Preparation and properties of high silica glass fiber reinforced benzoxazine composite①
2014-09-07WUKeWANGRuminZENGJinfangDONGDazhou
WU Ke , WANG Ru-min, ZENG Jin-fang, DONG Da-zhou
(1.Department of Applied Chemistry, School of Science, Northwestern Polytechnical University, Xi'an 710072, China; 2.Xi'an Aerospace Composites Research Institute, Xi'an 710025, China)
Preparation and properties of high silica glass fiber reinforced benzoxazine composite①
WU Ke1, WANG Ru-min1, ZENG Jin-fang2, DONG Da-zhou2
(1.Department of Applied Chemistry, School of Science, Northwestern Polytechnical University, Xi'an 710072, China; 2.Xi'an Aerospace Composites Research Institute, Xi'an 710025, China)
A novel composite composed of polybenzoxazines and high silica glass fiber was prepared successfully. Thermal properties, curing kinetics and mechanical properties were studied in this paper. The results show that controllable curing window change indistinctively with the increae of heating rate. Curing kinetic parameters of cured mixture were calculated by the methods of Kissinger and Crane, and the activation energy is 69.619 kJ/mol, reaction order is 0.95. The tensile strength of composite is 35.5 MPa, compressive strength is 196 MPa, and ablation rate is 0.14 mm /s.
benzoxazine; high silica glass fiber; thermal property; kinetic; mechanical property
0 Introduction
Polybenzoxazines (PBZS) is a relatively new class of thermosetting addition-cure phenolic resins developed in the recent years[1-5]. These newly developed resins possess special features, such as near-zero shrinkage upon curing, low water absorption, high char yield, no strong acid catalysts required for curing, and release of no by-products during curing[6]. Benzoxazines(BZS) can be prepared by the Mannich-like condensation of different types of phenol, formaldehyde and an amine, either by employing solution or solventless methods, so the molecular structure of polybenzoxazines offers enormous design flexibility, which allows the properties of the cured materials to be tailored for a wide range of applications. They have gained great interest because they have the capability to exhibit the thermal and flame retardance properties with mechanical performance and molecular design flexibility. PBZSresins are widely used in various applications to the needs of the high technology aerospace industry.
High silica glass fiber(HSGF) reinforced plastic composite has comprehensively excellent properties,which has been broadly used in aerospace, astronautic and transportation fields in the past few years.As structure material,high silica glass fiber are commonly combined with epoxy resin by molding process,the fibers impregnating with resin and molding on the tools deviate greatly from different resin content,which will influence the properties of the composite.
ZHANG Jian,et al[7]reported preparation of impregnating chopped glass fiber with different benzoxazine systems. amines catalyst or epoxy-amines catalyst decreased significantly benzoxazine thermal curing temperature. The flexural strength and flexural modulus of molding compound of the system is 565 MPa and 22.7 GPa respectively, and the glass transition temperature is 195.31 ℃ , higher than that of common phenolic.
In the present works, the properties and processing of high silica glass fiber reinforced PBZSresin composite are studied, using differential scanning calorimetry (DSC).
1 Materials and methods
1.1 Materials
High silica glass fiber is provided by Shaanxi HuaTe glass fiber co., LTD,China;BZSis purchased from Sichuan University, the structure of the BZSand PBZSis shown in Fig.1.All chemicals are used without further purification.
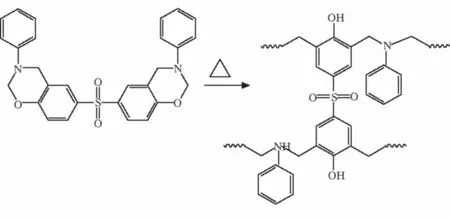
Fig.1 Preparation of polybenzoxazine from typical benzoxazine monomer
1.2 Preparations of samples
High silica glass fiber is dried in the oven at 105℃ for 2 h to reduce the moisture and cut into equal length of 30 mm. Certain amount of high silica glass fiber is added into the system composed of BZS. High silica glass fibers are homogeneously blended with the BZSto the weight ratio of 60/40. In order to achieve homogeneous dispersion of the high silica glass fiber into BZS, the resulting mixtures of HSGF and BZSare stirred for 24 h at room temperature and dried for 1 h at 80 ℃. In molding process, the mold, measured 165 mm×165 mm, is first coated with silicone. High silica glass fiber weighing 60% of the composite are filled in the mold and then cured at a higher temperature between 200~220 ℃ for a fixed time.
1.3 Thermal characterization
The curing behaviour of BZSand high silica glass fiber blends is evaluated by using Thermal Analysis-DSC7. A heating rate of 5 K/min,10 K/min,15 K/min,20 K/min in nitrogen atmosphere and a sample mass of 5~6 mg in aluminum pans is used. The blends are dried under vacuum at 323 K for 1 h before DSC analysis.
1.4 Strength characterization
The mechanical properties of the composites are evaluated by using the state standard of the People's Republic of China(GB) test procedure. The standard test specimens are machined from the composite. Tensile strength and compressive strength test are carried out on INSTRON Testing Machine Model No.4505.
1.5 Microstructure analysis
The microstructures of the tensile specimens are examined by using scanning electron microscope (SEM),Japan model JSM 6460LV.
1.6 Ablation characterization
Ablation properties(GJB-323A-96)are tested by the oxygen-acetylene ablation test, the test specimen size isφ30 mm×30 mm, ablative time is 20 s.
2 Results and discussions
2.1 Cure profile by DSC
2.1.1 Bzsmonomer and Bzs/ HSGF mixture
Thermal curing of the benzoxazine monomers forms the corresponding polymer PBzswith ring-opening of the oxazine of the two monomers. The curing behavior of Bzsmonomer in the present of HSGF is monitored by DSC. The DSC curves of Bzsand Bzs/HSGF mixture. The DSC thermogram of Bzsmonomer shows a typical sharp curing exotherm with a peak (Tp) located at 205 ℃ attributed to the benzoxazine ring-opening polymerization, and with a final cure temperature (Tf) located at 250 ℃. On the other hand, the Bzs/ HSGF mixture show a curing exotherm with a peak (Tp) located at 204 ℃, and with a final temperature (Tf) located at 250 ℃. This could attribute to the non-catalytic effect of HSGF surface group on the Bzsmonomer ring-opening reaction.
The enthalpy of reaction of Bzsmonomer and Bzs/ HSGF mixture are 134.4 J/g and 110.7 J/g, respectively.
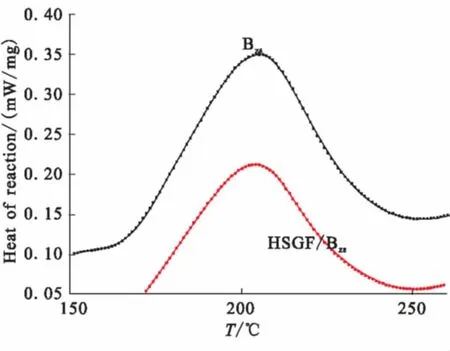
Fig.2 DSC of Bzs monomer and Bzs/ HSGF mixture at heating rate of 5 ℃/min
2.1.2 Bzs/ HSGF mixture
The cure behavior of Bzs/HSGF mixture investigated by DSC at different heating rate is shown in Fig.3. The endotherm that corresponds to melting is observed at 98 ℃. The initial curing temperature (Ti), peak curing temperature (Tp) and final temperature (Tf) rise with the increase of heating rate. The initial curing temperature at 154.9 ℃, 161.9 ℃, 167.7 ℃, 168.7 ℃ indicates a controllable curing window (CCW) of 77.9 ℃, 80.7 ℃, 81.4 ℃, 84.7 ℃, respectively. The CCW implies the control over processing. As the heating rate increases, the window don't change significantly. This could be attributed to the HSGF conductivity limiting the thermal effective transmission. From the results, the development tendency of the CCW are different from other reported works[8]. The curing characteristics of Bzs/HSGF mixture are summarized in Table 1.
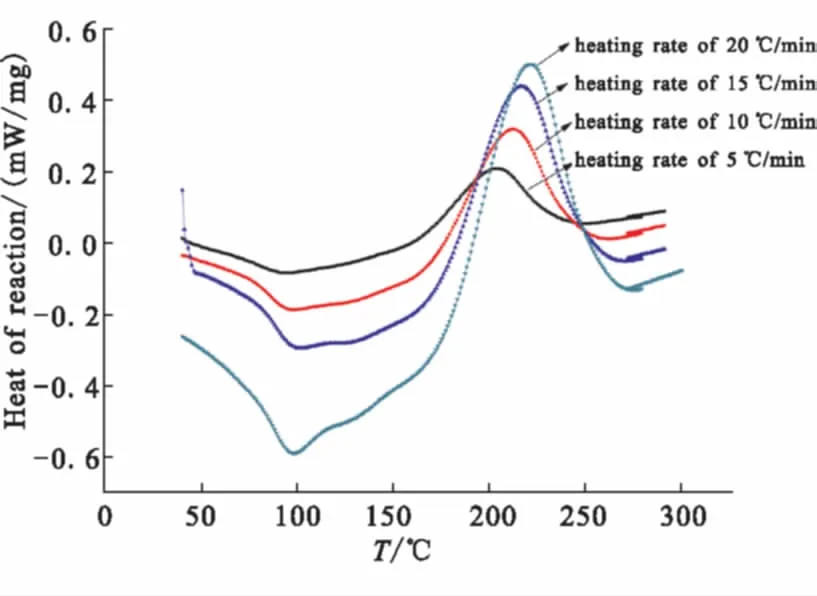
Fig.3 DSC of Bzs/ HSGF mixture at different heating rate
2.2 Curing kinetics
The nonisothermal kinetics for the Bzs/HSGF mixture are investigated by the Kissinger and Crane method,
through the following expression.
Kissinger method[9]:
(1)
Crane method[10]:
(2)
Whereβis heating rate;Tpis peak curing temperature;Eis the activation energy;nis reaction order;Ris the universal gas constant.
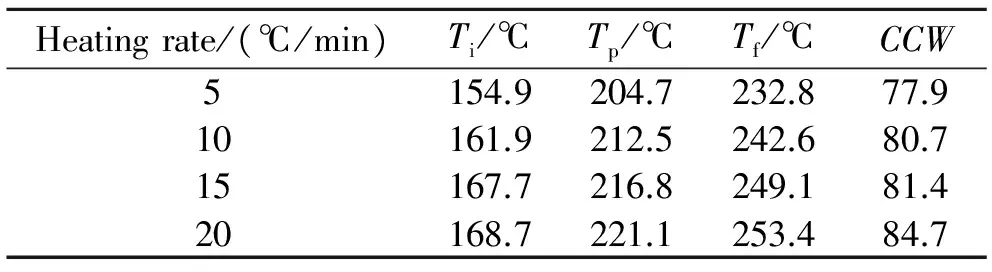
Table 1 DSC observations of Bzs/HSGF mixture
As the multiple heating rate methods for non-isothermal analysis proposed by Kissinger and Crane can be used as an alternative way of calculating the activation energy without assuming any model of kinetic parameters, the logarithm plots of heating rate versus the reciprocal of the absolute peak temperature of Bzs/HSGF mixture are given in Fig.4 and Fig.5. Results show that a good linear relationship between the heating rate and the reversal of the exothermic peak temperature can be obtained. The average activation energy values of Bzs/ HSGF mixture are calculated from the slopes of the plots. Bzs/HSGF mixture shows only one dominant curing kinetic process with the average activation energy of 69.619 kJ/mol, reaction order is 0.95, which mean fhat the reaction is complex. This mechanism is the heterocyclic ring opening polymerization of benzoxazine monomer since the oxazine ring is the reactive site for curing of the benzoxazine.
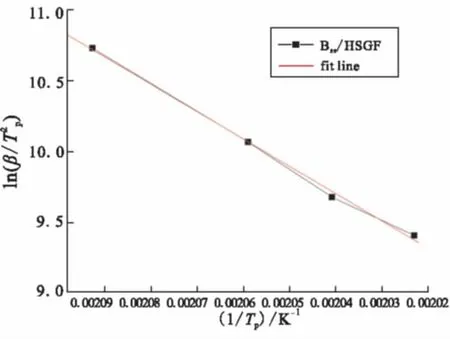
Fig.4 Curve of ln(β/Tp2) vs 1/Tp
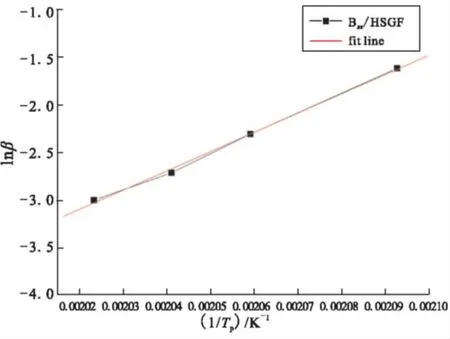
Fig.5 Curve of lnβ vs 1/Tp
2.3 Mechanical and ablation behavior of composites
The mechanical strength of Bzs/HSGF composite specimens are shown in Table 2. If the mechanical properties of Bzs/ HSGF composite are compared with a kind of structural phenolic resin/HSGF system commonly used, the compressive strength and ablation rate are in the same level,tensile strength of Bzs/HSGF composite is better than that of the phenolic resin/ HSGF composite.
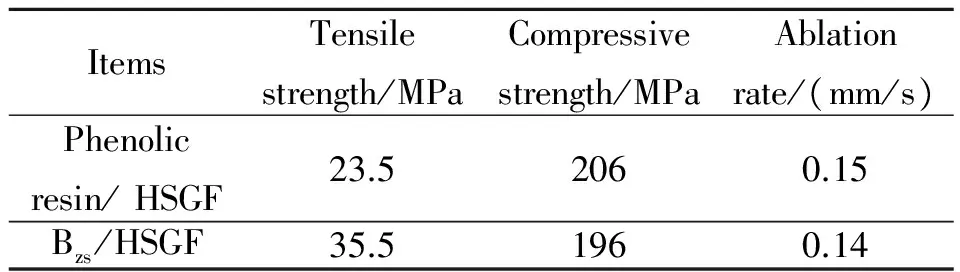
Table 2 Mechanical and ablation properties of Bzs/HSGF
The strength and mode of failure of a composite are not only dependent on the properties of the fibers and matrix, interfacial bond, and fiber volume, but also on the fiber orientation[11]. The prepreg of phenolic resin/HSGF and Bzs/HSGF employ same preparation technology, HSGF present disorder state,which mean fhat fiber show random orientation in the matrix. The appearance of the prepreg of Bzs/ HSGF is given in Fig.6. In the same preparing condition, the different resin properties become the key factor influencing composite mechanical properties.

Fig.6 Appearance of Bzs/ HSGF prepreg
Tensile and compressive properties are very important for a composite to structural applications, the function of the resin matrix in the composite is to bind the reinforce fibers together and transmit load and stress,the interface between matrix and fibers is the key factor influencing the property of composite.The fracture surface of the Bzs/HSGF composite and phenolic resin/ HSGF composite is observed by SEM to analyze stress states from the SEM pictures(Fig.7),the fibers are evenly dispersed in the matrix,the fibers and matrix break uniformly and the fracture surface of the specimens are well arranged,which show that the fibers and resin matrix can bear load together. Compared to the existing phenolic resin/ HSGF, tensile strength of the Bzs/HSGF is generally increased by 51.1%, compressive strength of the Bzs/HSGF is reduced by 4.8%. As the toughness of phenolic resin/HSGF is not very good,the stress loaded on the composite specimens would induce cracks in the matrix as well as the interface between fibers and resin. Cracks in the composite occur primarily by interfacial failure with little crack-tip deformation. The microstructure in Fig.7 shows that the fibre bundl, entangle or curl in the matrix, HSGF fibers are pulled out on the fracture surface binding with very few resin,which show that the interface condition between HSGF fiber and phenolic resin is not very good.
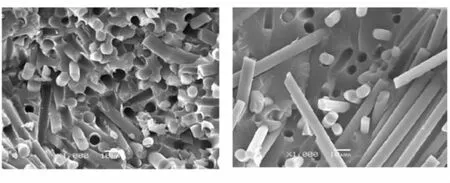
(a) Bzs/HSGF (b) Phenolic resin/HSGF
The ablation results of two kinds of composites are listed in Table 2. the data in Table 2 shows that the ablation rate of phenolic resin/HSGF and Bzs/HSGF composites is 0.15 mm/s, 0.14 mm/s, respectively. anti-ablative performances of Bzs/HSGF composites are slightly better than that of phenolic resin/ HSGF, the ablation morphologies of composites are plotted in Fig.8. The ablation process includes two stages, the first stage presents decomposing the primary products such as benzene derivatives, amines, phenolic comounds and Mannich base compounds, the mechanism involves chain scissions, such as the C—C, C—O and C—N cleavages[12], the second stage presents small molecules forming carbonization zone, isolating Bzs/ HSGF composites and high temperature gas, and effectively preventing ablation of composites expanding.
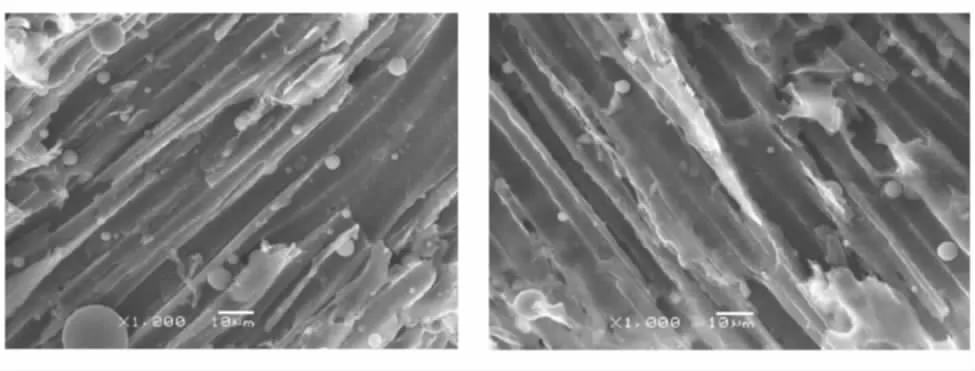
(a) Bzs/HSGF (b) Phenolic resin/HSGF
3 Conclusions
(1) Bzsmonomer and the Bzs/HSGF mixture show the initial curing temperature and final cure temperature without any catalytic effect.
(2) TheCCWof Bzsmonomer and the Bzs/HSGF mixture don't change significantly with the increase of heating rate.
(3) Bzs/HSGF mixture shows only one dominant curing kinetic process with the average activation energy of 69.619 kJ/mol, reaction order is 0.95.
(4) Bzs/HSGF composite has better mechanical and ablation properties: tensile strength is 35.5 MPa, compressive strength is 196 MPa, and ablation rate is 0.14 mm /s.
Referencee:
[1] Zdenka Brunovska, Richard Lyon, Hatsuo Ishida. Thermal Properties of phthalonitrile functional polybenzoxazines [J]. Thermochimica Acta,2000:195-203.
[2] Chen Qiao, Xu Ri-wei, Yu Ding-sheng. Mutiwalled carbon nanotube/polybenzoxazines nanocomposites: Preparation, characterization and properties[J]. Polymer,2006, 47,(3):7711-7719.
[3] Huang Jian-xiang, Zhang Jian, Wang Fan, et al. The curing reaction of enthynyl-functional benzoxazine[J]. Reactive & Functional Polymers.2006,66(12):1395-1403.
[4] Santhosh Kumar K S, Reghunadhan Nair C P, Sadhana R, et al. Benzoxazine-bismaleimide blends: Curing and thermal properties[J]. European Polymer Journal,2007,43:5084-5098.
[5] Bimlesh Lochab, Indra K Varma, Jayashree Bijwe. Thermal behaviour of cardanol-based benzoxaines[J]. J. Therm. Anal. Calorim,2010,102:769-774.
[6] Renhunadhan Nair C P. Advances in addition-cure phenolic resins[J]. Progress in Polymer Science,2004,29:401-498.
[7] Zhang Jian, Liu Xiang-yang, Gu Yi. Preparation and Properties of high performance benzoxazine molding composites [J]. China Plastics Industry,2009,39(3):78-81.
[8] Liao Dong, Yan Chun, Pan Li-jian, et al. Study on curing kinetics of benzoxazine resin using non-isothermal DSC method. [J]. Thermosetting Resin,2011,26(1):1-5.
[9] Su Yi-che, Yei Ding-ru, Chang Feng-chih. The kinetics of B and P-a type copolybenzoxazine via the ring opening process[J]. Journal of Applied Polymer Science,2005,95:730-737.
[10] Li Ling, Chen Jian-nan. Study on curing reaction and kinetics of modified BMI/benzoxazine resin[J]. Chian Adhesive,2008,17(10):23-26.
[11] Joseph S, Sreekala M S, Oommen Z, et al. A comparison of the mechanical properties of phenol formalddhyde composites reinforced with banana fibers and glass fibers[J]. Comp. Sci. Technol.,2002,62(14):1857-1868.
[12] Kasinee Hemvichian, Hatsuo Ishida. Thermal decomposition processes in aromatic amine-based polybenzoxazines investigated by TGA and GC-MS[J].Polymer,2002,43:4391-4402.
(编辑:吕耀辉)
高硅氧纤维增强苯并噁嗪树脂复合材料的制备及性能
吴 轲1,王汝敏1,曾金芳2,董大洲2
(1.西北工业大学 理学院应用化学系,西安 710072;2.西安航天复合材料研究所,西安 710025)
以苯并噁嗪为基体树脂,短切高硅氧纤维为增强材料,制备了一种模压用复合材料。研究了该树脂的热性能、固化动力学性能及力学性能。结果表明,苯并噁嗪增强高硅氧纤维混合物随固化温度升高工艺窗口未发生显著变化,利用Kissinger、Crane法得到固化反应活化能为69.619 kJ/mol,反应级数为0.95。复合材料拉伸强度为35.5 MPa,,压缩强度为196 MPa,线烧蚀率为0.14 mm /s.
苯并噁嗪;高硅氧纤维;热性能;动力学;力学性能
V258 Document Code:A Article ID:1006-2793(2014)05-0699-005
10.7673/j.issn.1006-2793.2014.05.022
①Received date:2014-06-01;Revised date:2014-06-25.
Foundation:Supported by the National Defense Foundational Research of China(C0320110006).
Biography:WU Ke (1973—), male, doctor, speciality: composite material science.
