低于300°C时两种氧化铈材料对稀燃阶段NO x存储性能的影响
2014-10-18朱金鑫沈美庆吕良方王建强
朱金鑫 沈美庆,2 吕良方 王 军,* 王建强,*
(1天津大学化工学院,绿色合成与转化教育部重点实验室,天津 300072;2天津大学内燃机国家重点实验室,天津 300072)
1 Introduction
Lean NOxtrap(LNT)catalysts,also known as NOxstorage reduction(NSR)catalysts,are one of the most effective technologies for removal of NOxemission from lean-burn diesel and gasoline engines.1-5Atypical LNT catalyst contains a noble metal component(NM,such as Pt,Pd,and Rh),a NOxtrapping component(alkali-or alkaline-earth oxide)and support oxide(e.g.,Al2O3).The NSR mechanism is generally assumed to include five steps:NO oxidation(NO oxidation to NO2over precious metal);NOxsorption(NOxsorption on the surface of storage material in the formation of nitrites/nitrates);reductant evolution;NOxrelease(NOxrelease from the nitrite/nitrate sites)and NOxreduction to N2.6,7
One of the primary challenges facing LNT catalysts is the inefficient NOxtrapping capacity at low temperature.8It is one of the reasons why the LNT has not been widely used in diesel enginepowered vehicles.The exhaust gas temperature is usually somewhat lower than the operating temperature window normally provided by the trap.For example,the exhaust-gas temperature of a light-duty diesel vehicle driving under the Federal test procedure(FTP)is typically less than 200°C,while the temperature window of operation of conventional LNT catalysts ranges from 200 to 500°C.9
However,most of studies about LNT catalysts are concentrated on Pt-BaO/Al2O3model catalysts,which show low NOxstorage efficiency at low temperature.10Hence,NOxremoval efficiency for LNT technology at low temperature needs to be enhanced to meet increasingly stringent emission regulations,which requires the researchers to develop some efficient low temperature NOxtrapping materials.
Some recent studies have showed that the support oxide is not only for dispersing noble metal and NOxstorage components,but is of great role for participating in NOxtrapping.10It is known that CeO2material shows the oxygen storage capacity,11,12due to the existence of available surface active oxygen species below 500°C.Although it is reported that addition of CeO2to LNT catalysts can improve the NOxstorage capacity at low temperature13-16and the sulfur-resistance.17,18However,sintering of CeO2at high temperatures is also noted.19In the diesel engine,the NOxemission is serious and presents at low exhaust temperature.20Thus,the application of CeO2in LNT catalysts is necessary to reduce the NOxemission via improving NOxtrapping capacity.However,the roles of physical-chemical properties of CeO2oxides in NOxtrapping capacity are still required to study intensively.Moreover,the influencing factors for NOxtrapping over CeO2materials are not clear.Finally,it needs to investigate whether metal support interaction(MSI)exists in Ce-based LNT catalysts and has influence on the NOxtrapping process.
In this work,two types of CeO2-X(X=S,I)mechanically mixed with Pt/Al2O3(PA)used as LNT catalysts have been investigated.X-ray diffraction(XRD),BET surface area measurement,and scanning electron microscope(SEM)were used to characterize the physical properties.X-ray photoelectron spectroscopy(XPS)and H2temperature-programmed reduction(H2-TPR)were employed to study the surface Ce3+concentration and the surfaceactive oxygen species.In-situ diffuse reflectance infrared Fourier transform spectroscopy(In-situ DRIFTS)was applied to analyze the surface adsorbed NOxspecies.By means of these technologies,the influencing factors for NOxtrapping process over Ce-based LNT catalysts were proposed.
2 Experimental
2.1 Sample preparation
2%(mass fraction,w)Pt was loaded on γ-Al2O3by incipient wetness impregnation method,using Pt(NO3)2solutions.The impregnated materials were dried in air overnight at 100°C and subsequently calcined in air at 500°C for 5 h to obtain the fresh Pt/Al2O3samples.Two commercial CeO2(S and I)materials were chosen,which were donated as FCS and FCI.In addition,these two CeO2materials were hydrothermally aged in 10%(volume fraction,φ)steam/air at 800 °C for 5 h,which are named as ACS and ACI,respectively.Finally,the CeO2oxides were mechanically mixed with PAto get the lean NOxtrapping catalysts,which were denoted as PA+CeO2.Besides,0.5%(w)Pt/CeO2sample was also prepared by incipient wetness impregnation method using Pt(NO3)2solutions and the aged CeO2-X supports,which were abbreviated as PACS and PACI.γ-Al2O3and CeO2were from Aldrich(U.S.).
2.2 Reaction tests and characterization
For lean NOxtrapping activity tests,0.5 g catalysts(0.2 g PA and 0.3 g CeO2)were mechanically mixed with 1.5 g quartz sand.The total flow rate was 1 L·min-1,with the space velocity of 30000 h-1.A NICOLET 380 Fourier transform infrared(FTIR)equipped with a 2-m gas cell was used to detect the outlet concentrations of NO,NO2,NH3,N2O,CO,CO2,and H2O(g).Prior to the evaluation,the samples were reduced at 450 °C in 7.5%(φ)CO/10%(φ)CO2/5%(φ)H2O/N2balance for 5 min.Then,cooling down to each testing temperature(150/200/250/300°C),the NOxtrapping tests were conducted in 5×10-4(φ)NO/7.5%(φ)O2/10%(φ)CO2/5%(φ)H2O/N2balance.

where,φ(NOx,inlet)is the inlet concentration of NOx(5×10-4(volume fraction,φ));φ(NOx,outlet)is the outlet concentration of NOx;L is the total flow rate(1 L·min-1);Vmis the molar volume of gas(22.4 L·mol-1);t is NO/O2adsorption time(40 min);mcatis the mass of catalysts(0.5 g).

where,φ(NOx,equilibrium)is the outlet concentration of NO2after adsorption equilibrium;φ(NOx,inlet)is the inlet concentration of NOx(5×10-4(φ)).
The powder X-ray diffraction(XRD)patterns were performed at room temperature with a Bruker D8 Focus,operating at 40 kV and 40 mA equipped with nickel-filtered Cu Kαradiation(λ=0.154056 nm),ranging from 10°to 90°with a step size of 0.02°.Crystal sizes of CeO2were calculated by JADE 5.0.
The specific surface areas and porous structures of the samples were measured by N2adsorption at 77 K with a Tristar 3000 Micromeritics apparatus.The morphologies of the fresh and aged CeO2were characterized by SEM,which was coated with Au-Pd and measured on a Hitachi S 4800 field emission microscope.
For H2temperature-programmed reduction(H2-TPR),0.2 g CeO2materials were treated at 500°C for 0.5 h in N2,cooling down to room temperature(RT)under the same gas condition.Then the 5%(φ)H2/N2was introduced.The catalysts were heated from room temperature to 900 °C at 10 °C·min-1.Thermal conductivity detector(TCD)was used.For the mixture of Pt/A2O3and CeO2,the mass ratio of PA and CeO2was the same as NOxtrapping activity tests,and the pretreatment was at 450°C in N2for 0.5 h.
The X-ray photoelectron spectroscopy(XPS)experiment was carried out on a PHI-1600 ESCAsystem by employing an Mg Kαsource operating at 250 W.The binding energy was calibrated internally by the carbon deposit C 1s binding energy(EB)at 284.8 eV.
In-situ DRIFTS was performed on NICOLET 6700 FTIR equipped with mercury cadmium telluride(MCT)detector conducting at a resolution of 1 cm-1and 10 scans for each spectrum.Powder samples were treated at 500 °C in 10%(φ)O2/N2balance for 30 min.Samples were cooled down to 300°C in N2purge for 30 min.Then the background was collected at 300 °C.Finally,5×10-4(φ)NO/7.5%(φ)O2/N2was introduced for NO/O2adsorption for 40 min.
3 Results and discussion
3.1 Lean NO xtrap performance
Lean NOxtrapping capacity oftwo(PA+CeO2)catalysts at150-300°Cwas calculated by Eq.(1).The results are displayed in Table1.The NOxtrapping capacity increases as the temperature rising.Compared with the fresh samples,the aged ones show lower NOxtrapping capacity.No matter for the fresh or aged catalysts,PA+CeO2-S samples present higher storage capacity than PA+CeO2-I.In addition,the decrease degree in trapping capacity after aging for PA+CeO2-S is lessthan thatfor PA+CeO2-I.

Table 1 NO xtrapping capacity of PA+CeO2-X(X=S,I)at each temperature during 40 min tests
The NO conversion for PA+CeO2-X(X=S,I)was calculated by Eq.(2).As Fig.1 shown,NO conversion of these samples is enhanced with the temperature elevating.After the samples were hydrothermally treated,their NO conversion decreases to various degrees.Furthermore,it is obvious that NO conversion of PA+CeO2-S is higher than that of PA+CeO2-I.
As a result,higher NOxtrapping capacity of PA+CeO2-S can be attributed to their higher NO conversion to some extent.For these two types of Ce-based NSR catalysts,the difference is that two different CeO2oxides were employed,whereas Pt/Al2O3components were completely identical.The reasons for different NOxtrapping performance between the two catalysts are probable the intrinsic physical-chemical properties of CeO2-X(X=S,I)or the different interaction between Pt/Al2O3and CeO2-X(X=S,I).Thus,physical textures and chemical properties are explored firstly as follows.
3.2 Structural properties and morphology of two types of ceria

Fig.1 NO conversion for fresh and aged PA+CeO2-X(X=S,I)samples at different temperatures

Table 2 Structural properties of the two types of CeO2
The structural properties of two different CeO2oxides are listed in Table 2.Fresh CeO2-S presents almost twice surface area as large as fresh CeO2-I.Likewise,fresh CeO2-S shows richer pore structure than fresh CeO2-I.After hydrothermal ageing treatment,the surface area of CeO2-I drops significantly to only 1.7 m2·g-1,while CeO2-S still exhibits higher surface area.The decrease degree of surface area for CeO2-S is 60.2%,which is lower than 97.7%for CeO2-I.It indicates that CeO2-S has relatively high hydrothermal stability.
Furthermore,the XRD patterns are displayed in Fig.2.All CeO2oxides show the same peak positions and cubic CeO2structure.These two fresh CeO2oxides exhibit similar full width at half maximum(FWHM),which are also verified by the comparable cell sizes of CeO2(Table 2).Nevertheless,after the samples were hydrothermally treated,CeO2-I sample presents smaller FWHM than CeO2-S.The cell size of the aged CeO2-I sample is 49.7 nm,which is far larger than that of the aged CeO2-S.These results suggest that hydrothermal treatment leads to the much severer sintering of CeO2-I particles.
In addition,the morphologies of these two CeO2oxides are described in Fig.3.The particle shape of fresh CeO2-S sample is globular,while fresh CeO2-I is flocculent and tabular.After the two types of CeO2oxides were hydrothermally treated,the particle morphology of CeO2-S samples does not show distinct changes.However,the aged CeO2-I samples present remarkable particle growth.
Therefore,the unique texture of CeO2-S oxides makes them possess excellent physical properties.In detail,CeO2-S oxides have much more rich physical structure(including higher surface area,rich porous texture,and unique globular particle shape)and higher hydrothermal stability than CeO2-I.
3.3 Chemical properties of two types of ceria
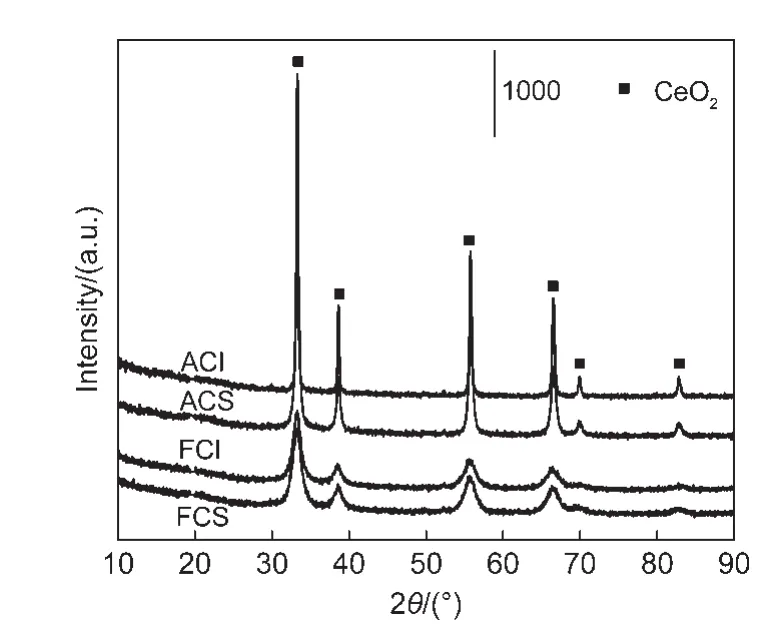
Fig.2 X-ray diffraction(XRD)patterns for the two different CeO2-X samples

Fig.3 Scanning electron microscope(SEM)images for the two different CeO2-X samples


Fig.4 X-ray photoelectron spectroscopy(XPS)spectra of Ce 3d on two different CeO2-X samples
The Ce 3d spectra for different CeO2oxides are shown in Fig.4.The peaks at v0,v′,u0,u′are attributed to Ce3+,and peaks at v,v″,v‴,u,u″,u‴ are ascribed to Ce4+.21By calculation,the surface Ce3+concentrations on fresh CeO2-S,aged CeO2-S,fresh CeO2-I,and aged CeO2-I samples are 35.3%,29.1%,29.3%,and 23.4%,respectively.It is worth noting that the fresh CeO2-S has the highest amount of surface Ce3+.Moreover,hydrothermal treatment leads to the decrease in Ce3+concentration on these samples.It is known that there is an oxygen vacancy on a Ce3+cation,while an active oxygen on a Ce4+cation.22So the NOxtrapping process can be described as follows:where,Ce3+-Δ represents an oxygen vacancy on Ce3+cations,and Ce4+-O*stands for an active oxygen on Ce4+cations.Ce3+-Δ can trap NOx(Eqs.(3)and(4)),22which is further oxidized by Ce4+-O*to cerium nitrite or nitrate(Eqs.(5)and(6)).Moreover,the formation of nitrates on ceria is a consecusive reaction:adsorption of NO to form nitrite species,followed by an oxidation to form nitrate species.23Hence,Ce3+plays an important role in the NOxtrapping process.Another reason for better NOxadsorption ability of CeO2-S catalysts is that these samples have higher Ce3+concentration.The relationship between NOxtrapping capacity at 250°C with the surface Ce3+contents is shown in Fig.5.It can be seen that the NOxstorage capacity presents linear increasing tendency with surface Ce3+contents increasing,which further verifies the role of Ce3+in NOxtrapping process.
In order to investigate the surface active oxygen on different CeO2oxides,H2-TPR is conducted and the results are shown in Fig.6.
As shown in Fig.6,the fresh CeO2oxides present mainly two reduction peaks around 500 and 800°C(Fig.6a),which are ascribed to the surface oxygen and bulk oxygen reduction,respectively.24By integrating these peak areas,the amount of surface oxygen on the fresh CeO2-S and CeO2-I oxides is similar.Differently,the surface oxygen on the fresh CeO2-S can be reduced at relatively lower temperature.After ageing treatment,the quantity of the surface oxygen on the aged CeO2-X(X=S,I)oxides decreases.In addition,the reduction of surface oxygen of the aged CeO2-I shifts to higher temperature at 567°C,which indicates that the surface oxygen becomes hard to be reduced.

Fig.5 Relationship between NO xstorage capacity at 250°C and surface Ce3+contents by XPS
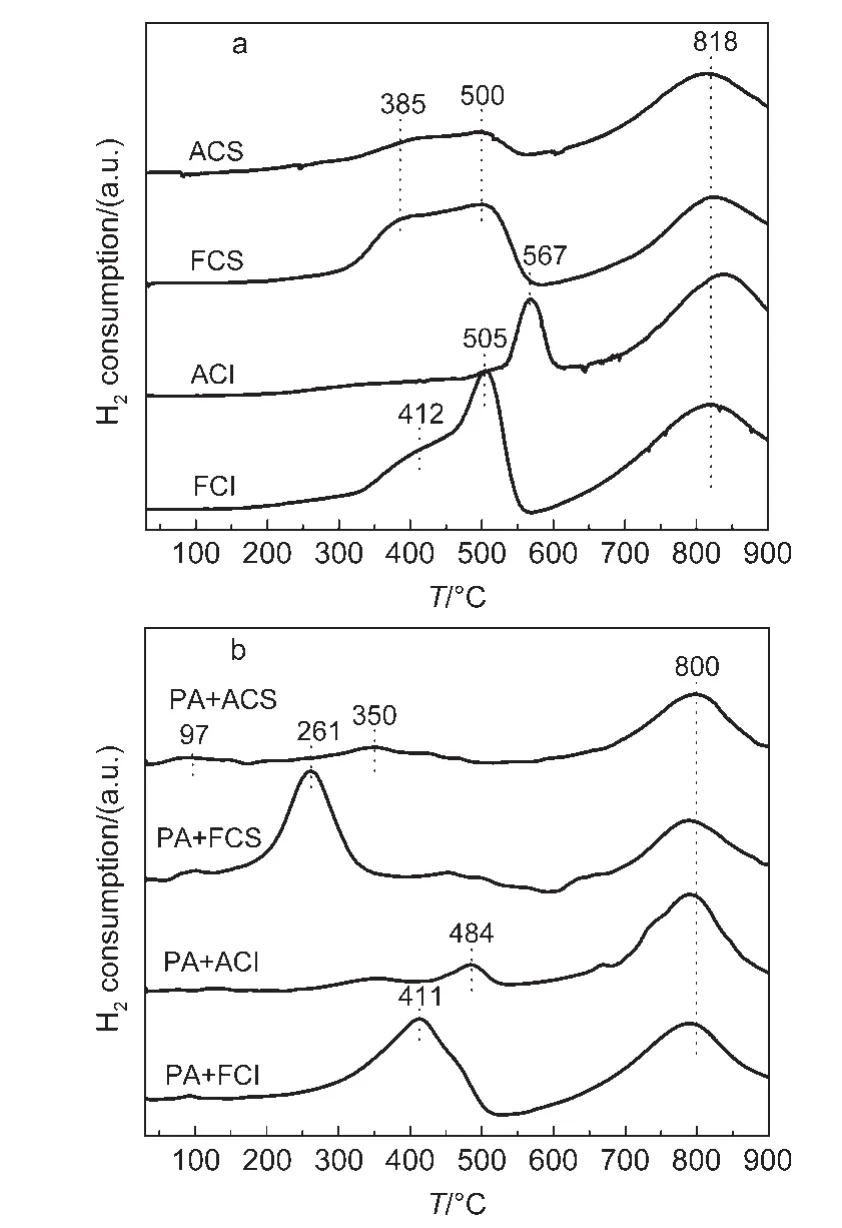
Fig.6 H2temperature-programmed reduction(H2-TPR)profiles for different samples
As to the reduction behavior for the mixture shown in Fig.6b,the surface oxygen reduction on PA+FCS samples appears at 261°C,while PA+FCI occurs at 411°C.Thus,compared with pure CeO2supports,the surface oxygen on these mixtures can be reduced at relatively low temperature.As to Pt/Al2O3,the weak reduction peak is usually below 100°C,which is ascribed to the PtOx.25In other words,when Pt/Al2O3was mechanically mixed with CeO2-X(X=S,I),the reduction of the surface oxygen on CeO2-X becomes easy to some extent.It infers that there is an interaction between Pt components and CeO2,which improves the surface oxygen reduction on CeO2.During the pre-treatment of each test,trace amount of Pt loaded on Al2O3may migrate from Al2O3to the surface of CeO2at high temperature due to a strong interaction between CeO2and Pt.26As a result,the pre-treatment probably leads to the interaction between Pt and CeO2even over Pt/Al2O3and CeO2mixture.The enhancement for surface active oxygen may play the critical role in NOxtrapping process at low temperature.For the aged CeO2,the interaction between Pt and CeO2shows weaker and less enhancement of the surface active oxygen.Besides the physical structure change for aged CeO2,the less active oxygen utilization may also be a factor which leads to low NOxtrapping capacity.Moreover,trace of Pt loaded on aged CeO2supports(PACS,PACI)are investigated to explore the modification effect for the NOxtrapping over aged CeO2supports.
Firstly,H2-TPR on PACS and PACI samples were conducted(Fig.7).As shown in Fig.7,one major reduction peak is found at 82°C on PACS samples,which is ascribed to the reduction of PtOxwith the surface oxygen on CeO2in close proximity to Pt species.25The surface oxygen species that are far away from Pt are reduced at relatively high temperature(at 340°C).For PACI samples,there are three reduction peaks at low temperatures,which are found at 164,327,and 490°C.Compared with PA+ACS(or ACI)samples,the reduction of surface oxygen species on PACS(or PACI)is further lowered.It indicates Pt loading on aged CeO2can significantly improve the surface active oxygen reduction of aged CeO2supports.However,the enhancement degree is different in both CeO2supports.PACI sample presents major peak at 490°C,which is far higher than the major peak at 82°C for PACS.In a word,the activity of surface oxygen species on CeO2-X is enhanced via this metal support interaction over PACS and PACI samples.

Fig.7 H2-TPR profiles for the Pt loaded on the aged CeO2-X samples
The NOxtrapping capacity is shown in Table 3.Interestingly,the NOxtrapping capacity of these samples is significantly enhanced(Table 3),which is comparable with PA+FCS(or FCI)catalysts(Table 1).
Similarly,NO conversion also obviously increases(Fig.8).The results suggest both NOxtrapping performance and NO conversion on PA+PACS(or PACI)samples are largely improved below 300°C,which is higher than that PA+ACS(orACI)samples.So it further infers that the surface oxygen species on CeO2play an important role in the NOxstorage process,22which facilitates NOxtrapping to some extent.Thus,the surface oxygen is able to more efficiently participant in NO oxidation.In general,the amount of NO2formation has a significant influence on NOxadsorption at low temperature.Therefore,the increase in NO2amount can greatlyimprove the NOxtrapping capacity.
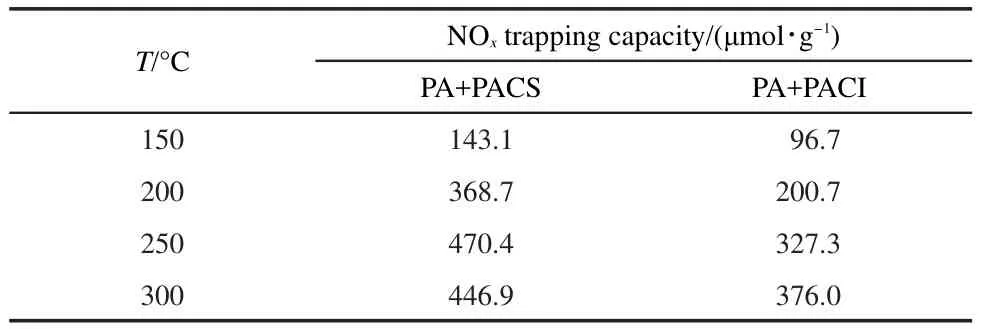
Table 3 NO xtrapping capacity of PA+PACS(or PACI)sample at differnt temperatures during 40 min NO/O2adsorption

Fig.8 NO conversions for PA+ACS,PA+PACS,PA+ACI,and PA+PACI samples at different temperatures
In addition,the characterization of physical structure of PACS and PACI are listed in Table 4.It is found that their structure properties are similar to that ACS and ACI sample.Thus,these results are further confirmed that the increase in NOxtrapping capacity of PA+PACS(or PACI)is attributed to the direct interaction between Pt components and the aged CeO2-X oxides,which can improve the active oxygen utilization and nitrate formation.
3.4 NO/O2adsorption by in-situ DRIFTS
In order to gain insights into the adsorption processes,timeresolved in-situ DRIFTS spectra were measured upon the NO/O2admission over different catalysts at 300°C(Fig.9).
According to literature,22,27,28the bands assignments are described in Table 5.For PA+FCS and PA+FCI catalysts,the surface NOxadsorbed species are similar,which are mainly attributed to different nitrates species.During the initial adsorption stage,the major bands are bidentate nitrates at 1563 and 1530 cm-1on PA+FCS samples,and around 1554,1521,and 1214 cm-1on PA+FCI samples.With the time prolonging,new adsorption bands associated with various nitrates appear and grow gradually.
For the aged samples,the surface NOxadsorbed species shift to higher wavenumbers,which may be due to the decrease in surface area of these samples and high species coverage.29Moreover,the overall bands intensity of adsorbed NOxspecies on the aged samples is less than that of the fresh samples.It indicates the amount of adsorbed NOxspecies declines.While as to PA+ACI samples,the types of NOxadsorbed species diminishes,which is probably caused by its very inferior physical-structures and loss of NOxtrapping sites.
However,it is found that the adsorption process on PA+PACS samples is significantly accelerated.In detail,a large amount of nitrate species on PA+PACS is observed at the beginning of 5 min,then less growth of the peak intensity.Thus,NOxcan be rapidly trapped on the surface of PA+PACS samples by fast nitrate formation and active oxygen utilization.It indicates that the active oxygen on the surface of CeO2indeed promotes NOxtrapping capacity.Just as shown in Eqs.(3)-(8),the active oxygen participates in nitrate and nitrite formation on the CeO2supports.Therefore,the modification of Pt on aged CeO2can make the active oxygen utilization easier and fast nitrates formation,thus improve the NOxtrapping capacity.

Table 4 Structure properties for Pt loaded on aged CeO2samples

Table 5 IR assignments of adsorbed NO xspecies

Fig.9 In-situ diffuse reflectance infrared Fourier transform spectroscopy(in-situ DRIFTS)spectra for NO/O2adsorption at 300°C on different catalysts
4 Conclusions
Lean NOxtrap performance below 300°C over two types of CeO2materials mechanically mixed with 2%(w)Pt/Al2O3were investigated.Compared with CeO2-I samples,CeO2-S samples have excellent physical-chemical properties,such as higher surface areas,richer porous texture,stronger aging-resistant ability,and higher concentration of surface active oxygen species.As a result,PA+CeO2-S samples exhibit outstanding NOxtrapping capacity.Moreover,the interaction between Pt and CeO2exists on PA+CeO2-X mixture,which facilitates NO oxidation and NOxtrapping capacity due to the enhancement of the activity of surface active oxygen on CeO2.This interaction on PA+CeO2-S samples is stronger than that on PA+CeO2-I.After aging treatment,the interaction between Pt and aged CeO2becomes weak.The results indicate that surface Ce3+contents and active oxygen on CeO2play a significant role in NOxtrapping process.Furthermore,Pt modified aged CeO2-X(PA+PACS or PA+PACI)presents higher NOxtrapping capacity than aged PA+ACS(or SCI)samples,which is attributed to easier active oxygen utilization and then fast nitrates formation.
(2)Takahashi,N.;Shinjoh,H.;Iijima,T.;Suzuki,T.;Yamazaki,K.;Yokota,K.;Suzuki,H.;Miyoshi,N.;Matsumoto,S.;Tanizawa,T.;Tanaka,T.;Tateishi,S.;Kasahara,K.Catal.Today 1996,27,63.doi:10.1016/0920-5861(95)00173-5
(3)Casapu,M.;Grunwaldt,J.;Maciejewski,M.;Baiker,A.;Eckhoff,S.;Gobel,U.;Wittrock,M.J.Catal.2007,251,28.doi:10.1016/j.jcat.2007.07.019
(4)Buchel,R.;Strobel,R.;Krumeich,F.;Baiker,A.;Pratsinis,S.J.Catal.2009,261,201.doi:10.1016/j.jcat.2008.11.016
(5)Yang,M.;Li,Y.P.;Wang,J.;Shen,M.Q.J.Catal.2010,271,228.doi:10.1016/j.jcat.2010.01.018
(6)Zhang,Q.Q.;Lv,L.F.;Zhu,J.X.;Wang,X.Q.;Wang,J.;Shen,M.Q.Catal.Sci.Technol.2013,3,1069.doi:10.1039/c2cy20775c
(7)Xian,H.;Ma,A.J.;Meng,M.;Li,X.G.Acta Phys.-Chim.Sin.2013,29,2437.[贤 晖,马爱静,孟 明,李新刚.物理化学学报,2013,29,2437.]doi:10.3866/PKU.WHXB201309052
(8)Wang,X.Q.;Lv,L.F.;Zhang,Q.Q.;Zhang,Y.W.;Wang,J.;Shen,M.Q.Catal.Sci.Technol.2013,3,200.doi:10.1039/c2cy20547e
(9)Xu,L.F.;Graham,G.;McCabe,R.;Hoard,J.;Yang,J.L.The Feacibility of an Alumina-Based Lean NOxTrap(LNT)for Diesel and HCCIApplications.SAE Technical Paper,2008-01-0451,2008.
(10)Ji,Y.;Toops,T.J.;Crocker,M.Catal.Lett.2007,119,257.doi:10.1007/s10562-007-9226-2
(11)Aneggi,E.;Boaro,M.;de Leitenburg,C.;Dolcetti,G.;Trovarelli,A.J.Alloy.Compd.2006,408,1096.
(12)Pang,X.J.;Gong,M.C.;Wang,M.;Ren,Y.G.;Zhao,M.;Chen,Y.Q.Acta Phys.-Chim.Sin.2004,20,1155. [庞秀江,龚茂初,王 敏,任屹罡,赵 明,陈耀强.物理化学学报,2004,20,1155.]doi:10.3866/PKU.WHXB20040919
(13)Ji,Y.;Toops,T.J.;Pihl,J.A.;Crocker,M.Appl.Catal.B:Environ.2009,91,329.doi:10.1016/j.apcatb.2009.06.002
(14)Ji,Y.;Fisk,C.;Easterling,V.;Graham,U.;Poole,A.;Crocker,M.;Choi,J.S.;Partridge,W.;Wilson,K.Catal.Today 2010,151,362.doi:10.1016/j.cattod.2009.12.009
(15)Ren,Y.J.;Harold,M.P.ACS Catal.2011,1,969.
(16)Ji,Y.;Choi,J.S.;Toops,T.J.;Crocker,M.;Naseri,M.Catal.Today 2008,136,146.doi:10.1016/j.cattod.2007.11.059
(17)Easterling,V.;Ji,Y.;Crocker,M.;Ura,J.;Theis,J.R.;McCabe,R.W.Catal.Today 2010,151,338.doi:10.1016/j.cattod.2009.12.007
(18)Corbos,E.C.;Elbouazzaoui,S.;Courtois,X.;Bion,N.;Marecot,P.;Duprez,D.Top.Catal.2007,42-43,9.
(19)Roy,S.;Baiker,A.Chem.Rev.2009,109,4054.doi:10.1021/cr800496f
(20)Liu,H.;Henein,N.;Bryzik,W.Simulation of Diesel Engines Cold-Start.SAE Technical Paper,2003-01-0080,2003.
(21)Zhang,F.;Wang,P.;Koberstein,J.Surf.Sci.2004,563,74.doi:10.1016/j.susc.2004.05.138
(22)Azambre,B.;Zenboury,L.;Koch,A.;Weber,J.V.J.Phys.Chem.C 2009,113,13287.
(23)Philipp,S.;Drochner,A.;Kunert,J.;Vogel,H.;Theis,J.;Lox,E.S.Top.Catal.2004,30-31,235.
(24)Yao,H.C.;Yao,Y.F.Y.J.Catal.1984,86,254.doi:10.1016/0021-9517(84)90371-3
(25)Golunski,S.E.;Hatcher,H.A.;Rajaram,R.R.;Truex,T.J.Appl.Catal.B:Environ.1995,5,367.doi:10.1016/0926-3373(94)00057-3
(26)Nagai,Y.;Hirabayashi,T.;Dohmae,K.;Takagi,N.;Minami,T.;Shinjoh,H.;Matsumoto,S.J.Catal.2006,242,103.doi:10.1016/j.jcat.2006.06.002
(27)Atribak,I.;Azambre,B.;Bueno López,A.;García-García,A.Appl.Catal.B:Environ.2009,92,126.doi:10.1016/j.apcatb.2009.07.015
(28)Lv,L.F.;Wang,X.Q.;Shen,M.Q.;Zhang,Q.Q.;Wang,J.Chem.Eng.J.2013,222,401.doi:10.1016/j.cej.2013.02.084
(29)Hadjiivanov,K.I.Catal.Rev.Sci.Eng.2000,42,71.doi:10.1081/CR-100100260
