resorted to template methods for tuning pores in porous carbon,especially mesopores[6-8].As for micropores,afterwards activation[9,10]provides some possibility.
Activation of carbonaceous materials is another widely used method for the preparation of porous carbons.Compared with templated carbons,activated carbons have a wide pore size distribution,which includes both micropores and mesopores.Usually,the ratio of micropores to mesopores not only depends on the structure of the precursor but also the activation conditions.A variety of activated carbons were prepared from different precursors including isotropic pitches[11],mesophase pitches[12,13],carbon aerogels[14],biomass carbon[15,16],and green coke[17-19].Although some of the samples have high surface areas,more and more researchers have convinced that not all the micropores detected by BET surface analysis method are available for electrolyte ions due to the transport resistance.Reasonable porous structure seems to be more crucial for the EDLC performance.
In previous work[20],we reported an amphiphilic oxidized carbon material having great potential to be an ideal precursor for porous carbon material.No doubt,pre-oxidation of the precursor is indispensable for activation to get a well-developed porous structure which is appropriate as the electrode material for EDLCs.But how and why does the oxidation affect the ultimate porous structure as well as their electrochemical properties are still ambiguous,therefore different kinds of oxidized carbonaceous materials are firstly prepared from green needle coke (GNC)and a comparative study was conducted.Herein,all these oxidized carbonaceous materials are called intermediates,considering that the total synthesis route are from GNC precursor to oxidized carbonaceous materials and finally to porous carbons.We try to figure out the relationship between the final EDLC performance and both surface properties and structure character of intermediates.Also activation conditions,especially the KOH dosage was optimized.The electrochemical performance of EDLCs are compared with those reported in recent literatures.
2 Experimental
2.1 Preparation
GNC derived from residual oil was provided by Jinzhou Petrochemical Co.in China.The coke was prepared around 450-520℃in a delayed coker,it was pulverized to particles of 50-100 μm.A calcined coke(CC)with the same particle size was prepared by calcination of the GNC at around 1 300 ℃,and used for comparison.Their elemental compositions were listed in Table 1.The GNC contains a larger amount of heteroatoms (O,H,N and S)than the CC.
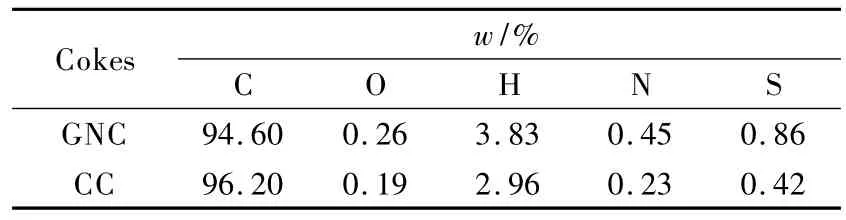
Table 1 Elemental analysis of carbon precursors.
10 g GNC powder was oxidized at room temperature for 1 h by either 50 or 100 mL (Vacid/mcoke=5 or 10)of a concentrated acid mixture consisting of concentrated nitric and sulfuric acid with a ratio of Vnitric/Vsulfuric=3/7.After oxidation,the suspension was poured into cold water and the oxidized coke was separated by centrifugation and washed with water until the oxidized coke reached a pH value of 3.Then the oxidized coke was added into a 1 mol/L NaOH aqueous solution,stirred for 1 h and separated into two components through centrifugation,water dispersable part and precipitated part.The non-dispersible part was obtained through washing the precipitated part with water until the pH value reached 3 and dried at 80 ℃,then pulverized to particles with almost the same size as the starting coke.This non-dispersible part is labeled using R with the amount of acid used(Vacid/mcoke),e.g.GNC-R10.The dispersible part was recovered from the filtrate by adding 1mol/L HCl until the pH of the solution was below 2,followed by washing with water until the pH value reached 3 and was dried to obtain a fine powder.This dispersible part is labeled using A with Vacid/mcoke,e.g.GNCA10;A is used since it is assumed to be amphiphilic according to the literatures[21,22].The CC was oxidized under the same conditions with Vacid/mcoke=10 and separated into two components labeled as CC-R10 and CC-A10.The yield of the intermediates was calculated from the equation (1):
The resultant oxidized coke powders (intermediates)were activated by mixing with KOH (mKOH/mintermediate=1,3 or 5)and heating to 800 ℃at a rate of 5 ℃/min,then maintained at 800 ℃ for 1 h and cooled to room temperature.The cooled activated products were then washed several times with HCl aqueous solution and distilled water consecutively.Finally,the products were dried at 110 ℃for 12 h.For CC,only the non-dispersible part,CC-R10,was activated because the yield of the dispersible part CCA10 was very small.For comparison,GNC and CC were also activated by using 3∶1 KOH/coke ratio (labeled as GNC-0-3 and CC-0-3,respectively).In Fig.1,a flow chart for the preparation procedure of the intermediates and activated carbons is shown.The prepared samples are in bold type.For example,GNC-R10-3 is the non-dispersible part of the GNC after oxidation in 100mL of mixed acid and activated by KOH with a ratio of 3 ∶1 (KOH/intermediate)at 800 ℃.

Fig.1 Flow chart for the preparation of the intermediates and activated carbons.
2.2 Characterization
The pore structures of the final nanoporous carbon materials were characterized by N2adsorption/desorption at 77 K using Micrometrics ASAP 2020.Before measurements,the samples were degassed below 1.33 Pa at 90 ℃ for 1 h and heated to 300 ℃(10 ℃/min)and maintained at this temperature for 5 h.The specific surface area SBETwas calculated by the Brunauer-Emmett-Teller (BET)method at the relative pressure ranging from 0.1 to 0.3.The mesoporous surface area Smes and mesopore volume Vmes were calculated using the Barrett-Joyner-Halenda(BJH)method from the desorption branch.The total pore volume Vtotwas calculated from the amount of N2adsorbed at a relative pressure (p/p0)of 0.99 and the pore size distributions were calculated using a cylindrical density function theory (DFT)method.
The contents of C,H,N,S were determined using an elemental analyzer (Elementar Vario MICRO,Germany).The contents of O was determined using an elemental analyzer (Elementar Vario EL cube,Germany).XPS was performed with a PHI-1600ESCA electron system (America PE Company)with Al Kα (1 486.6 eV)radiation over an area of 0.8 mm2to get information on the atomic compositions of the sample surface.X-ray diffraction (XRD)was used as a complimentary method to determine the essential differences between the R-and A-type intermediates.The microstucture of the resultant nanoporous carbons was investigated by high-resolution transmission electron microscopy (HR-TEM,Philips Tecnai G2F20).
2.3 Electrochemical measurements
The electrodes for electrochemical measurements were prepared by mixing 80% nanoporous carbon with 10% acetylene black and 10% polytetrafluoroethylene (PTFE)in mass ratios,and then pressing mixture into sheets.The sheets were punched out in the required size (φ 13 mm)as test electrodes and then pressed onto foamed nickel.A coin-type cell was fabricated using the prepared electrodes and a polypropylene membrane as the separator.Cyclic voltammetry (CV)test was carried out on PARSTAT2273 electro-chemical system.The galvanostatic chargedischarge characterization was performed on a Arbin battery test instrument.The gravimetric capacitance Cgof the EDLC was calculated according to the formula Cg=It/Vm,where I is the discharge current,t is the discharge time,V is the potential change during the discharge process and m is the mass of the active electrode material.The volumetric capacitance Cv,capacitance per unit bulk volume of carbon,can be obtained by multiplying the gravimetric capacitance by bulk density of electrode sheet.
3 Results and discussion
3.1 Characterization of the intermediates
Two kinds of intermediates were obtained after the oxidation.There is no noticeable difference of XPS surface element contents between A-and R-type intermediates (Table 2).However,A-type intermediates can be dispersible in water while R-type intermediates cannot,which are assumed to be attributed to the difference of their N1s species.

Table 2 Yields of the intermediates and atomic percentage of various elements by XPS.
The deconvolution of N1s spectra revealed three kinds of carbon-nitrogen bonding structures,which are N1 (399.1 ±0.5 eV),N2 (400.7 ±0.1 eV),and N3 (405.4 ±0.2 eV)(Fig.2).Peak N1 is assigned to the cyanide functional group (C≡ N :the N atom gets bonded to a C atom without replacing it),peak N2 represents graphite-like substituted structure,in which N is bonded to three C atoms in the aromatic graphene structure,and the peak N3 is attributed to chemisorbed nitrogen oxide species.N in Rtype intermediates (GNC-R5 and GNC-R10)mainly exists as the cyanide functional group.Only a small quantity of N is in the chemisorbed nitrogen oxide group,which is mainly -NO2.As the quantity of acid oxidant increases,for example from 5 to 10 (Vacid/mcoke),percentage of N3 increases from 9.09 (GNCR5)to 20.71% (GNC-R10).In all,C≡ N species are still dominant in R-type intermediates.On the contrary,N2 and N3 are dominant N species on the surface of A-type intermediates.The total atom fractions of such two species are 58.38% in GNC-A5 and 78.65% in GNC-A10.Based on the dispersibility of A-type and R-type and the their N functional groups,it can be found that the atom fraction of N2 and N3 on the surface is the key factor for the water dispersibility of such oxidized intermediates.It can be envisioned that the more intermediates disperse in water,the less KOH is needed in KOH activation because of a full and nearly homogeneous contact of KOH with carbonaceous surfaces.
In addition,R-type and A-type intermediates have different structural characteristics.Compared with A-type,R-type intermediates retain the lamellar structure originating from green needle coke to some extent (Fig.3a).Calculated from XRD patterns,Rtype intermediates have larger Lcsize and thus have more statistic stacking carbonaceous layers than Atype.In fact,R-type and A-type intermediates both lose their (100)diffraction peaks,which means that their lamellar layers were broken into smaller pieces compared with that of GNC.Thus it is indicated that during the chemical transformation from GNC to intermediates,two main structural changes occur.One is that lamellar layers in precursor GNC were broken into smaller pieces and the other is that stacking numbers were decreased,especially for A-type intermediates.

Fig.2 High-resolution N1s XPS spectra of (a)GNC-A10,(b)GNC-A5,(c)GNC-R10,(d)GNC-R5 and (e)GNC.
3.2 Porous structure
The yields of the intermediates are listed in Table 2.Only 7% water dispersible components was obtained under the condition Vacid/mcoke=5.Increasing the oxidant concentration up to Vacid/mcoke=10%,57% water dispersible component was got.However,if CC was used as the starting material,only 7% was dispersible in water.So CC is not a suitable precursor for this method.
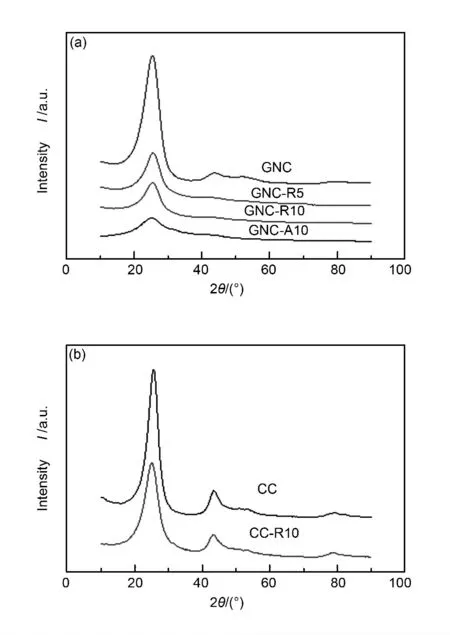
Fig.3 X-ray diffraction patterns of (a)GNC and its intermediates,(b)CC and CC-R10.
The N2adsorption/desorption isotherms of the nanoporous carbons measured at 77 K are shown in Fig.4a.The different amounts of KOH usage affected the appearance of the isotherms:the isotherms of GNC-0-3,GNC-R10-1,GNC-A10-1,GNC-R10-3 and GNC-A10-3 are type I,which indicates that the prepared carbons are typical microporous structure,whereas GNC-R10-5 and GNC-A10-5 are type IV,suggesting the coexistence of micropores and mesopores.The two samples made under KOH/Intermediate ratio of 5 show much larger N2adsorption capabilities than GNC-0-3,GNC-R10-3 and GNC-A10-3.As the consumption of KOH increased from 1-,to 3-to 5-times,the N2adsorption capability of the nanoporous carbons increased significantly.Additionally,many mesopores appeared during the activation.Fig.4b shows the pore size distributions of the GNC-derived nanoporous carbons.The GNC-0-3,GNC-R10-1 and GNC-A10-1 samples exhibit a microporous character with a dominant pore size less than 2 nm.GNC-R10-3 and GNC-A10-3 have more micropores than GNC-0-3,as well as some mesopores between 2 to 3.6 nm.GNC-R10-5 and GNC-A10-5 have much larger pore volumes and the size distributions are broadened to 6.8 nm.

Fig.4 (a)Adsorption/desorption isotherms of GNC-derived carbon samples and(b)pore size distribution calculated by DFT method.
To objectively present the porous structure,particularly the connectivity of pores,TEM images and inverse fast Fourier transform (IFFT)images of GNC-A10-3 are shown in Fig.5.Masking step and IFFT were taken place on the power spectrum to analyze the pores.The specific frequency regions in reciprocal space were chosen by ring-shaped mask patterns.After IFFT,images corresponding to pore size 1-2 and 2-4 nm could be obtained.The real space images were then transformed to binary pictures to observe the pore shapes more clearly.GNC-A10-3 is amorphous and abundant micropores as well as mesopores were created during the activation.From the transformed images,both micropores (1-2 nm)and mesopores (2-4 nm)are well inter-connected.These micropores provide abundant adsorbing sites for the ions,and inter-connecting mesopores facilitate the electrolyte ion diffusion in the material.Thus GNCA10-3 may have great potential as EDLC electrode material.
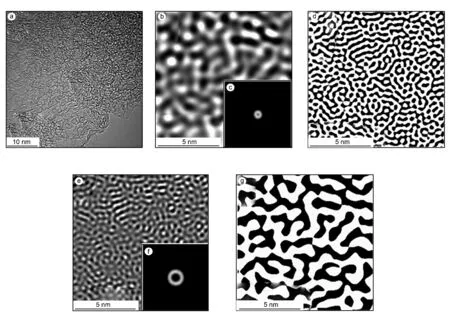
Fig.5 (a)TEM image of GNC-A10-3,(b)IFFT image of GNC-A10-3 after filtering of the power spectrum in(a),inset (c)is the filter pattern corresponding to pore size 1-2 nm and (d)is binary image of(b),(e)IFFT image of GNC-A10-3 after filtering of the power spectrum in (a),inset(f)is the filter pattern corresponding to pore size 2-4 nm and (g)is binary image of (e).
Combining the information given by Fig.4 and Table 3,it is true that both R-type and A-type intermediates promote the pore development.GNC-R10-3 and GNC-A10-3 have much higher SBET(as high as 3000 m2/g)than that of GNC-0-3.Even the intermediates prepared by using less amount of acid,nanoporous carbons prepared from GNC-R5 and GNC-A5 also possess better-developed porous structure (GNCR5-3 and GNC-A5-3)than the porous carbon sample by directly activation from GNC (GNC-0-3).However,nanoporous carbons derived from A-type and Rtype intermediates are different because all GNC-Aderived carbons have a little larger Vtotthan the corresponding GNC-R-derived ones.
As for the KOH dosage,with the help of oxidized intermediates,nanoporous carbons with high SBETwere obtained with less KOH consumption.Four kinds of carbons activated by using 3∶1 KOH consist of mainly micropore,but the carbons prepared from the same intermediates (GNC-R10 and GNC-A10)by using 5 ∶1 KOH (GNC-R10-5 and GNC-A10-5)are mesoporous and have higher Smes,about 1900 m2/g,than the carbons activated by less KOH(GNC-R10-3,-A10-3,-R10-1 and -A10-1).Therefore,activation with 3∶1 KOH is sufficient to generate micropores and more KOH acts as a pore-widening agent.On the other hand,porous structures of nanoporous carbons prepared by 1 ∶1 KOH (GNCR10-1 and GNC-A10-1)were similar with sample GNC-0-3,which indicates oxidized GNC are superior to GNC as activation precursors.
If the GNC was calcined in advance (i.e.,CC),no porous carbon was obtained even after activation by 3 ∶1 KOH (CC-0-3).The same results were obtained for CC-R-derived carbon (CC-R10-3),which indicates that this method is not suitable to precursors with relatively ordered structures.
Above results show the following points:the GNC derived intermediates are very helpful for preparing well-developed porous carbons by KOH activation,in particular,facilitating the connectivity of pores and decreasing the KOH dosage;A-type and Rtype intermediates have different effects in the porous structure development.
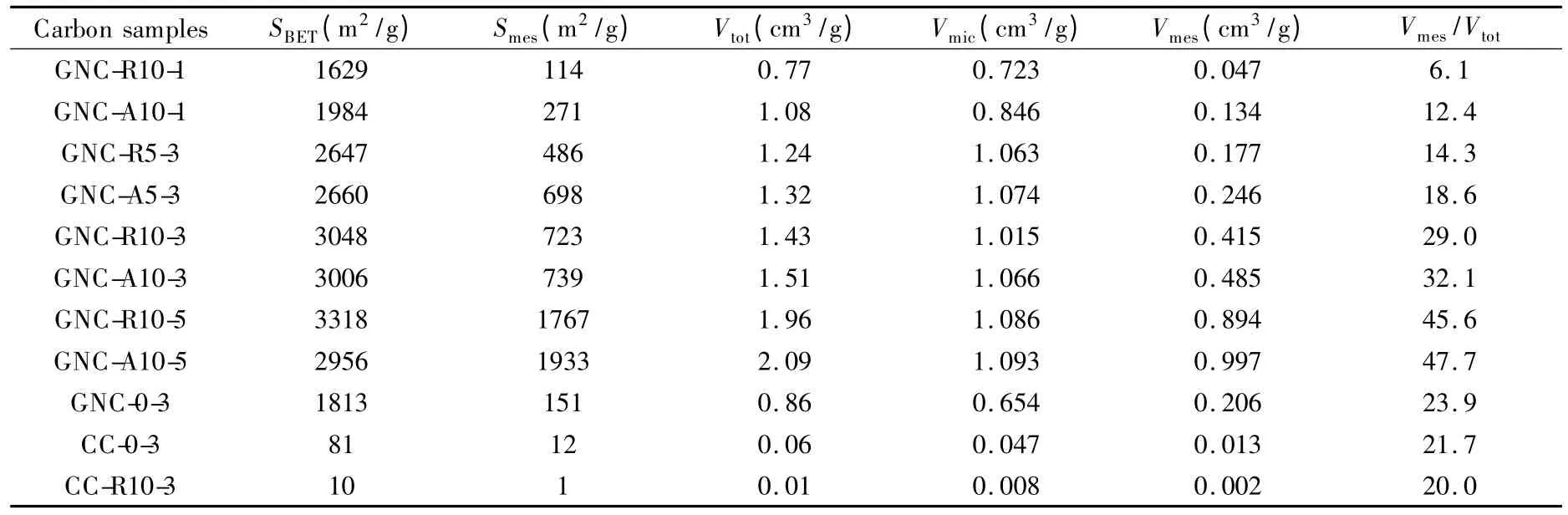
Table 3 BET surface area and pore structure parameters of GNC-derived porous carbons.
Based on the abovementioned discussion about the surface functional groups and the structural changes during the oxidation treatment,it can be concluded that the oxidation of GNC would definitely help the pore formation.Consequently,only a small amount of KOH is needed to develop the porous structure with surface area over 3000 m2/g as well as good pore connectivity.We attribute it to the coeffect of the hydrophilicity of intermediates’surface and the decrease of microcrystalline size.Chemisorbed nitrogen oxide groups are hydrophilic,but carbonaceous layers of these oxidized intermediates are hydrophobic.Since these intermediates possess both hydrophilic and hydrophobic groups,the force balance will determine their water-dispersibility.A-type owns less hydrophobic stacking layers and more hydrophilic groups,so it gives KOH more accesses to carbonaceous body than R-type.Consequently,the A-type derived porous carbons own better pore-connectivity and more mesoporous channels than the Rtype.
If the precursor is a calcined needle coke (CC),such oxidation cannot break its lamellar layer owing to its ordered structure.However,owing to the introduction of oxygen functional groups,the d002increases a little.
3.3 Electrochemical performance
Fig.6a and b show CV curves of the nanoporuos carbons in 6 mol/L KOH aqueous electrolyte at scan rates of 50 and 300 mV/s.All the samples present typical rectangular CV curve without obvious redox peaks except for GNC-0-3 and GNC-R10-3.The results demonstrate that GNC-A10-3 and GNC-A10-5 electrodes exhibit remarkable rate performances,because the inter-connecting mesopores can significantly reduce the ion-transport resistance.Additionally,Atype derived porous carbons exhibit better rate performance than those from R-type.Since the R-type intermediate has more stacking layers than the Atype,the tunnel length in the R-type derived porous carbon is longer.The short length pores make ions transport from mesopores to micropores quickly,which favors for high rate performance.
As shown in Fig.6c,GNC-A10-3 has the gravimetric specific capacitance about 340 F/g at a current density of 50mA/g.When the current density was increased to 40 A·g-1,the capacitance can still retain 248 F/g (76%).High specific capacitance is attributed to the abundant micropores,and the high rate performance is due to the fast movement of electrolyte ions through the connected mesopores.
The volumetric specific capacitances of R-type intermediates derived carbons are larger than GNC-Aderived carbons,as shown in Fig.6d.GNC-R10-3 possesses volumetric capacitance of 195 F/cm3at a current density of 50 mA/g,which is larger than that of GNC-A10-3.GNC-R10-5 also exhibited larger volumetric capacitance than GNC-A10-5 at current densities ranging from 0.05 to 40 A/g.Obviously,this is caused by the moderate pore volume of the GNC-R-derived carbons.
Both GNC-A10-3 and GNC-R10-3 electrode exhibit remarkable performance in long-term cycling tests,retaining 98% specific capacitance after 900 cycles at a current density of 100 mA/g.The Nyquist plots of GNC-0-3,GNC-A10-3 and GNC-R10-3 show capacitive behavior (Fig.7b).In the high frequency range,the semicircle is related to the charge transfer resistance,i.e.,the migration rate of ions at the interface between the solution and the electrode surface.The charge transfer resistance of GNC-0-3 is relatively higher than that of other two samples,indicating that narrow pore-size distribution and low surface area are not favorable to the access of ions in KOH aqueous electrolyte.The results of EIS show the improved electrochemical capacitive properties and the suitability of the GNC-A10-3 and the GNC-R10-3 as the electrode materials for EDLCs.
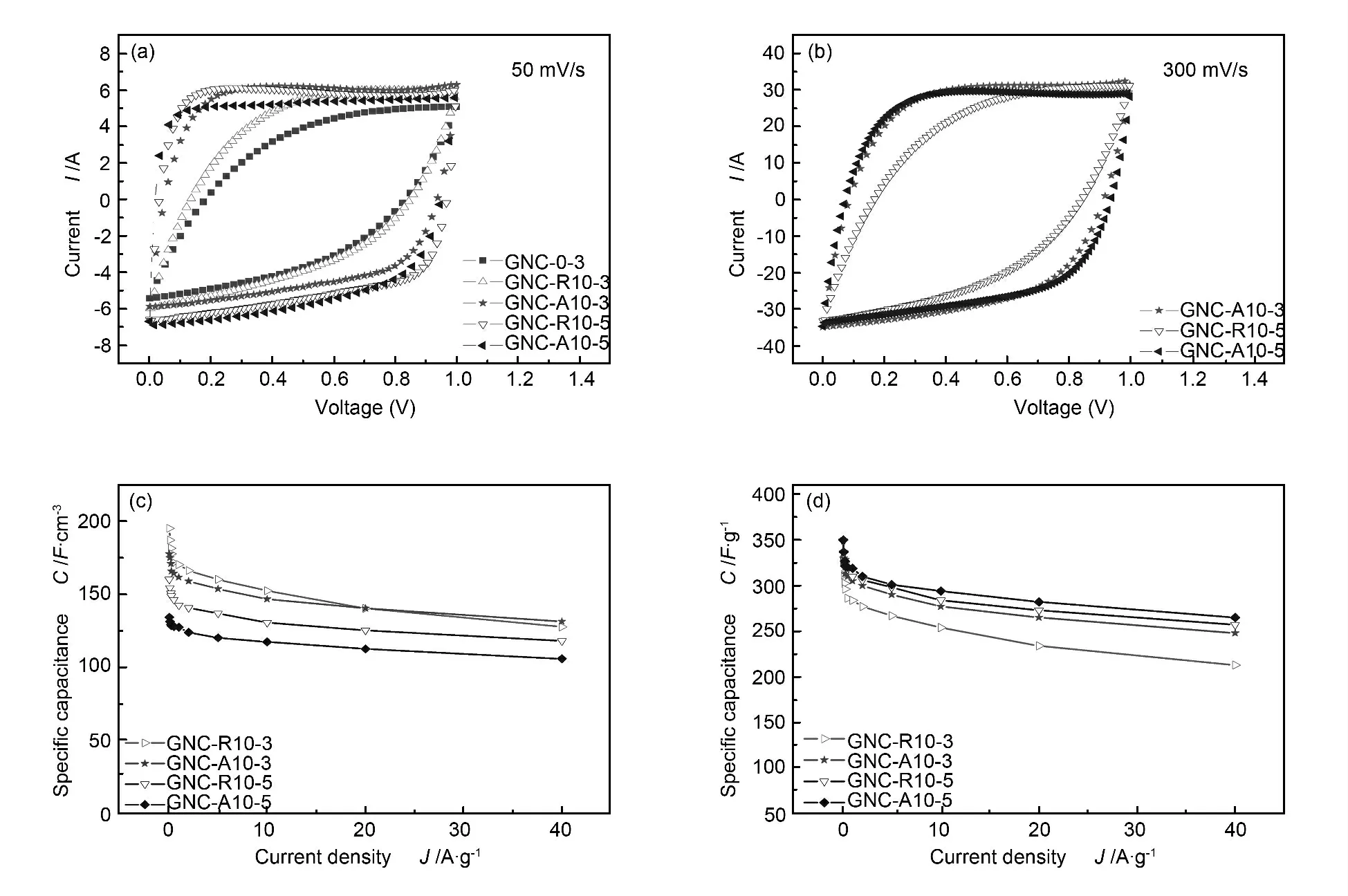
Fig.6 (a)CV curves of GNC-derived activated carbons at 50 mV/s,(b)CV curves of GNC-A10-3,GNC-R10-5 and GNC-A10-5 at 300 mV/s,(c)gravimetric specific capacitance as a function of the current density,(d)volumetric specific capacitance as a function of the current density.
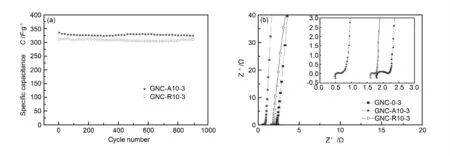
Fig.7 (a)Cycle life data of GNC-R10-3 and GNC-A10-3 at a current density of 100 mA/g,(b)complex-plane impedance plots of the GNC-0-3,GNC-R10-3 and GNC-A10-3 electrodes.
4 Conclusions
For oxidized intermediates prepared from green needle coke,the amount of hydrophilic groups on their surfaces determines their water dispersibility.Such surface properties together with the intermediates’structure characteristics affect the final pore structure of the resultant nanoporous carbons.Both hydrophilic and hydrophobic oxidized intermediates derived from green needle coke are helpful for the development of pore structure which facilitates the balance of rate performance and specific capacitance of electric double layer capacitors (EDLCs).In 6 mol/L KOH electrolyte,sample GNC-A10-3 has the best performance as EDLC electrodes in keeping a balance of gravimetric specific capacitance of 248 F/g at a current density of 40 A/g with excellent rate performance expressed in the capacitance retention C40/0.05of 76%.Sample GNC-R10-3 has great potential to improve the volumetric specific capacitance.
[1]Inagaki M,Konno H,Tanaike O.Carbon materials for electrochemical capacitors[J].J Power Sources,2010,195(24):7880-7903.
[2]Conway BE.Electrochemical Supercapacitors:Scientific Fundamentals and Technological Applications[M].New York:Kluwer Academic/Plenum,1999.
[3]Wang L,Morishita T,Toyoda M,et al.Asymmetric electric double layer capacitors using carbon electrodes with different pore size distributions[J].Electrochim Acta,2007,53(2):882-886.
[4]Morishita T,Soneda Y,Tsumura T,et al.Preparation of porous carbons from thermoplastic precursors and their performance for electric double layer capacitors[J].Carbon,2006,44(12):2360-2367.
[5]Chmiola J,Yushin G,Gogotsi Y,et al.Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer[J].Science,2006,313(5794):1760-1763.
[6]Gao W,Wan Y,Dou Y,et al.Synthesis of partially graphitic ordered mesoporous carbons with high surface areas[J].Adv Energ Mater,2011,1(1):115-123.
[7]Fuertes AB,Lota G,Centeno TA,et al.Templated mesoporous carbons for supercapacitor application[J].Electrochim Acta,2005,50(14):2799-2805.
[8]Xing W,Qiao S,Ding R,et al.Superior electric double layer capacitors using ordered mesoporous carbons[J].Carbon,2006,44(2):216-224.
[9]Xia K,Gao Q,Jiang J,et al.Hierarchical porous carbons with controlled micropores and mesopores for supercapacitor electrode materials[J].Carbon,2008,46(13):1718-1726.
[10]Jin J,Tanaka S,Egashira Y,et al.KOH activation of ordered mesoporous carbons prepared by a soft-templating method and their enhanced electrochemical properties[J].Carbon,2010,48(7):1985-1989.
[11]Vilaplana-Ortego E,Lillo-Ródenas MA,Alcañiz-Monge J,et al.Isotropic petroleum pitch as a carbon precursor for the preparation of activated carbons by KOH activation[J].Carbon,2009,47(8):2141-2142.
[12]Mora E,Blanco C,Pajares JA,et al.Chemical activation ofcarbon mesophase pitches[J].J Colloid Interface Sci,2006,298(1):341-347.
[13]Ramos-Fernandez JM,Martínez-Escandell M,Rodríguez-Reinoso F.Production of binderless activated carbon monoliths by KOH activation of carbon mesophase materials[J].Carbon,2008,46(2):384-386.
[14]Contreras MS,Páez CA,Zubizarreta L,et al.A comparison of physical activation of carbon xerogels with carbon dioxide with chemical activation using hydroxides[J].Carbon,2010,48(11):3157-3168.
[15]Seredych M,Hulicovajurcakova D,Lu G,et al.Surface functional groups of carbons and the effects of their chemical character,density and accessibility to ions on electrochemical performance[J].Carbon,2008,46(11):1475-1488.
[16]Cardoso B,Mestre AS,Carvalho AP,et al.Activated carbon derived from cork powder waste by KOH activation:preparation,characterization,and VOCs adsorption[J].Ind Eng Chem,2008,47(16):5841-5846.
[17]Mitani S,Lee S-I,Saito K,et al.Activation of coal tar derived needle coke with K2CO3into an active carbon of low surface area and its performance as unique electrode of electric doublelayer capacitor[J].Carbon,2005,43 (14):2960-2967.
[18]Lu C,Xu S,Wang M,et al.Effect of pre-oxidation on the development of porosity in activated carbons from petroleum coke[J].Carbon,2007,45(1):206-209.
[19]Qiao W,Yoon S-H,Mochida I.KOH activation of needle coke to develop activated carbons for high-performance EDLC[J].Energy Fuels,2006,20(4):1680-1684.
[20]Wang J,Chen M,Wang C,et al.Preparation of mesoporous carbons from amphiphilic carbonaceous material for high-performance electric double-layer capacitors[J].J Power Sources,2011,196(1):550-558.
[21]Esumi K,Takahashi S,Sugii H,et al.Characterization of organo-carbonaceous gel[J].Carbon,1993,31 (8):1303-1306.
[22]Tateishi D,Esumi K,Honda H.Preparation of organo-carbonaceous gel[J].Carbon,1992,30(6):944-945.
