Ecological adaptation of Reaumuria soongorica root system architecture to arid environments
2014-10-09LiShanShanYiLiDongMeiGengQiuLianDong
LiShan Shan, Yi Li , DongMei Geng, QiuLian Dong
College of Forestry, Gansu Agricultural University, Lanzhou, Gansu 730070, China
1 Introduction
Glimskär (2000) analyzed the topological structure of roots for five species of plants under controlled and natural conditions, and concluded that nutrient restriction produced a herringbone-like root branching pattern.Boumaet al. (2001) researched the topological structure of plant roots in a flooded area and found a theoretical relationship between predicted root diameter, branch number and root architecture. Through the fractal theory,wheat roots was researched by Yang and Luo (1994), and noted that fractal dimensions of roots changed along with root’s age and moisture conditions. When root development is higher, the fractal dimension is also high. In contrast, with a simple root development, the fractal dimension is small. According to research applying topology and fractal theory on the root architecture of several desert plants in the Taklamakan desert hinderland, Yanget al. (2008a,b; 2009) found that adaptability of several desert plant root structures produced two different root branching patterns. The root branching pattern ofTamarix taklamakanensiswas dichotomous, while the root branching pattern ofApocynum venetumandCalligonum taklamanensistended to be herringbone-like, and the plants had very good fractal characteristics. This shows how adaptability of root architecture to the fractal theory and topology has become an important topic (Martínez-Sánchezet al., 2003). Unfortunately, this method is mainly focused on the response of plant root architecture to changes of a single or a few factors under controlled conditions. Based on an ecological factor playing a dominant role, quantitative research on the change of root architecture are few under natural conditions.
Gansu Province is located in arid and semi-arid areas,and moisture gradient from east to west is the most significant environmental gradient in this region, thus, differentiation of the vegetation zone is very obvious, with desert grassland, grasslamd desertification, and typical desert vegetation types are formed from east to west(Chen and Pan, 2001). Therefore, Gansu is a complicated ecological transition zone and special natural ecological background, which made the region become a major hub of desert shrub.
Reaumuria soongoricais a super-xerophytic shrub,widely distributed, and a dominant species in this region(Liuet al., 1982).Reaumuria soongoricawith its ecological plasticity is highly resistant to drought, salt and sand burial which play an important role in protecting the desert ecology (Huang and Nie, 1988; Ma and Kong,1998). At present, there are numerous studies on the physiological ecology ofR. soongoricaadapting to a dry environment (Chonget al., 2010a,b; Zhouet al., 2010;Zhouet al., 2012). However, this research is mainly focused on above-ground parts and very little on underground parts, and even fewer studies on the ecological adaptation of root architecture with fractal theory and topology. Due to the distribution features ofR. soongoricain this region, and based on the fractal theory and topology in a larger-scale natural area, the present studies examined the ecological adaptation ofR. soongoricaroot architecture to an arid environment under a natural moisture gradient. Differentiation phenomenon with an increase degree of drought on the underground root architecture was analyzed from several aspects and multilayer.This study analyzed the regulative ability ofR. soongoricato drought in aspect of root morphology and provided a theoretical foundation for revealing the cause for a rich diversity of shrubs on the ecological transition zone of the northwest arid and semiarid areas.
2 Materials and methods
2.1 Materials
Three typical distribution areas whereR. soongoricais the dominant constructive species, from west to east in Gansu Province include Jiuzhoutai of Lanzhou, Minqin County, Wuwei City and Zhangye, and were chosen to be the study area. Jiuzhoutai of Lanzhou (LZJ) belongs to a typical loess hill and is located in the easternmost tip of selecting the section of the study area,Zhangye (ZY) is in a continental desert grassland climate and is located in the westernmost tip of selecting the section of the study area. Minqin of Wuwei (WWM)is located in the northeastern Hexi Corridor, and is a typical temperate continental desert climate. Standard samples plots of 20m×20m were set up within each habitat, and recordedR. soongoricameasurements of height, crown size and basal diameter. We selected three standard samples ofR. soongaricain the samples plots in order to excavate the roots and investigate the root architecture. Environmental characteristics of the root sampling sites are listed in table 1, and plant community characteristics are listed in table 2.

Table 1 Environmental characteristics of the Reaumuria soongorica root sampling sites
2.2 Methods
2.2.1 Excavation methods of root system
The combined trenching with root-tracking method was used to excavate the entire root system of the sample, and root architecture was investigated. The procedure was as follows: dig a 2m×0.6m×1.6m trench at 2 m distance around the plants, then using a small shovel slowly remove the sand from around the plants to the trench, constantly removing the sand from the trench.This continued until the whole root system of >3 mm was exposed, because it is difficult to reconstruct root architecture for roots with a diameter <3 mm (Oppeltet al., 2000, 2005). We tried to keep the roots in their natural position. After the root system was completely exposed,50cm×50cm grids were used to confirm the distribution location (until roots were not found). According to the proportion of 1:50, a map of the root system was accurately drawn on a 35cm×25cm coordinate paper.
2.2.2 Measure the root system parameters
镇痛效果较好,无牵拉反应视为优。镇痛效果一般,有轻微疼痛视为良。镇痛效果差,孕妇疼痛感明显视为差。优良率为优秀率与良好率之和。
After the root system is completely exposed, a vernier caliper and measuring tape were used to measure the diameter, internal and external connection number and connection length of every level root branching before and after.
2.2.3 Calculate the parameters of root architecture parameters
1) Topological index
Fitter (1986, 1987), Boumaet al. (2001), Fitteret al.(1991), Fitter and Stickland (1991) put forward two plant root topological structures which are dichotomous and herringbone-like branching patterns (Figure 1), and topological indices were used to reflect root branching pattern of different plants. The topological indexTI= lgA/lgM(Mis the total number of external root connections,Ais the total number of internal connection of the longest root) is a typical herringbone-like branching pattern while close to 0.5 is a dichotomous branching pattern, and most research shows that these patterns are typical for plant roots.
The topological index of typical dichotomous branching patterns which depends onMtends to be 0.5 in Fitter’s topological model (Fitter and Stickland, 1991;Fitteret al., 1991), thus the new modified topology parameters proposed by Oppeltet al. (2001) illustrate the middle transition form of root branching conditions.The number of root connections from the base to the root terminal is called topological length (a), maximum topological length is indifferent with gradeAin the Fitter model, andbwhich is associated with total number of connections from root base to terminal channel(Pe) is called the average topological length of the roots. The following equation can be used to expressb=Pe/v0(v0is equivalent toMwhich is total number of terminal connections in the Fitter model).aandbare variable with changes ofv0, soqaandqbare obtained by linear transformations ofaandb, and the range is from 0 to 1. The specific method is presented in the following equations:in the formula,lbv0=lnv0/ln2, thus, branching pattern corrections areqa=qb= 1 for the herringbone-like patterns, andqa=qb= 0 for the dichotomous patterns.
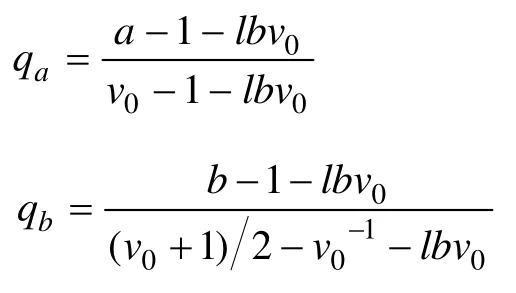
2) Fractal dimension
According to the box-counting method of fractal theory, the self-similarity of roots was studied, and the complexity of root branching is discussed in this paper. The method is described as follows: the side of the square of 18.4 cm is drawn on the root distribution map which is classified into 0–5 squares with its side asr= 18.4/2nand then calculated the number of squares which is expressed asNr. The number of squares which was cut by the root increased with decreasing side length of the square, and a group ofNrandrvalues was obtained. A linear regression was made with lgras abscissas and lgNras ordinate;the equation is lgNr= -Dlgr+ lgK, negative number (D)of the slope of the regression line is fractal dimension(FD), and the range is 1–2.FDincreased with increasing number of rootlets, and a higher number of fine roots indicated a higher fractal dimension of fine roots and more advanced roots. In contrast, a smaller fractal dimension showed that the meristic ability of roots was relatively feeble and the number of fine root was few and the main root was comparatively advanced. lgKis called fractal abundance which is expressed by FA(Atsumiet al., 1989; Ketipearachchi and Tatsumi, 2000;Yanget al., 2009).
3) Root branching rate
According to the methods of Strahler (1952) and Berntson (1995), the root sequence in the root cap layer is determined from outside to inside. The branching pattern of roots is such that the outermost small root is known as the first level root, and the root was known as the second level root when the two first level root met on a root, and the root was known as the third level root when the two second level root met on a root and so on. If different level roots met, the higher level is known as the root level.Root length and diameter are determined with a ruler and vernier caliper, and we calculated the number (Ni) of each level root (i) withias the abscissas and lgNias the ordinate,and branch rate (Rb) is the antilogarithm of the slope of the regression line. The ratio of one root branching to the next root branching is gradually branching rate [Ri: (Ri+1)],and formulaRi: (Ri+1) =N i: (N i+1),Niare the number ofi-level roots.
4) Data statistics
Analysis of variance, LSD (Least Significant Difference) and the correlation analysis were used on the data with SPSS software and significant difference was compared atP= 0.05 level.
3 Results and analysis
3.1 The comparison of topological indices and topological structure of Reaumuria soongorica roots in different habitats
The topological indices of roots can reflect the differences of topological structure ofR. soongoricaroots in different habitats. We can see from table 3, in different habitats, that topological indices (qaandqb) ofR. soongoricaroots are low, the maximum of which is 0.243,and theTIis close to 0.5 and far from 1 (TIis 0.733,0.687 and 0.631, respectively), which indicates the root branched structure ofR. soongoricais close to the dichotomous branching pattern. With a decrease of rainfall and increase of drought-stress, topological indices (qa,qbandTI) ofR. soongoricaroots increased significantly,which indicates that drought has a tendency of transforming the branching pattern from dichotomous to herringbone-like pattern. Variance analysis shows that topological indicesqaandTIdiffer (qa:F= 6.891,P= 0.022;TI:F= 6.413,P= 0.026) in different habitats. Using LSD in multiple comparisons, we found that topological indices(qaandTI) in loess hilly gully area (Jiuzhoutai of Lanzhou)with higher rainfall are significantly higher than those in Gravel Gobi District of the Hexi Corridor (Zhangye) with lower rainfall. Root branches ofR. soongoricaare highest in the semiarid loess hilly gully area, while significantly decreased in the arid Gravel Gobi District due to inhibited growth and higher soil bulk density. This indicates adaptation and strong plasticity of the branching pattern ofR.soongoricaroots in different habitats.
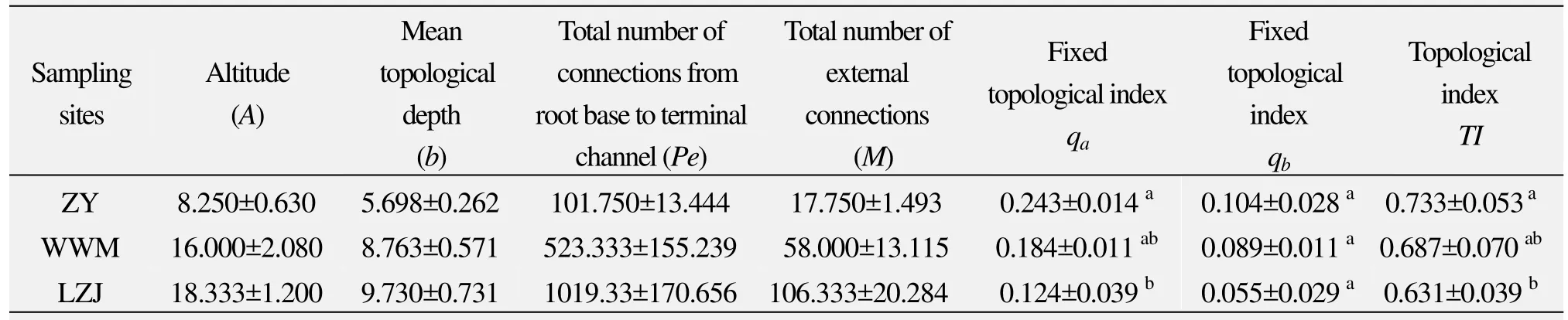
Table 3 The parameters of root system topology in different habitats
3.2 The comparison of root fractal characteristics in different habitats
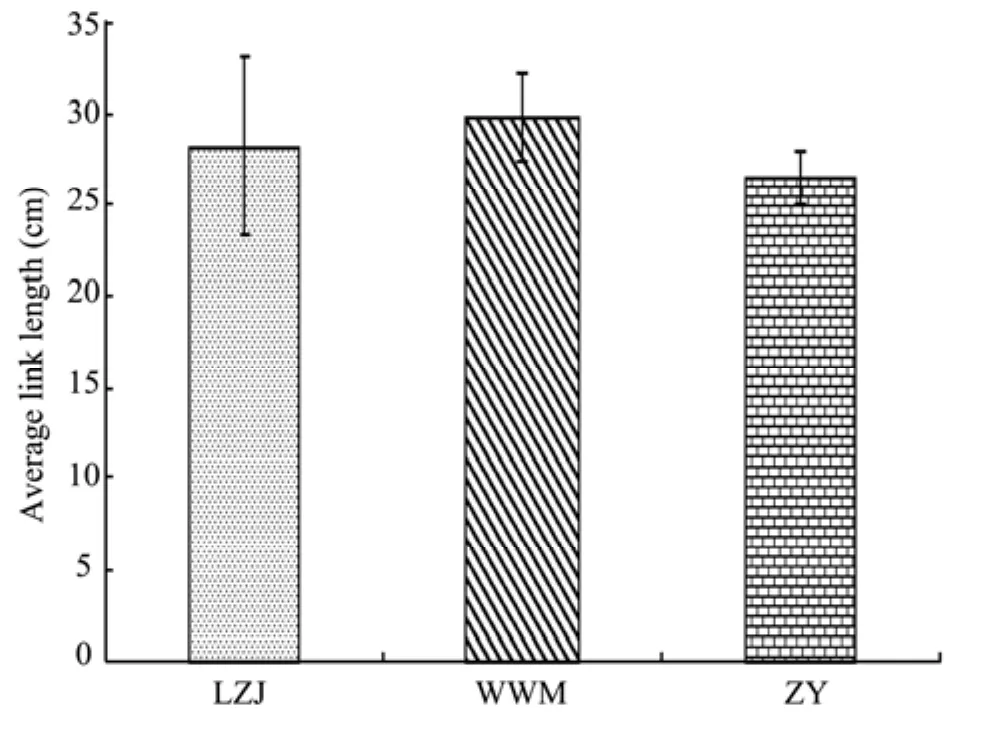
The fractal dimension of root morphology directly reflects differences of environmental factors. A higher fractal dimension (1 Root fractal abundance can reflect root extended volume in the soil (Atsumiet al., 1989; Quijano-Guertaet al., 2002). Thus, high fractal abundance indicates a larger volume range of the root system. From table 4 we find that, under different habitats, the extended volume ofR. soongoricaroots is different. Extended volume ofR.soongoricaroots in the semiarid loess hilly gully area is the highest which is probably related to a higher soil water content and lower soil bulk density (Table 2). Table 4 The parameters of root fractal characteristic in different habitats From table 5, we note that the total branching rate ofR. soongoricaroots are different in the various habitats,with a sequence of Lanzhou Jiuzhoutai >Wuwei Minqin>Zhangye, that is, the branching ability ofR. soongoricaroots in the arid Hexi Corridor are smaller than those in the semiarid loess hilly gully area. These results show that root system branching ability decreases with increasing drought. From table 5, we also find that there were only three levels root amongR. soongoricaroots in the arid gravel Gobi region, and root branching ability is relatively weak. This may be due to higher soil bulk density and soil compaction in the Gobi region compared to the loess hilly gully area and Minqin windy desert region.In the Minqin windy desert region with less rainfall,R1:R2is relatively high, that is, in this area the number of first order roots ofR. soongoricais higher. This may be due to larger soil porosity and scarce upper soil moisture in the dry windy desert region. In a drought environmentR. soongoricaroots can increase the number of first order roots and expand root space to absorb deep-layer soil water. Analysis of variance shows that in different habitats,total branching rate,R1:R2andR2:R3ofR. soongoricaroots are not significantly different. Table 5 Branching ratio and stepwise branching ratio of the root systems in different habitats In figure 2, average connection length ofR. soongoricaroots is the largest in the arid Minqin windy desert region while smallest in the Zhangye gravel Gobi region.This may be the result of large soil bulk density (Table 2).Analysis of variance shows that there was no significant difference among average connection length ofR. soon-goricaroots, which indicates that drought has no significant effect on average connection length ofR. soongoricaroots. From figure 2 we also found that in different habitats average connection length are all large, with the smallest average connection length of 26.46 cm. These results indicate that, in northwest arid and semiarid areas,in order to adapt to arid environmentsR. soongoricaincreases the root connection length to expand the distribution range, which enlarges the nutrition space. Figure 2 Average connection lengths of root system in different habitats Topological architectural feature is the most important part of root architecture which decides space distribution of roots in the soil and the root’s nutrient absorption capacity and fixation (Berntson, 1997). Root topology is used to discuss if habitat change varies the branching and extension strategy of roots (Oppeltet al.,2001). Many scholars have analyzed the dichotomous and herringbone-like root branching patterns on nutrient competition and found that herringbone-like roots have less secondary branching and less overlap or interior competition, while dichotomous roots are the opposite.Herringbone-like roots to have a higher surface area under the input of unit carbon and absorb more nutrients compared to dichotomous branching, thus herringbone-like roots are more suitable to arid environments(Fitter, 1987; Fitteret al., 1991; Glimskär, 2000; Boumaet al., 2001). This study found that the root branching patterns ofR. soongoricapopulations all tended to be dichotomous in the northwest arid and semiarid regions.This root branching pattern is the same asTamarix taklamakanensisunder extreme drought conditions, but in a different habitat condition with varied topological indices (Yanget al., 2008a). Topological index ofR.soongoricaroot system gradually increased in the arid area of Hexi Corridor, suggesting that droughts in this area led to a herringbone-like root branching pattern.This is consistent with findings of Glimskär (2000), that is, the limitation of nutrients is beneficial to herringbone-like root branching patterns. Also, we found that to adapt an arid environment, the root system ofR.soongoricadecreased secondary branching and root overlap or interior competition, and increased connection length to extend the surface area. Fractal dimension is an index to reflect the growth conditions of root systems (Liao and Yu, 2001), which can accurately represent the difference of plant root development and change of root structure under a variety of stress conditions (Yang and Luo, 1994; Yanget al., 1999).Gaoet al. (2006) and Chenet al. (2006) studied the numerical fractal dimension of root systems for potted peach and silver linden under different water conditions.They found that water logging treatment and drought stress produced a minimal root system fractal dimension,but rehydration after drought produced a greater fractal dimension. Different nutrient supply can also change the branching and distribution of the root system, changing the fractal dimension (Kaiet al., 1999; Wanget al., 2008).Our study found that the fractal dimension ofR. soongoricaroots shows a significant difference with change of environmental conditions in northwest arid and semiarid regions, with lesser values of 1.1778 and 1.1169, respectively, in the sand-wind area and Gravel Gobi region of the Hexi Corridor. Namely, fractal characteristics were not obvious, root meristem ability was relatively weak,the number of fine roots was less, and the main root was relatively developed. In the semiarid loess hilly region,root fractal dimension was larger and the root system had good fractal characteristics and relatively high development which is conducive for roots to absorb water and nutrients from the soil. The fractal abundance of roots is related to the distribution scope, expanding-space ability of the root system and its absorption efficiency of water and nutrients(Yanget al., 2009), and the larger the fractal abundance,the larger the volume of roots in the soil (Wanget al.,2008). Chenet al. (2006) noted that drought and water logging gradually reduced the fractal abundance of silver linden roots. Our study found that the fractal abundance ofR. soongoricaroots in the arid Hexi Corridor was smaller than in the semiarid Loess Hilly and Gully,and shows that the expanding distribution scope in Loess Hilly and Gully region was larger than in the arid Hexi Corridor. Different root branching is the plant’s adaptability to heterogeneity resources environment, and directly reflects the root system’s capability of adapting to an environment. Our study shows that the root branching rate ofR. soongoricagradually reduces in drought stress, thus adapting to the environment by reducing the capability of root branching. The root branching rate differed under different habitats.R1:R2was greater in the windy desert area of Minqin with relatively less rainfall. This shows that in drought windy desert areas with large soil porosity,soil moisture content is reduced.Reaumuria soongoricaroot system increased the quantity of one-level roots and expanded the root space to absorb soil water, thus adapting to a drought prone environment. In the drought gravel Gobi area (Zhangye),R. soongoricalacked the fourth-level root system, possibly because the weakness of the secondary branch ability of the root was caused by rather large soil bulk density. There is a possibility that soil space was so large that the capability of secondary branching was reduced. This result is similar to the growth and distribution ofPopulus euphraticaroots affected by high density of deep soil (Mubarekeet al., 2011). From the point of root connection length, increasing length is an important strategy for plants to expand the root system distribution in the soil which improves the ability to obtain nutrients (Yanget al., 2008b). Fitter(1987) and Fitteret al. (1991) found that higher absorption efficiency of roots was interrelated with the herringbone-like structure and longer length of root connection.Under different habitat conditions, branching patterns ofR. soongoricaroot was dichotomous, but its branch pattern changed from dichotomous to herringbone-like pattern with increasing drought. The average length ofR.soongoricaroot connections in dry and sandy areas was longer than in the loess hilly and gully region. This shows that in the loess hilly and gully region, inside root compe-tition for nutrients decreased, because soil moisture and nutritional situation were relatively good, the root topological index was smaller, and the root branching structure tended to be dichotomous. In the Minqin sand area,due to stress of the arid barren nutrition environment,R.soongoricamainly increased the length of root connections to reduce the overlapping of roots and decrease the inside root competition for nutrients. This improved the absorption efficiency of the root to nutrients, ensured effective nutrition space thereby the plant absorbed enough water and nutrients to ensure normal physiological needs of the plant in resource-poor environment. This is similar withAlhagi sparsifolic Shap. andSalix psammophilain drought expended length of the root and increasing the length of root connections, occupying a larger soil moisture resource space to adapt to an environmental stress (Liuet al., 2010; Zhanget al., 2011). In the arid Gobi of Zhangye, root average connection length was smaller than in the loess hilly region, but the difference was not significant. This might be due to the large soil bulk density in the region. This study was funded by the National Natural Science Foundation of China (41361100, 31360205), International Science and Technology Cooperation Program of China (2012DFR30830), the Gansu Science and Technology Support Program (1204NKCA084). We would like to thank Dr. Yi Li for his support, and DongMei Geng,QiuLian Dong for their help. Atsumi J, Yamauchi A, Kono Y, 1989. Fractal analysis of plant root systems. Annals of Botany, 64(5): 499–503. Berntson GM, 1995. The characterization of topology: a comparison of four topological indices for rooted binary trees. Journal of Theoretical Biology, 177(3): 271–281. Berntson GM, 1997. Topological scaling and plant root system architecture: developmental and functional hierarchies. New Phytologist,135(4): 621–634. Bouma TJ, Nielsen KL, Vanhal J,et al., 2001. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Functional Ecology, 15(3): 360–369. DOI:10.1046/j.1365-2435.2001.00523.x. Chen JH, Yu XX, You XL,et al., 2006. Fractal characteristics ofTilia tomentosa’s root system under different water conditions. Science of Soil and Water Conservation, 4(2): 71–74. Chen P, Pan XL, 2001. The floristic characteristics in the area of the Hexi Corridor. Plant Research, 21(1): 24–29. Chen SH, Hang H, Wang LQ,et al., 2001. The Plant Roots of Northern China in the Grass. Jilin University Publishing House,Changchun. Chong PF, Li Y, Su SP, 2010a. Diurnal change in chlorophyll fluorescence parameters of desert plantReaumuria soongoricaand its relationship with environmental factors. Journal of Desert Research,3(30): 539–544. Chong PF, Li Y, Su SP,et al., 2010b. Photosynthetic characteristics and their effect factors ofReaumuria soongoricaon three geographical populations. Acta Botanica Sinica, 30(4): 914–922. Fen B, Yang PL, 2000. Simulation of the root growth by using the image and fractal growth technology. Journal of China Agricultural University, 5(2): 96–99. Fitter AH, 1986. The topology and geometry of plant root systems:influence of watering rate on root system topology inTrifolium pratense. Annals of Botany, 58(1): 91–101. Fitter AH, 1987. An architectural approach to the comparative ecology of plant root systems. New Phytologist, 106(1): 61–77. Fitter AH, Sticklabd TR, 1991. Architectural analysis of plant root systems 2. Influence of nutrient supply on architectural contrasting plant species. New Phytologist, 118(3): 383–389. DOI:10.1111/j.1469-8137.1991.tb00019.x. Fitter AH, Stickland TR, Harvey ML,et al., 1991. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytologist, 118(3): 375–382. DOI:10.1111/j.1469-8137.1991.tb00018.x. Gao ZQ, Zhang XC, Wang XW, 2006. The root fractal character of peachin different water conditions. Tianjin Agricultural Sciences,12(3): 20–22. Glimskär A, 2000. Estimates of root system topology of five plant species grown at steady-state nutrition. Plant and Soil, 227(1–2):249–256. DOI: 10.1023/A:1026531200864. Huang PY, Nie XP, 1988. Habitat ofReaumuria soongoricacommunity in the middle part of Junggar Basin. Journal of Xinjiang University,5(3): 66–71. Kai LN, Carter RM, Douglas B,et al., 1999. Fractal geometry of root systems: Field observations of contrasting genotypes of common bean (Phaseolus vulgaris L.) grown under different phosphorus regimes. Plant and Soil, 206(2): 181–190. Ketipearachchi KW, Tatsumi J, 2000. Local fractal dimension and multifractal analysis of the root system of legumes. Plant Production Science, 3(3): 287–295. DOI: 10.1626/pps.3.289. Liao CZ, Yu XH, 2001. Application of fractal theory on studies of the root structure of plant. Journal of Jiangxi Agricultural University,23(2): 192–196. Liu J, He X, Bao HL,et al., 2010. Distribution of fine roots ofSalix psammophilaand its relationship with soil moisture in Mu Us Sandland. Journal of Desert Research, 30(6): 1362–1366. Liu JQ, Qiu MX, Pu JC,et al., 1982. The typical extreme xerophyte—Reaumuria soongoricain the desert of China. Acta Botanica Sinica, 24(5): 485–488. Lynch J, 1995. Root architecture and plant productivity. Plant Physiology, 109(1): 7–13. DOI: 10.1104/pp.109.1.7. Ma MH, Kong LS, 1998. The bioecological characteristics ofReaumuria soongoricaon the border of oasis at Hiutubu Xinjiang. Journal of Plant Ecology, 22(3): 237–244. Ma XM, Xi L, Xiong SP,et al., 2006. Dynamic changes of morphological parameters of tobacco root in field. Chinese Journal of Applied Ecology, 17(3): 373–376. Martínez-Sánchez JJ, Ferrandis P, Trabaud L,et al., 2003. Comparative root system structure of post-firePinus halepensisMill. andCistus monspeliensisL. saplings. Plant Ecology, 168(2): 309–320. DOI:10.1023/A:1024406029497. Mubareke Ayoupu, Chen YN, Li WH,et al., 2011. Fine root distribution ofPopulus euphraticaOliv. and its relations with soil factors under extremely arid environment. Journal of Desert Research, 31(6):1449–1458. Oppelt AL, Kurth W, Dzierzonb H,et al., 2000. Structure and fractal dimensions of root systems of four co-occurring fruit tree species from Botswana. Annals of Forest Science, 57(5–6): 463–475. DOI:10.1051/forest:2000135. Oppelt AL, Kurth W, Godbold DL, 2001. Topology, scaling relations and Leonardo’s rule in root systems from African tree species. Tree Physiology, 21(2–3): 117–128. Oppelt AL, Kurth W, Godbold DL, 2005. Contrasting rooting patterns of some arid-zone fruit tree species from Botswana-II. Coarse root distribution. Agro forestry Systems, 64(1): 13–24. DOI:10.1007/s10457-005-2403-7. Quijano-Guerta C, Kirk GJD, Portugal AM,et al., 2002. Tolerance of rice germplasm to zinc deficiency. Field Crops Research, 76(2):123–130. DOI: 10.1016/S0378-4290(02)00034-5. Strahler AN, 1952. Hypsometric (area altitude) analysis of erosional topology. Geological Society of America Bulletin, 63(11): 1117–1142.DOI: 10.1130/0016-7606(1952)63[1117:HAAOET]2.0.CO;2. Sun DY, Zhao WY, Wang LJ,et al., 2011. Progress in the study on architecture of desert plants. The Study of Soil and Water Conservation, 18(5): 281–287. Tsakaldimi M, Tsitsoni T, Ganatsas P,et al., 2009. A comparison of root architecture and shoot morphology between naturally regenerated and container-grown seedlings ofQuercus ilex. Plant and Soil,324(1–2): 103–113. DOI: 10.1007/s11104-009-9974-4. Wang H, Jin JY, Shan NZ, 2008. Fractal analysis of root system architecture by box-counting method and its relationship with Zn accumulation in rice. Acta Agronomica Sinica, 34(9): 1637–1643. Wang YQ, Zhang HJ, Bai KZ,et al., 1999. Application of fractal geometry in the studies of plant root systems. Journal Nature, 21(3):143–145. Wang ZQ, Guo DL, 2008. Root ecology. Journal of Plant Ecology,32(6): 1213–1216. Yang PL, Luo YP, 1994. The root morphology fractal characteristics of winter wheat. Chinese Science Bulletin, 39(20): 1911–1913. Yang PL, Ren SM, Luo YP, 1999. Measurement of fractal curve and express of root. Scientia Aricultura Sinica, 32(1): 89–92. Yang XN, Zhang XM, Dan LS,et al., 2008a. Analysis on root structure ofTamarix taklamakanensisin the Hinterland of the Taklimakan Desert. Arid Zone Research, 25(5): 660–667. Yang XN, Zhang XM, Li YL,et al., 2008b. Analysis of root architecture and root adaptive strategy in the Taklimakan Desert area China.Journal of Plant Ecology, 32(6): 1268–1276. Yang XN, Zhang XM, Li YL,et al., 2009. Root fractal characteristics at the hinterland of Taklimakan Desert. Arid Land Geography, 32(2):249–254. Zhang XL, Zeng XF, Liu B,et al., 2011. Effects of irrigation on root growth and distribution of the seedlings ofAlhagi sparsifoliaShap. in the Taklimakan Desert. Journal of Desert Research, 31(6): 1459–1466. Zhou HY, Tan HJ, Zhang ZS,et al., 2012. Physiological response and adjustment mechanism ofReaumuria soongoricaandSalsola passerinato extreme environment. Journal of Desert Research, 32(1):24–32. Zhou SH, Liu YB, Tan HJ,et al., 2010. The photoprotective mechanism of desert plantReaumuria soogoricaunder progressive soil drying.Journal of Desert Research, 30(1): 69–73. Zhou YS, Wang LQ, 2011. Ecological adaptation of root architecture to grassland degradation inPotentilla acaulis. Journal of Plant Ecology,35(5): 490–499.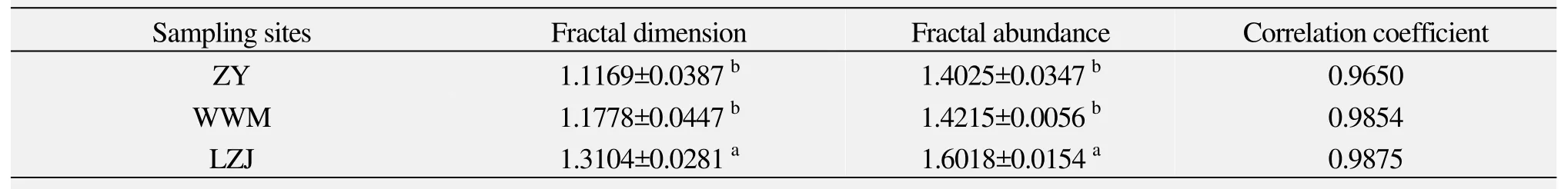
3.3 The comparison of Reaumuria soongorica roots branching rate in different habitats

3.4 The comparison of Reaumuria soongorica root connection length in different habitats
4 Discussion and conclusions
4. 1 The impact of drought to the root system topology
4.2 The impact of drought on root fractal characteristics
4.3 The influence of drought stress on roof system
4.4 The impact of drought on length of root connection
猜你喜欢
杂志排行
Sciences in Cold and Arid Regions的其它文章
- The spatial and temporal distribution and characteristics of inorganic ion concentrations of TSP in the Tarim Basin
- The atmospheric circulation patterns influencing the frequency of spring sand-dust storms in the Tarim Basin
- Earthquakes as a potential contributing factor to climate change at multi-decadal scale
- Role of blowing snow in snow processes in Qilian Mountainous region
- Greenland Ice Sheet surface melt: A review
- A new MODIS daily cloud free snow cover mapping algorithm on the Tibetan Plateau
