Analysis of bromate and bromide in drinking water by ion chromatography-inductively coupled plasma mass spectrometry
2014-11-26HANMeiJIANaLIKeZHAOGuoxingLIUBingbingLIUShenghuaLIUJie
HAN Mei, JIA Na, LI Ke, ZHAO Guo-xing, LIU Bing-bing, LIU Sheng-hua, LIU Jie
Institute of Hydrogeology and Environmental Geology, Zhengding, Hebei 050803, China
Abstract: A method for the detection of bromate and bromide in drinking water by ion chromatography-inductively coupled plasma mass spectrometry (IC-ICP-MS) was developed.The optimized conditions of IC including pH and concentration of the effluent were studied.The results showed that the above two species of bromine were baseline separated within nine minutes under the optimized conditions. The detection limits (S/N=3) of bromate and bromide were 0.23 and 0.12 μg/L, respectively. The RSD (n=6) of the peak areas was 1.2%-3.5%. The method has been successfully applied to the determination of the type bromide in drinking water samples. The recoveries were 95%-109%. The method can be used for the regular analysis of bromate and bromide in real drinking water samples.
Keywords: Ion chromatography (IC); Inductively coupled plasma mass spectrometry (ICPMS); Analysis; Bromate; Bromide
Introduction
In the past decades, there attention has increasingly been paid has been an increase of attention to the quality of drinking water. It is well known that much of drinking water passes through treatment processes such as filtration, deionization,reverse osmosis, and ozonation, to ensure its quality (Y Kurokawa et al. 1990; Blsz Divjak et al.1999). Ozonation is a disinfection method that can destroy microorganisms, reduce the color, odor and total organic carbon, etc (J T Creed et al.1996). However, during the ozonation procedure,because bromide occurs naturally in source water and may not be entirely removed during the treatment processes, bromide is oxidized to hypobromous acid and hypobromite, which are further oxidized to bromate (Oscar Quinones et al.2006). In drinking water, the main forms of bromine species are bromate and bromide ions(YANG Zhen-Yu and DENG Xiao-Jun, 2009;YING Bo et al. 2006). The presence of bromate in drinking water has attracted much attention because it is an animal carcinogen (Stalberg O et al.2002), The maximum contaminant level of bromate allowed by the European Union is 10 μg/L (WANG Ying-Feng et al. 2008). But as it is almost harmless to humans due to the presence of bromide, it is very important to know the concentration of bromate and bromide in drinking water.
Generally, techniques such as ion chromategraphy could be used for the determination of bromate and bromide in drinking water (WANG Ying-Feng et al. 2008; LI Shu-Min et al. 2006;SHEN Jin-can et al. 2005). However the application of ion chromatography is limited because of the one fold method of qualitative and quantitative sampling. Also, this technique is susceptible to the interference of chlorite and chloridion. As a result,in recent years, ion-chromatography has been commonly employed alongside inductively coupled plasma mass spectrometry (IC-ICP/MS) for the analysis of bromate and bromide (J D Keith et al.2006; SHI Hong and Craig Adams, 2009; F Sacher et al. 1999).
In this study ion-chromatography was utilized to selectively separate the compounds of interest from the drinking water. Detection was accomplished by simultaneously measuring atomic bromine isotopes using inductively coupled plasma mass spectrometry. A method for the detection of bromate and bromide in drinking water by ion chromatography-inductively coupled plasma mass spectrometry (IC-ICP/MS) was developed. A new type of chromatographic column was adopted and the separation of bromate and bromide by IC was studied.
1 Experiment
1.1 Reagents
All reagents used were of analytical reagent grade unless specified. The standard supplies of bromate and bromine were bought from China Metrology Academy of Sciences. The stock solutions were stored in refrigerator at 4 ,℃ and the standard solutions were prepared daily by serial dilution of the stock solution prior to use. The running buffer solution of 30 mmol/L of NH4NO3(pH=8.0) was prepared by dissolving analytical grade ammonium nitrate (NH4NO3), which were purchased from Tianjin Reagents Co. Ltd. (Tianjin,China), in Milli-Q water. All solutions were filtered through a 0.22 μm membrane filter before use. All experiments were performed at temperature 20-22 ℃ regulated by an air conditioner,and water used in this experiment is Milli-Q water(18.2 MΩ) prepared by a Milli-Q equipment(Millipore, Bedford, USA). The super-pure grade of nitric acid (HNO3) and ammonium hydroxide(NH3·H2O) were purchased from Tianjin Reagents Co. Ltd. (Tianjin, China).
1.2 IC-ICP/MS system
The IC-ICP/MS system consists of a Dionex IC-2 500 (Dionex Technologies, USA) system and a Bruck 820 ICP-MS (Bruck Technologies, USA).The ion chromatography system equipped with a GP50 gradient pump was connected to a 250 mm ×4 mm (IonPac AS19) analytical column and 50 mm × 4 mm (AG19) guard column. An 8-min isocratic elution with 30 mmol/L ammonium nitrate was utilized for all chromatographic separations. The eluent from the IC column was introduced directly into the spray chamber of the ICP/MS. A time resolved elemental signal was obtained for bromine masses 79 m/z, which was used for quantitation as it yielded a higher signal to noise ratio and improved sensitivity. The time resolved signal was converted into chromatographic data and analytes quantitated from extrapolation of peak areas against those of calibration standards. Separation and detection parameters are outlined in Table 1 and Table 2.Bromate and bromide showed excellent chromategraphic signals in water resolution samples using this method.
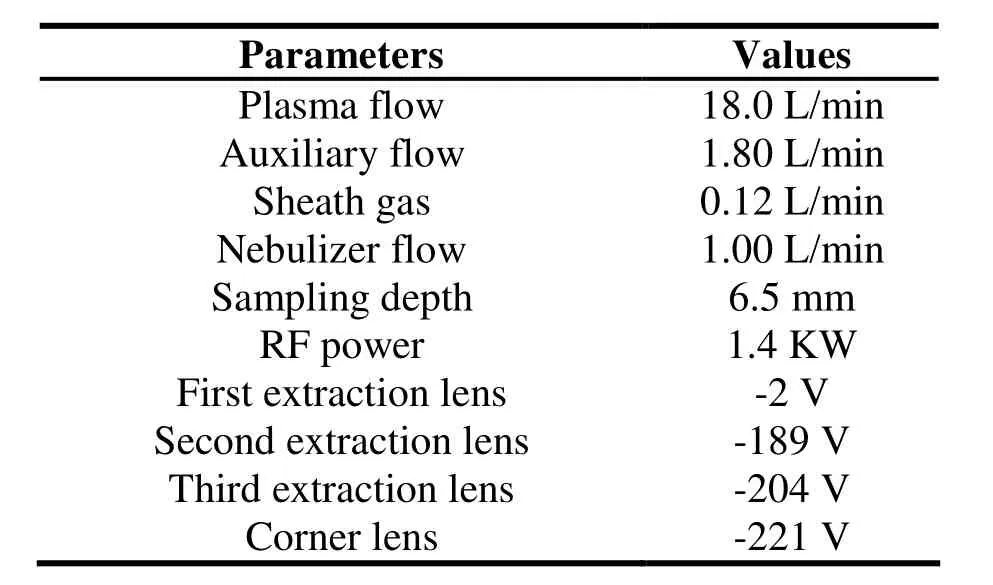
Table 1 Detection parameters of ICP-MS
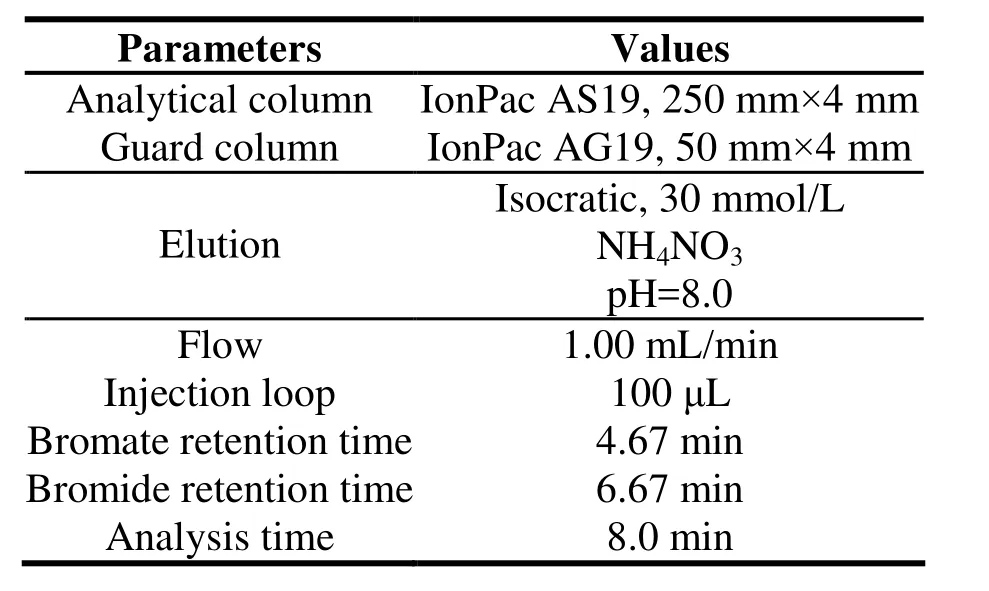
Table 2 Separation parameters of IC
2 Results and discussion
2.1 Optimization of IC-ICP/MS conditions for the analysis of bromate and bromide
2.1.1 Selection of analytical column
In the process of drinking water disinfection,the bromate background levels were generally low,due to the lower dosage of ozone. In order to achieve effective separation of different forms and low detection limits, a high capacity analysis column, such as IonPac AS19, was chosen for this study.
2.1.2 Option of eluent and its concentration
The solution of sodium salt, such as sodium carbonate/sodium bicarbonate and sodium hydroxide can be utilized to elute bromate and bromide in the ion chromatography system.However, sodium salt can jam the sampler cone and skimmer cone, thereby changing the ionizing condition. Therefore, a volatile solution is usually used as eluent in IC-ICP/MS system. In this study, ammonium nitrate solution was selected,since it has certain advantages including absence of corrosions to the simple cone and complete atomization.
The pH and concentration of ammonium nitrate solution were optimized as they can affect the separation migration time of analyses drastically.The tests with 10, 15, 20, 30 and 40 mmol/L ammonium nitrate were conducted for the eluent concentration. The peak shapes of bromate and bromide were improved and the migration times became shorter with increasing eluent concentration (Fig. 1). Considering the stability of base line and the influence of sampler/skimmer cone, 30 mmol/L of ammonium nitrate solution was selected as the running eluent solution.
The relationship between migration times according to resolution and eluent pH was studied.As shown in Fig. 2, the peak shape of bromide improved with an increasing pH, and the migration time was mostly improved when the pH is over 6.0.We also tested eluent with a pH higher than 8.0,but this produced a large signal fluctuation, which was possibly due to the presence of high concentration of ammonium hydroxide. Finally, pH=8.0 was chosen to be the optimum pH value for bromate and bromide separation. The optimal separation effect for IC-ICP/MS system was shown in Fig. 3.
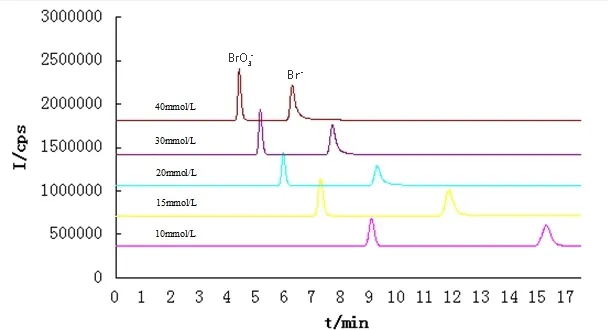
Fig. 1 Separation effect of different eluent concentration

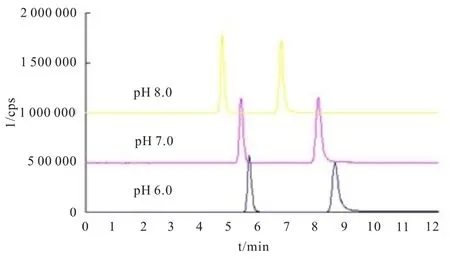
Fig. 2 Separation effect of different pH
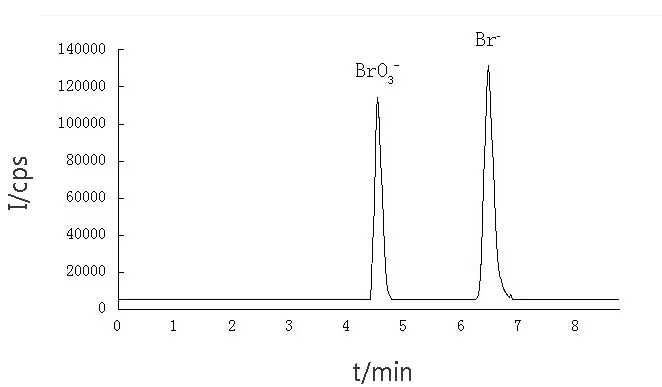
Fig.3 IC-ICP-MS chromatogram of a mixed solution of BrO3- and Br-(10 μg·L-1)
2.2 Method parameters
At above optimum conditions, bromate and bromide were baseline separated within 7.5 minutes. The same experiment was repeated six times, and the RSD (relative standard deviation,n=6) of peak areas was calculated to be a range of 1.2%-3.5% for bromate and bromide, and the results were listed in Table 3. The detection limit(3σ, the concentration necessary to yield a net signal equal to three times the standard deviation of the background) was calculated with counts 0.23 μg/L and 0.12 μg/L for bromate and bromide,respectively. A calibration curve was constructed from standards in reagent water with a linear range from 1.0 μg/L to 100.0 μg/L for both bromate and bromide. The linearity (R2) of all calibration curves exceeded 0.9995.
2.3 Method recovery
Mixed standard solutions of low, medium and high concentrations of bromate and bromide were added into background samples, and tested by the method established. The recovery rates were listed in Table 4. The recovery rates of bromate and bromide are 95%-102% and 97%-109% across the concentration range, respectively.
2.4 Sample analysis
Table 5 illustrates the test results of bromate and bromide in different drinking water. The results by IC-ICP/MS for five samples were 31.1 μg/L, 59.0 μg/L, 49.6 μg/L, 25.5 μg/L, 44.6 μg/L,respectively. It was coincident to the values by ion chromatography-electrical conductivity detector,which were 32.3 μg/L, 58.2 μg/L, 50.3 μg/L, 24.2 μg/L, 43.5 μg/L, respectively. The results of this method agree well with the ion chromatographyelectrical conductivity detector, so the method is reliable.

Table 3 Precision of the method (n=6)
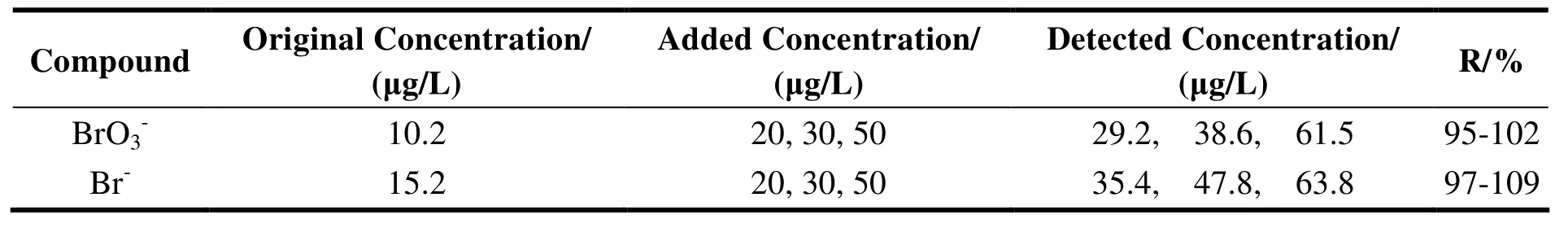
Table 4 Recoveries of bromate and bromide from drinking water sample at three spiked levels
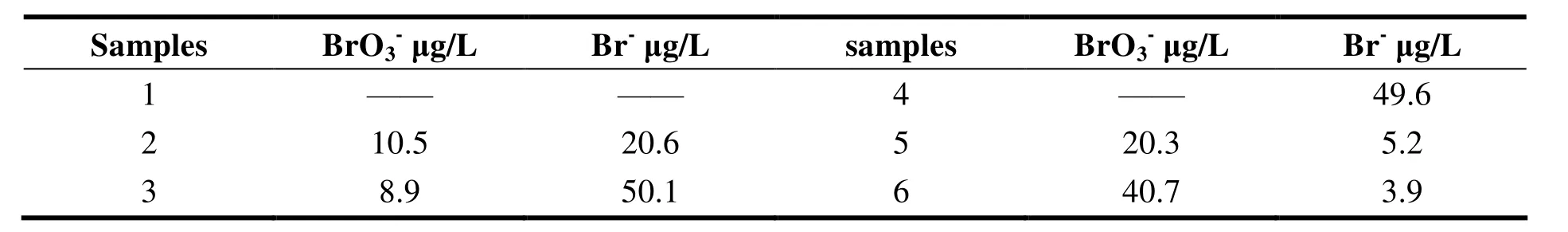
Table 5 Contents of bromine in drinking water
3 Conclusions
The method presented here provided a fast and sensitive technique for the analysis of bromate and bromide in drinking water. Bromate and bromide are more commonly analyzed by IC with an electro-conductivity detector or colorimetric detection. However, these methods have limited sensitivity and are prone to show false positives due to matrix interferences typical in natural water samples. Instead we studied a method which has been previously published, to analyze bromate and bromide in water using IC-ICP/MS. A 7.5 minute isocratic elution of bromate and bromide combined with the high sensitivity and selectivity of the ICP-MS detector for elemental bromine, yielded a simple yet robust method for analysis of bromate and bromide at trace levels. The large sample loading capacity of the IC column allows for the injection of increased sample volumes to achieve low detection limits. Excellent recovery and reproducibility was achieved.
Acknowledgements
This study is supported by Basal science research fund from Institute of Hydrogeology and Environmental Geology (Grant No. SK201406).
杂志排行
地下水科学与工程(英文版)的其它文章
- Stability assessment and risk analysis of aboveground river in lower Yellow River
- Establishment of an assessment method for site-scale suitability of CO2 geological storage
- Comparison of hydrogeological characteristics between the Sanjiang Plain and the Amur River Basin
- Study of moisture migration in clay soils considering rate of freezing
- Characteristics-based classification research on typical petroleum contaminants of groundwater
- Sustainable utilization measures of groundwater resources in Beijing
