Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
2014-09-06WeiMAYanhongLIUYujunSUNJasonGrabosky
Wei MA · Yan-hong LIU · Yu-jun SUN · Jason Grabosky
ORIGINAL PAPER
Carbon stock in Korean larch plantations along a chronosequence in the Lesser Khingan Mountains, China
Wei MA · Yan-hong LIU · Yu-jun SUN · Jason Grabosky
Received: 2013-09-28; Accepted: 2013-11-17
© Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
Carbon (C) dynamics are central to understanding ecosystem restoration effects within the context of Grain for Green Project (GGP). GGP stared in China since 2003 to improve the environment. Despite its importance, how total forest ecosystem C stock (FECS) develops following land-use changes from cropland to plantation is poorly understood, in particular the relationship of C allocation to pools. We quantified C pools in a chronosequence ranging from 0 to 48 years, using complete above- and below-ground harvests based on detailed field inventory. Stands were chosen along a succession sequence in managed plantations of Korean larch (Larix olgensis Henry.), a native planting species in the Lesser Khingan Mountains, Northeast of China. The FECS of Korean larch plantation (KLP) were dynamic across stand development, changing from 88.2 Mg·ha-1at cropland, to 183.9 Mg·ha-1as an average of forest C from 7- through 48-year-old plantation. In a 48-year-old mature KLP, vegetation comprises 48.63% of FECS and accounts for 67.66% of annual net C increment (ANCI). Soil is responsible for 38.19% and 13.53% of those, and with the remainders of 13.18% and 18.81% in down woody materials. Based on comparisons of our estimate to those of others, we conclude that afforestation of Korean larch plantation is a valid approach to sequester carbon.
Korean larch plantation, forest ecosystem, carbon stock, chronosequence
Introduction
Recent studies on global carbon (C) cycle suggest that land sinks account for 30% of total anthropogenic C emissions from 2000 to 2006 (Canadell et al. 2007). The FAO (2010) declared that C storage in terrestrial vegetation exceeds 650 Pg for the period 1990 to 2010 and continues to be part of the active discussion regarding greenhouse gas emissions (GHGs) and afforestation-reduction commitments (UNFCCC 2011a). Forests have a higher C density than other types of ecosystems (Silver et al. 2004; Pibumrung et al. 2008) and are estimated to contain more than 56% of terrestrial C (Bonan 2008). Their management therefore could play an important role in reducing atmospheric CO2(Johnson and Kern 2003; Stinson et al. 2011). Widespread interest in terrestrial C management may change the dynamics of current land use by influencing the relative importance of specific management objectives (Lal R 1999; Feng et al. 2006).
Goodale et al. (2002) summarized data from the northern hemisphere and concluded that boreal forests sequester about 0.6−0.7 Pg C year−1, allocated as 0.25 Pg C of vegetation biomass, 0.11 Pg C of dead materials, 0.13 Pg C of soil, and 0.08 Pg C of wood product. Zheng et al. (2011) suggested disturbances from nature and human activities reduced 36% of forest C, mainly determined by harvest (63%), land-use change (33%), and fire suppression (4%). A relatively large proportion of the reduction was due to land-use change in the conversion of forest into agricultural land (Erb 2004). Smith and Heath (2008) presumed that 80% of the aboveground forest C would be lost during a conversion to non-forested land use.
Carbon may be recaptured through afforestation projects, which would increase aboveground biomass and replenishing the soil C pool (Guo and Gifford 2002; Pibumrung et al. 2008; Tobias et al. 2010). With that understanding, the Grain for Green Project (GGP), the largest afforestation program in China, en-ables the transition of lands from agricultural use as a proposed method to restore and rebuild the degraded ecosystems as farmland land (Wu et al. 2008; Wang et al. 2010a).
However, it is important to recognize that it can take decades to achieve the original level of carbon stock (CS) after land-use changes. Because GGP is occurring on a large scale and will last for decades, it is important to monitor and assess the development of newly established forests. As various studies have reported, C dynamics are central to understanding ecosystem-restoration efforts; not only in the context of GGP, but also as part of solving the serious problem of global warming (Chastain et al. 2006; Woodbury et al. 2007; Meelis et al. 2009).
Estimating C allocation to sectors or pools over a long time scale is challenging because forest C should be based on both initial and continuous assessments of biomass extraction and be multiplied by the appropriate factor as carbon concentration. Long-term inventories are hard to keep consistent, comparable, and accurate for forest heterogeneity, natural factors, and human activities (Magnani et al. 2007; Stinson et al. 2011).
Due to such challenges, the research record within major forest types with regard to carbon concentration (c) is still rudimentary, and precise data on c are scant (Didier and Frédéric 2006; Tolunay 2009; Zhang and Wang 2009). High degrees of uncertainty are associated with most available estimates (Grace 2004; Bradford et al. 2009). In many present studies, C in forest ecosystems addresses only the aboveground tree and aspects of soil, but lacks an absolute estimate of belowground and understory biomass (Pregitzer 2003; Giardina et al. 2005; King et al. 2007). An additional limitation, the estimation of biomass that has been removed is necessary to trace its release or continued capture as C because it is also based on the content and productivity of the forest (Houghton 2005; Heath et al. 2011). Furthermore, less experimental evidence is available on the effect of forest practices, such as thinning, on the C pools (Smith and Heath 2002; Birdsey and Lewis 2003; IPCC 2006).
The goal of this study is to describe and compare the dynamics of net CS of Korean larch (Larix olgensis Henry.) forest ecosystem, as an afforestation transition from farmland use, along a chronosequence. Korean larch is regarded as a commercially important planting species in northeast China; thus Korean larch plantations (KLP) are representative of a large part of the Lesser Khingan Mountains and the region beyond. Stand-level inventories were based on forest inventory analysis (FIA) fixed-radius plots. The conventional biomass-carbon method was employed, using complete above- and below-ground harvests with a chronosequence approach.
We developed land-based estimates of CS in specific subsets of the total forest ecosystem. Highly detailed, species- and region-specific conversion factors as c for CS have been developed for refined estimates of FECS. Analysis of ongoing reporting for these plantations indicates that spatial identification of C sinks as well as explicitly estimating growth and forest practices for their contribution to forest C can provide information to managers for altering or creating management plans (Brown 2002; Oscar et al. 2004).
Materials and methods
The chronosequence
Our study was carried out at the Dongzhelenghe forest farm (46°31'−46°49' N; 128°55'−129°15' E), which represents the typical forest types and landscape of the Lesser Khingan Mountains. This area is classified in temperate mixed broadleaf-conifer forest region based on the system of Chinese national vegetation regionalization (Cao and Li 2007). A large proportion of the original forest had been destroyed by industrial logging long ago, and was subsequently reclaimed for farming.
Since the mid-1960s, the corn, soybean, and abandoned fields which occurred beside or surrounded by the forest, had been taken back gradually for planting KLP. In order to study the CS dynamic in KLP after returning from cropland, we used a space-for-time substitution (Carolina and Belén 2005). To set a benchmark, we first choose two plots of cropland to estimate CS. Ten stands along a sequence in the managed KLP were sampled.
The chronosequence for both cropland and forests plots ranged in age from 0 to 48 years (Table 1). Elevations at all stands are similar, predominately occurring on south-facing lower slopes with modest variation in slopes from 6° to 10°. Soils at all sites are sandy loams on hill land forms that are similar in depth. All stands were established with an initial planting density of 2883 trees·ha-1, had been tended and thinned (intensity did not exceed 30%) at intervals of 8−10 years, and the stand tree densities necessarily declined over time. For each experiment stand, two plots were assigned simultaneously in the summer of 2009, and the mean value used for data analysis is shown in Table 1. Mean DBH (diameter at breast height) across all stands ranged from 3.0 to 27.4 cm, mean tree height ranged from 3.0 to 29.2 m, and stand volume ranged from 3.917 to 283.712 m3·ha-1. In current stands, similar site condition and similar management practices reflected that stand-to-stand variation was modest. Therefore, we can assume that the ecological dynamics of forest growth and CS accumulation followed the same pattern.
Field measurement and sampling treatment
In our study, the CS of cropland was based on soil C measures and sampling of crops as the benchmark surrogate for C status before the afforestation land use change. With slight modifications to the application of previous researches (Skog et al. 2004; Smith et al. 2006; Woodbury et al. 2007), FECS can be divided into seven distinct storage sectors: live trees, understory vegetation, snags, down dead wood (DDW, included slash pile and coarse woody debris), forest floor (included fine woody debris, litter and duff), soil, and removed wood. Specially, removed wood C refers to a portion of live tree C initially that is sequestered as logs and periodically removed out forest, but other residues remain on the site.
These sectors were included in three major pools, as vegetation (live biomass), dead woody materials (DWM, dead biomass)and soil. Also, they can be roughly divided into two types of non-soil (vegetation & DWM) and soil. Fixed-radius plots were designed for use in FIA programs for forest surveys to obtained inventory data and original samples for biomass and CS (Bechtold and Patterson 2005; USDA Forest Service 2007). We employed small-scale and highly accurate surveys on sub-plots for trees and residual piles (RP), micro-plots for shrubs, quadrats for herbs and forest floor, and transects for fine woody debris (FWD) and coarse woody debris (CWD) within each plot (described below).
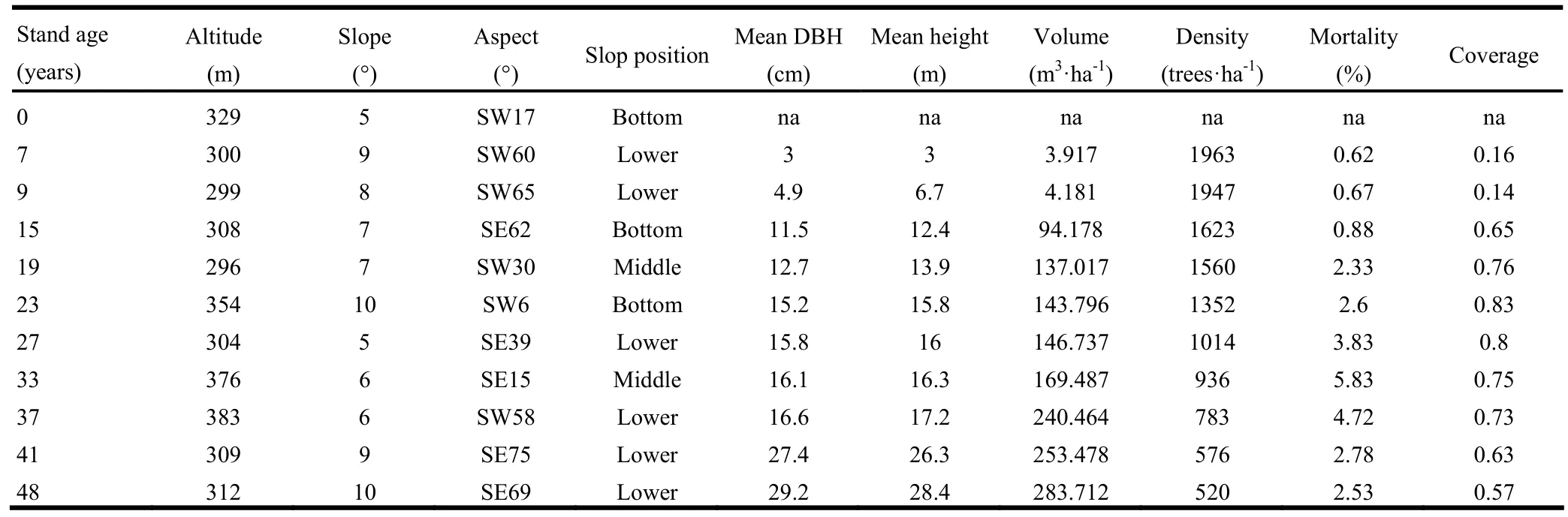
Table 1: Physical characteristics of the stands used in the study of C stock dynamic of Korean larch plantations along a chronosequence for cropland and stands 0−48 years of age in the Lesser Khingan Mountains of China.
Vegetation
In sub-plots, all rooted individual trees were tagged and characterized by DBH, height, and crown width. The understory vegetation was surveyed in two categories for more efficient sampling: woody plants (vines, shrubs, bushes, and tree saplings) and herb layer plants (herbaceous plants, grasses). Plant species, numbers, and dimensions for woody plants (vines, shrubs, and tree saplings) were measured in micro-plots. Inside quadrats nested within each sub-plot, each individual tree (by species) was measured by height as well as the extent of the foliage along its widest dimension and perpendicular to herbaceous plants. Total vegetation cover and the average height of all plants were visually estimated for graminoid grasses (including corn and soybeans in cropland).
Vegetation biomass was determined by using complete aboveand below-ground harvests, and/or applying allometric regressions. Based upon the results of the tally survey, two to three representative trees near the mean size from trees of each plot were destructively sampled for development of allometric regressions to estimate biomass across the chronosequence. A tree was divided into roots and shoots, and shoots were separated into foliage, live branches, and bole components. Seven to 30 individuals of each species were collected to represent the range of sizes common in understory vegetation plants, and they were separated into foliage, stem, and roots or simply aboveground and root components by species. After clipping down and root excavation, the harvested materials were separated and weighed. Between 500−1000 g of fresh material was randomly sampled from each tissue group in the dissected trees, and taken to the lab.
Down dead materials
DWM was distinguished for five decay classes (DC) as defined by FIA methods (Woodall et al. 2008; Woodall and Monleon 2008). Measurements included branch diameter at the point of transect intersection, total branch length, small and large end diameter, DC, and species, were collected for every CWD piece that intersected a sub-plot transect (USDA Forest Service 2005; Woodall and Williams 2005; Woodall and Monleon 2008). Dimensions (height, width, length) of ground coverage were measured. FWD was sampled with methods described in Woodall et al (2008), and FWD samples for determinations of volume and density were collected in each DC following methods described in Woodall and Monleon (2008) and Catharina et al. (2008). We assumed litter and duff resulted in homogeneous distribution. Tallies for each layer as weight and thickness were conducted on three 50 × 50 cm2quadrants located at the end of each CWD transect, and a fraction of composite samples were collected.
Soil
Data on soil was obtained from profile layers with the general approach described by John et al. (2008). Three 1 m deep profiles of the soil (including humus and mineral soil) adjacent to subplots were used for collecting samples with a soil core sampler (100 cm3). Whole-plot blended batch sub-samples for soil density were dried for 24 hours at 105°C, and other sub-samples were air-dried for 48−72 hours before being processed for testing the physical and chemical characteristics.
Biomass estimation
After oven drying at 85°C for 24−48 hours to reach constant weight, dry weights of all non-soil samples were measured to determine the fresh mass to dry mass conversion factors (p) to apply toward respective components of the whole-sample field mass.
For living plants, raw data was initially graphed to provide a visual assessment of the relationships between foliage, branch, bark, stem, root biomass, and the independent variables. A summation for whole-tree biomass regression models was also developed. Least-square regression models were then developed for individual variables using curve forms. Estimates of these organs biomass were regressed against independent variables, and corresponding biomass allometric equations were developed (Table 2). Models were selected on the basis of the best combination of predictor significance, improvement of fit (R2), and analysis of residuals. Additional equations—as research results from Chen and Zhu (1989)—were provided for estimating the biomass of other tree species in the stand.
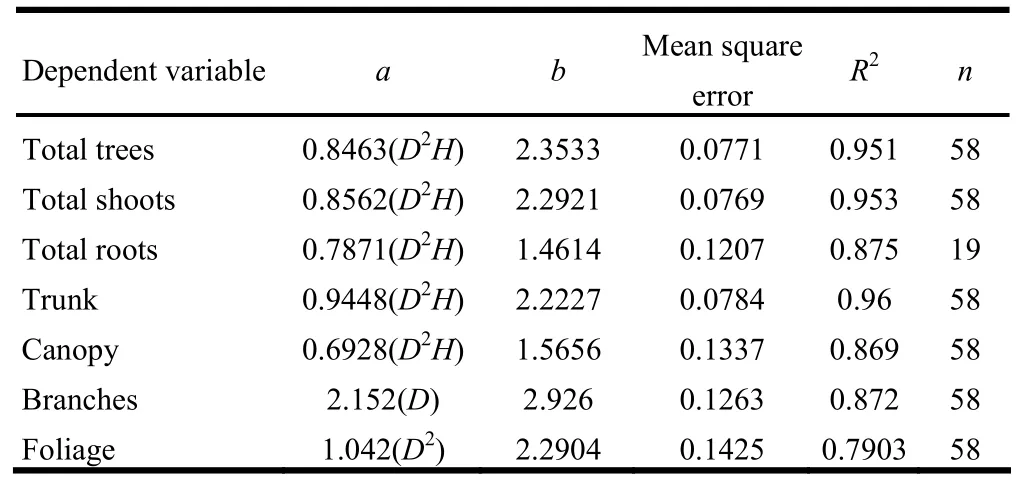
Table 2: Parameters of the allometric equations used to predict tree-component biomass of Korean larch along a chronosequence for stands 7−48 years of age in Lesser Khingan Mountains of China.
Volumes of DDW and forest floor were estimated by diameter, length and thickness of samples for each respective DC. The measured volume per unit plot area data was combined with bulk density (BD, dry mass/ wet volume) measures to develop the DWM biomass estimator. For snags, volume was calculated as live tree, and taken as CWD for biomass. We computed the biomass of DWM (BDWM) using a simple linear equation: where n is the total number of individual DWM samples, f is the conversion factor for per-unit area values, BDiis the bulk density (kg·m-3) of ith individual per decay class, Viis cubic volume (m3) of ith individual.

The entire plot biomass was summarized from all categories, and quantified as biomass density (Mg·ha-1) after scaling from sample area to hectare. The average of plots in each stand was used as a standard value and defined as stand biomass. We chose this method to avoid analysis with skewed data.
Carbon concentration determination
Converting biomass in non-soil sectors to C was based on c, which represented the proportion of C in dry organic matter. To quantify biases in CS estimates, we determined species-specific c values for each collected tissue or component type in the forest plot. After biomass measurements, three oven-dry subsamples were finely ground and homogenized. The resulting powder was sent to for c determination. Powder subsamples of 50−60 mg were determinated by instantaneous combustion with a Multi CN Analyzer (ELEMENTAR Vario EL III, Germany), and c expressed as mg C mass per 100-mg dry mass (%). For QA/QC, every c power subsample was run in triplicate with an error rate of less than 0.01%, and recorded as the mean value c of tissue to eradicate any discrepancies. Weighted mean c (WMC) of biomass tissue or components were weighted by biomass, taking into account their architecture for plants (Bert and Danjon 2006; Tolunay 2009; Zhang and Wang 2009).
Carbon stock and change
To derive CS for all non-soil sectors, c was applied to biomass estimates in the plot, summed, and scaled to an area basis (Mg·ha-1). For soil, we do not include relatively inert inorganic C in this study, but soil organic matter (SOM) in well-mixed oven dried samples of each individual soil layer was determined by employing a potassium dichromate volumetry technique (Man et al. 2010). We corrected the original SOM value for unreacted SOM by multiplying a fixed modification factor of 1.1. After that, SOM was converted to soil organic carbon (SOC) by multiplying c of 0.58 (the Van Benmmelen conversion factor). Thus, soil C stock (SCS, Mg·ha-1) was calculated directly as:
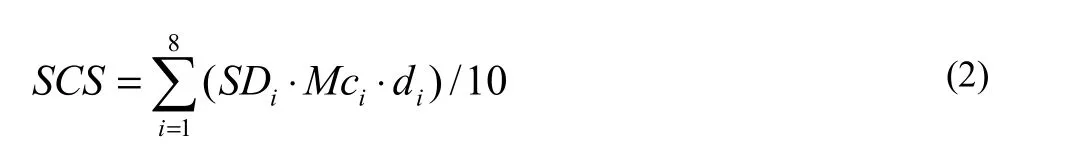
where, SDi(g·cm-3) was the soil density of the ith layer, Mci(g·kg-1) was SOC, di(cm) was soil thickness of ith layer. Given the relatively small area of sample coverage and virtually no gravel (larger than 2-mm grain size) in the clayey loam soil samples, we ignored volume percentage of gravel.

Individual estimates for each C pool were aggregated into FECS (Mg·ha-1) of all stands along the chronosequence. After that, the ANCI (Mg·ha-1·a-1) for the whole ecosystem can be determined by dividing stand age: where, t1was the current stand age (and thus along the chronosequence), C1was FECS of stand at t1, and C2was CS of cropland. The positive value for stock change indicates a net increasein C over the time interval. Alternatively, a negative value would indicate a decrease within the time interval.
Results
Carbon concentrations
Mean tissue c across the Korean larch varied from 40.8% in fine roots to 49.9% in live branches (Table 3). Variations in c within organs across the chronosequence was low, with coefficients of variation (CV) ranging from 0.9% to 8.4%, and live branches and fine roots being the most variable. The WMC of Korean larch reached 48.8% in the crown, 47.6% in the bole, and 45.3% in the roots, which resulted in 47.6% at tree level. Compared to other species as Zhang et al (2010) described, this 47.6% c of Korean larch was smaller than the Amur cork-tree (55.1%), Korean pine (53.2%), Manchurian ash (52.9%), and Manchurian walnut (52.4%) by 5.3−7.5 percentage points, but close to Mongolian oak (47.6%), and slightly larger than Dahurian larch (46.9%), Mono maple (46.4%) and white birch (46.1%). The mean WMC of all tree species was 48.4%.
We figured out the WMC for the sectors: trees (48.4%) >shrubs (46.9%) > herbs (40.8%)> DWM (43.5%) > forest floor (36.8%), all which averaged to 45.9% at stand level.

Table 3: Carbon concentrations (%) of all organs from a chronosequence of Korean larch in Lesser Khingan Mountains of China.
Carbon stock
Tree CS typically increased in a double sigmoid curve across the chronosequence, from 2.670 Mg·ha-1at the 7-year-old stand to 122.863 Mg·ha-1at the 48-year-old stand (Fig. 1 a). The live tree CS ratio was low in the youngest stands, increased sharply to a sub-maximum at age 19, briefly suffered a modest drop, and then ascended asymptotically from 33 to 48 years of age (Table 4). There was a significant difference (Fig.1, p <0.05), in CS between the individual stands of different development stages. The value of C stored in removed wood did not have a marked effect on FECS in the early stages (years 0−19) (Fig. 1 b), but reached maximum (22.416 Mg·ha-1) at 41-year-old stand, and was responsible for 7.83% over the entire chronosequence. The under-story CS displayed a U-shaped curvilinear pattern (Fig. 1 c). Though it recovered gradually, the understory contained no more than 2.54% of FECS. CS dynamics of DDW and standing dead trees are generally similar with S-shaped curves (Fig. 1 d, e) of the tree, while the forest floor rose in a linear fashion (Fig. 1 f). Total CS of DWM in the 48-year-old stand amounted to a sink of about 38.756 Mg·ha-1.
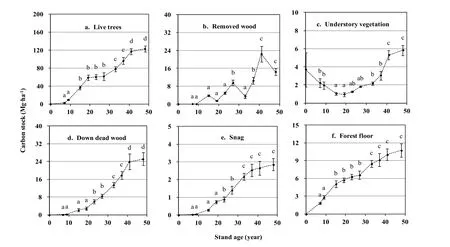
Fig. 1: Carbon stocks of sectors in farmland and Korean larch plantations along a chronosequence for cropland and forest stands 7−48 years of age. The process data were supported with X-axis gaps since relative regular stand age intervals. Error bars represent stand deviations of the means. The different small letters indicate significant difference by Duncan's multiple test (p =0.05).
Soil properties changed with increasing time from the transition of cropland to mature forest. Values of pH ranged from 6.89 to 6.23, generally decreasing over time. Soil BD decreased from 1.263 g cm-3at cropland to 0.955 g cm-3at the 27-year-old stand to less than 0.955 g cm-3in the oldest forest plots. SOC increased from 16.39 g Kg-1to 23.81 g Kg-1as plot age increased (Fig. 2). In Fig. 2, it can be seen that SCS of 84.491 Mg·ha-1at cropland was higher than that at the following forest stands until it was approximately equal to the 23-year-old stand. Since then, SCS increased significantly and the highest CS of 112.363 Mg·ha-1appeared at the 48-year-old stand. As measured across the increasing age of forest plots, the fraction of SCS decreased from 95.82% to 65.59% in the 19-year-old stand, remained constant at 53.72%−51.01% in the 23 to 33-year-old stands, and decreased from 46.75% to 38.19% in the 48-year-old stand.
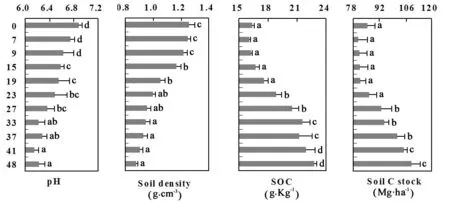
Fig. 2: The properties of soils in farmland and Korean larch plantations along a chronosequence of 7−48 years. Error bars represent stand deviations of the means. The different lower case letters within the same property indicated significantly different at p <0.05.
Carbon stocks from all different sectors were pooled to obtain a FECS combined within vegetation, soil, and DWM. Fig. 3 illustrates the time-trajectory of FECS follows a relative flat sigmoid pattern. At 87.553 Mg·ha-1, the FECS of the 7-year-old
stand almost recovered to the original level of cropland with 88.177 Mg·ha-1. In the 48-year-old stand, FECS reached a maximum of 294.192 Mg·ha-1, which was 3.34 times than that in cropland. Specially, vegetation comprised 48.63% of FECS, SCS comprised 38.19%, and with the remainders of 13.18% in DWM. On average, soil was the biggest sink with 91.847 Mg·ha-1, which was 1.37 times than 66.881 Mg·ha-1of vegetation and represents more than 49.96% percent of FECS.
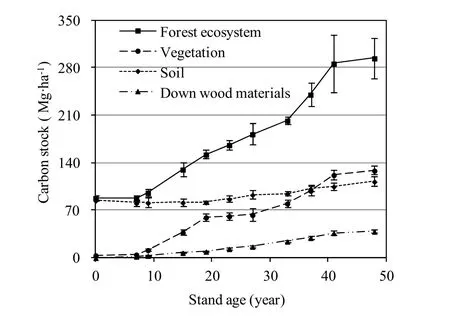
Fig. 3: Carbon stock growth curves by age of ecosystem and each layer of farmland and Korean larch plantations along a chronosequence 7−48 years of age.
Carbon accumulation
Shortly after the transition of land-use types, as seen in the 7-year-old stand, ANCI was below zero as -0.089 Mg·ha-1·a-1due to the considerable SCS loss of 0.554 Mg·ha-1·a-1(Fig. 4). In all of the older plots, ANCI was much higher because C was sequestered in the soil as well as in the non-soil pool. In this example, the 9-year-old stands were sequestering C at an ANCI of 0.913 Mg·ha-1·a-1. Latter, ANCI increased rapidly to 2.83 Mg·ha-1·a-1in the 15-year-old stand, and then it increased slowly thereafter till 41-year-old stand of 4.847 Mg·ha-1·a-1and 4.302 Mg·ha-1·a-1in 48-year-old stand.
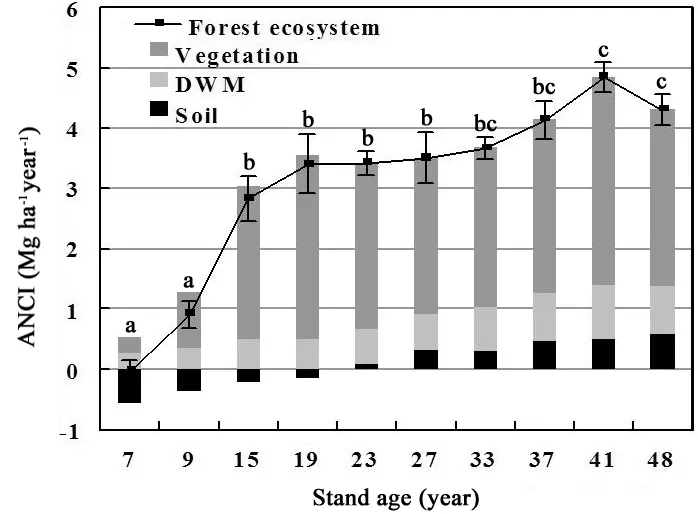
Fig. 4: Annual net carbon increment to farmland of Korean larch plantations along a chronosequence of 7−48 years.
C accumulated largely in vegetation, the proportion that held in ecosystem total ANCI, was as high as 72.66 % of the total (see Table 4). Of the average net C accumulation in all stands, 18.26% is attributed to DWM on the average. For SOC, the accumulation increased from -0.554 Mg·ha-1·a-1in the 7-year-old stand to 0.581 Mg·ha-1·a-1in the 48-year-old stand, presumably through long-term humification processes.
Discussion
Estimate methodology
As such, the cumulative data set and approach reflects a viable and available method for developing stand-level CS estimates of forest ecosystems converted from cropland. The development of FIA plot investigation and the process of estimating biomass and c determination enabled us to accurately quantify FECS including the seven sectors. The stratified sampling of organs or tissues provided an excellent basis for capturing the variation in their dimensions or decay classes in the respective components. The excavation profile to 1 m deep allowed systemic soil sampling. Fresh mass to dry mass conversion factors and c were determined for all tissues or soil samples.
We found that models based on diameter and height provided a good fit to biomass for trees of all ages. Carbon stocks of DWM and soil were calculated based on measurements needed for available sampling formulas. In the case of two plots in the same stand age, values for all sectors in each stand were averaged to obtain one estimate per stand. Although many studies have included ecosystem-level assessments of C standing stocks (Mund et al. 2002; Albaugh et al. 2004), the current study provides precise accounting of all sectors and components rather than calculating one sector of C based on simply the roots versus shoot ratio or constant allocation coefficients. Simultaneously, we estimate the FECS dynamic contained in cropland, immature forest to mature forest, which permitted thorough CS dynamic. Carbon concentration variation
Generally, the c values (i.e., 0.50 for wood, 0.45 for understory vegetation, 0.37 for DWM) are widely accepted as a constant factor for conversion of biomass to CS (IPCC 2006). Mass-based constant values may not be the best for accurate estimation of CS in forest. Variations between and within compartment in c had been considered in previous works (Tolunay 2009; Zhang and Wang 2009). Zhang et al. (2009) presumed that failing to account for the inter- and intra-specific variations in c would introduce a relative error of 6.7% to +7.2% in estimates of biomass CS from inventory data, of which >93% was attributed to ignoring the inter-specific variation in C. For the Korean larch forest ecosystem, the overestimation of C storage reached 4.89% if the usual 0.50, 0.45 and 0.37 conversion factors were used.
Carbon stock partitioning and trend over time
Croplands with degraded vegetation cover contained a lowerbiomass density than the maximum potential value for the site and type of vegetation, established in this study as Korean larch plantations. Vegetation played a significant role as a primary producer in the KLP, which promoted the sustainable FECS growth along the chronosequence. Live standing trees amounted to 86.98% of the total amount of vegetation (which affected 34.99% of the FECS). The CS of thinning removals (as wood products in our study) accounts for a little more than 3.84% of all forest-related C stored by a forest. This is consistent with the statement that "wood products formed only a modest fraction of the total C stock" by other authors (Birdsey and Lewis 2003; Woodbury et al. 2007).
DWM provided corresponding responses to the vegetation changes, and the partitioning of it is proportional to levels at the time of thinning and added logging residue. Unlike reforestation, afforestation accounts for lower C densities of DDW and forest floor C in the initial years after forest establishment on non-forest post-cropland (Smith and Heath 2002). The DWM CS as we estimated represented a noteworthy percentage of the total ecosystem, approximately 9.82%, which was treated as an important transition layer between vegetation and soil (Jandl et al. 2007; Hudiburg et al. 2009).
It is clear that the conversion of agricultural land into KLP forest increased SCS by 32.99%, which is within the range of values from 18% to 53% associated with SCS after cropland was changed to plantation and secondary forest (Guo and Gifford 2002). Afforestation is likely to initially decrease SCS, as a result of disturbance during site preparation associated with erosion, loss of fertilizer regime, and root uptake (Jackson et al. 2002; Paul et al. 2002; Vesterdal et al. 2002; Susan et al. 2006). Undoubtedly, chemical and physical properties of soil used as indicators of soil strength and/or mechanical root impedance, can thus affect SCS (Drewry et al. 2008; Wang et al. 2010b).
Meanwhile, growing vegetation tend to maintain SCS level by continuously supplying C from root turnover (Jobbagy and Jackson 2002; Prévost 2004). Root CS specifically accounted for more than 4.67% of that in KLP ecosystem. According to our estimate, SCS is generally restored to the original stock after 20 years, and the soil C pool approached a new equilibrium, substantially higher than under cropland. Other chronosequence studies also support this theory (Romanyá et al. 2000; Zerva et al. 2005).
Given the slowing down of the development of trees, the associated slowing in total vegetation CS and the saturations of DWM and SCS, FECS will not expand quickly. We do wonder whether the increased growth rate can be sustained for an extended period during the over-mature stage, which has also been questioned by Adrien et al. (2006). Thus, we tend to identify the trajectory of FECS as a sigmoid pattern (Fig. 2).
Comparison with other forests
Our observed sequence of living tree biomass in KLP is generally consistent with that in the nearby Changbai Mountains (Wang et al. 2010a), higher than Larix gmelinii var. plantations (Du et al. 2009), but substantially lower than Larix kaempferi plantations growing in south of China (Shen et al. 2005) (Fig. 5). It is notable that our estimate for the initial stage is lower than previously published estimates for much smaller stand densities. Compared to studies in larch natural forests, the 229.00 Mg·ha-1of average biomass in KLP is close to 250.96 Mg·ha-1of 120 year-old natural Korean larch forest, and also falls to the range of other natural larch forests in China (199.876 Mg·ha-1of Larix gmelinii Rupr.; 277.89 Mg·ha-1of Larix principis rupprechtii and 244.63 Mg·ha-1of Larix sibirica (Wu et al. 1995).
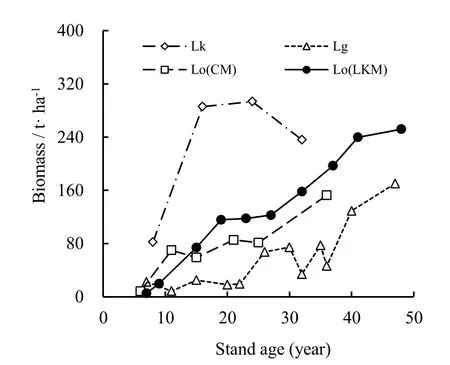
Fig. 5: Comparison of tree biomass with previous estimates of other larch plantations (does not include removed wood). Lk - Larix kaempferi plantation (Shen et al. 2005); Lo(CM) - Larix olgensis plantation (Wang et al. 2010a); Lo(CM) - Larix olgensis plantation we estimated; Lg - Larix gmelinii var. plantation (Du et al. 2009).
Even though biomass CS of living trees and understory accounted for 77.23% and 19.35% of the content in these natural forests, DWM levels were found to be 4.47 times higher than in the comparative natural larch forests mentioned. In explanation, we observed that more logging residues from thinning were left on the site, which was unusual occurrence in natural forests.
Our estimates can also be compared with recent estimates from the literatures for CS in context of forest ecosystem (see Table 5). In comparison to the CS of a 36-year-old KLP stand in the Changbai Mountains (Wang et al. 2010a), our estimates in a 37-year-old stand are obviously higher except C in soil. It can be presumed that extensive management slowed tree development, provided low understory vegetation populations, and a lack of logging residues in KLP. Substantial differences in vegetation and SCS emerged when our estimates are compared with the CS estimates for a 35-year-old maple-beech-birch stand after a clear cut harvest in northeast U.S., based on USDA Forest Service FIA data (Smith et al. 2006). On average, the vegetation CS is above that of national-wide larch forests estimated by Zhou et al (2000), and very nearly the same in DWM CS, while soil in this study demonstrated a relatively low CS.

Table 5: Comparison of our estimates with previously published estimates of C stocks for each C pool.
The major difference between our estimates of SCS and previous ones is that SCS, as we estimated, is close to KLP in Changbai Mountains but comparatively low for the average levels of national-wide Larix forests. Actually, this has been borne out in research on C stocks with a land-use gradient as forest, reforestation, and agricultural land, which indicate that a relatively large proportion of the C loss in soil is due to forest conversion to agricultural land (Pibumrung et al. 2008).
In addition to the numerical estimates discussed above, proportional distribution of CS reported by Woodbury et al. (2007) for U.S. forests and FAO for the whole terrestrial vegetation (FAO 2010) is shown in Table 5. Our estimates are fairly similar, but under those of earlier study, which reported that soil contained 62.9% and vegetation 37.1% of mid-latitude forest C pool (Dixon et al. 1994), and the reference proportion of SCS in mid-latitude temperate forests of China was 63% (Chen 2003). What this suggests is that the afforestation soil of KLP has huge potential for C sequestration and may become an even greater C sink over the long term.
For the annual net C increment, different researchers show various levels by forests; e.g., between 1.483 and 6.402 Mg·ha-1·a-1of C for afforestation (U.S. EPA 2005). KLP served as a net C sink of about 3.076 Mg·ha-1·a-1once established, which is a little greater than 2.65 Mg·ha-1·a-1of Larix gmelinii Rupr. plantation (Jiang and Zhou 2002). It must be noted that ANCI of KLP peaked in a 41-year-old stand and amounted to a net C increase in forest ecosystem of 4.9 Mg·ha-1·a-1, which is a comparable to 4.8 Mg·ha-1·a-1of that in deciduous broad-leaved forest in the warm temperate zone of China (Sang et al. 2002).
Implication of stand management
Associated with shifts in land use, large natural disturbances, such as extensive insect outbreaks or large fires were not found in our study region, but all stands experienced management activities, as described above. Also, density-dependent mortality had not occurred at the stand level; most trees in the plot appeared vigorous, probably a response to forest practices. It is undeniable that artificial regeneration includes greater dominance of commercially desirable tree species, greater control over the number of trees established, and more rapid establishment of trees, all of which increase growth of the desired trees. Jandl et al. (2007) confirmed that land-use changes—such as those resulting from afforestation and management of fast-growing tree species—have an immediate effect on the regional rate of C stock by incorporating CO2in plant biomass.
Young KLP stands increased total C storage in dense stands, except where severe competition significantly reduced growth rates (David et al. 1997). Tending, pruning, and the predominant forest management practices, like pre-commercial thinning and commercial thinning, were conducted to focus growth on fewer trees, and to reduce competition among plants. These interventions concentrated stand growth on fewer larger trees, with little overall change in C storage.
The greatest CS accumulation was found in a small number of live trees from 41 ages on, which dominated in term of FECS. This confirmed that FECS had increased not only in quantity but also in quality, as fewer large trees in the 48-year-old stand concentrated more C than a greater number of smaller trees. Some have calculated that harvesting timber from an over-mature forest can sequester substantial additional C because the forest is currently sequestering little additional C and the timber can continue to store C for decades in long-term solid wood products (Gorte 2009). As a management cycle, after the harvest rotation, the newly established stand can sequester large amounts of C through its vigorous growth.
After cutting some of the existing vegetation on a site, slash treatments (like rolling, chopping and crushing) are not designed to compact the biomass and accelerate DWM deterioration. DWM accumulation was a little greater in KLP with mean value 18.051 Mg·ha-1, and lower in natural Korean larch forests and Korean pine forests, with mean values of 15.12 and 17.64 Mg·ha-1respectively (Wang et al. 2010a). Depending largely on the local climate, it would typically take several months to years for the fallen materials begins to deteriorate, and to release C to atmosphere and incorporates C as organic matter in the soil. DWM turnover is also affected by the C: N (nitrogen) ratio and other determinants of DWM quality (Guo and Gifford 2002).
With regard to forest soil cycles, soil C is lost following thinning and timber harvesting, reducing canopy cover, and disturbing the surface and thus accelerating decomposition rates (Robert et al. 1991; David et al. 1997). Additionally, a portion of the C sequestrated by soil is then quickly absorbed by new growth resulting from the treatment (Gorte 2009). By leaving the thinning residues on the soil surface, however, their input into the soil may compensate for losses (Heleen and Sheila 1999). The initial C accumulation occurs in the forest floor. The dynamic pattern of long-term equilibrium of SCS depends on climate, which influences plant growth and the turnover rate of organic matter. Litter-derived C was moved into the mineral soil, but it remained unstabilized and was lost rapidly through decomposi-tion (Susan 2000; Hagedorn et al. 2003).
After several decades, more C is moved to the mineral soil (Hooker and Compton 2003; Johnson et al. 2003; DeGryze et al. 2004). Other studies have shown that some old-growth forests, even during the later stages of succession, continue to accumulate C in their soils overtime (Silver et al. 2004; Zhou et al. 2006). Rationally, forest land has the potential to increase soil C sequestration if proper practices and management are implemented, such as reduced thinning or harvest residue removal (Richardson et al. 2002).
Conclusion
Our research supports a growing consensus that afforestation of cropland increases CS, and shows certain predictable effects after implementing GGP in China. The estimated forest ecosystem carbon stock of Korean larch plantations across chronosequence is useful for CS estimate on (two-way) transitions between agriculture and forest, and dynamics following afforestation. In this study, the amount of total C storage in the agricultural land was estimated at 88.2 Mg·ha-1. We estimated the distribution of CS to stand-level, individual-sector, and forest ecosystem of KLP ranging in age from 7 to 48 years, for afforestation conversions between forestland and agricultural land based on plot-level investigation data and samples.
Though Korean larch has a lower c than some broadleaved species, the fast development of KLP brings along high growth of CS, making the appropriate species selection for afforestation crucial in the Lesser Khingan Mountains area. Overall KLP forest ecosystem C was estimated at 183.9 Mg·ha-1, combined within vegetation (73.9 Mg·ha-1), DWM (18.1 Mg·ha-1) and soil (91.9 Mg·ha-1). Worth noting, the 48-year-old forest sequestered 290.3 Mg·ha-1net C, or 4.3 Mg·ha-1·a-1, showing superior capacity in C sequestration.
The growth of vegetation biomass does create a massive influx of C, which does increase the C accumulation in DWM and soil. Therefore, differences in CS between communities were primarily the result of differences in vegetation biomass, soil constitutes the largest pool, and the increasing BD and decreasing of SCS will both make more soil C gains. Furthermore, these appropriately managed native ecosystems will have great potential for sequestration in the future.
Acknowledgements
We express our sincere appreciation to Xiao-Yu Guo, Yao Fu, Feng-Jiao Liu, Yu Dong, Tian-Bo Wang, Yi-Fu Wang for their field work assistance, the members of Langxiang Forestry Bureau for their friendship and assistance. We also thank the Research Center for Eco-Environmental Sciences of Chinese Academy of Sciences for its support during the study.
Adrien CF, David JPM, Evan HD, John L, Kirsten SH, Robert BJ, Hyun SK, Roser M, Heather RM, Ram O, Jeffrey SP, William HS. 2006. Progressive nitrogen limitation of ecosystem processes under elevated CO2in a warm-temperature forest. Ecology, 87(1): 15−25.
Albaugh TJ, Allen HL, Doughtery PM, Johnsen KH. 2004. Long term growth responses of loblolly pine to optimal nutrient and water resource availability. Forest Ecol Manag, 192: 3−19.
Bechtold WA, Patterson PL (Eds.). 2005. The enhanced forest inventory and analysis program—national sampling design and estimation procedures. Gen. Tech. Rep. SRS-80. US Department of Agriculture, Forest Service, Southern Research Station, Asheville, NC.
Bert D, Danjon F. 2006. Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). Forest Ecol Manag, 222: 279−295.
Birdsey RA, Lewis GM. 2003. Carbon in U.S. forests and wood products, 1987-1997: state-by-state estimates. Gen. Tech. Rep. NE-310. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station. 42 p
Bonan GB. 2008. Forests and climate change: forcing, feedbacks, and the climate benefits of forests. Science, 320(5882): 1444−1449.
Bradford JB, Weishampel P, Smith ML, Kolka R, Birdsey RA, Ollinger SV, Ryan MG. 2009. Detrital carbon pools in temperate forests: magnitude and potential for landscape-scale assessment. Can J For Res, 39: 802−813.
Brown S, Swingland IR, Hanbury TR, Prance GT, Myers N. 2002. Changes in the use and management of forests for abating carbon emissions: issues and challenges under the Kyoto Protocol. Philos. Trans. Math Phys Eng Sci, 360(1797): 1593−1606.
Brown S. 2002. Measuring, monitoring, and verification of carbon benefits for forest-based projects. Phil Trans R Soc London Ser A: Math Phys Eng Sci, 360: 1669−1683.
Canadell JG, Le QC, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G. 2007. Contributions to accelerating atmospheric CO2growth from economic activity, carbon intensity, and efficiency of natural sinks Proc. Natl Acad Sci, 104(47): 66−70.
Cao W, Li JY. 2007. Flora and distribution in Lesser Khingan Mountains, China. Beijing: Science Press (In Chinese)
Carolina MR, Belén FS. 2005. Natural revegetation on topsoiled mining-spoils according to the exposure. Acta Oecologica, 28: 231−238.
Catharina JE, Schulp GN, Peter HV, Rein WW. 2008. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. Forest Ecol Manag, 256: 482−490.
Chastain JRA, Currie WS, Townsend PA. 2006. Carbon sequestration and nutrient cycling implications of the evergreen understory layer in Appalachian forests. Forest Ecol Manag, 231: 63−77.
Chen CG, Zhu JF. 1989. Biomass manual of main trees in northeastern China. Beijing: China Forestry Press, 528 p (In Chinese)
Chen XL. 2003. Researches on carbon sequestration functions of main forest types in northern China. Doctoral Thesis: Beijing Forestry University (In Chinese with English abstract)
David MS, Bruce CL, Matthew JK, Mark SAP. 1997. The Practice of Silviculture: Applied Forest Ecology. New York, NY: John Wiley & Sons, Inc..
DeGryze S, Six J, Paustian K, Morris SJ, Paul EA, Merckx R. 2004. Soil organic carbon pool changes following land-use conversions. Global Change Biology, 10: 1120−1132.
Drewry JJ, Cameron KC, Buchan GD. 2008. Pasture yield and soil physicalproperty responses to soil compaction from treading and grazing - a review. Aust J Soil Res, 46: 237−256.
Du HM, Wang C, Gao HZ. 2009. Carbon-sink function of artificial Larix principis - rupprechtii plantation. Chinese Journal of Eco-Agriculture, 17(4): 756−759. (In Chinese with English abstract)
Dixon RK, Brown S, Houghton RA. 1994. Carbon pols and flux of global forest ecosystems. Science, 263: 185−190.
Erb KH. 2004. Land-use related changes in aboveground carbon stocks of Austrias terrestrial ecosystems. Ecosystems, 7: 563−572.
FAO. 2010. Global forest resources assessment. FAO Forestry Paper 163. FAO, Rome
Feng RF, Yang WQ, Zhang J. 2006. Artificial forest management for global change mitigation. Acta Ecologica Sinica, 26(11): 3870−3877 (In Chinese with English abstract).
Grace J. 2004. Understanding and managing the global carbon cycle. J Ecol, 92(2): 189−202.
Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, Houghton RA, Jenkins JC, Kohlmaier GH, Kurz W, Liu SR, Nabuurs, GJ, Nilsson S, Shvidenko AZ. 2002. Forest carbon sinks in the Northern Hemisphere. Ecol Appl, 12: 891−899.
Gorte RW. 2009. Carbon Sequestration in Forests. Congressional Research Service [online] [viewed on September 12, 2011]. Available on the Internet: http://www.fas.org/sgp/crs/misc/RL31432.pdf
Guo LB, Gifford RM. 2002. Soil carbon stocks and land use change: a meta analysis. Global Change Biology, 8: 345−360.
Heath LS, Smith JE, Skog KE, Nowak DJ, Woodall CW. 2011. Managed forest carbon estimates for the US greenhouse gas inventory, 1990-2008. Journal of Forestry, 109(3): 167−173.
Heleen AD, Sheila K. 1999. Carbon stocks in Norwegian forest soils and effects of forest management on carbon storage. Rapport fra skogforskningen (Supplement 14): 52 s.
Hooker TD, Compton JE. 2003. Forest ecosystem carbon and nitrogen accumulation during the first century after agricultural abandonment. Ecological Applications, 13: 299−313.
Hudiburg T, Law B, Turner DP, Campbell J, Donato D, Duane M. 2009. Carbon dynamics of Oregon and Northern California forests and potential land-based carbon storage. Ecological Applications, 19(1):163−180.
IPCC. 2006. IPCC Guidelines for National Greenhouse Gas Inventories, Vol.2. Edited by S Eggleston, L Buendia, K Miwa, T Ngara and K Tanabe (Japan: IGES). Available at: www.ipcc-nggip.iges.or.jp/public/2006gl/index.html (26 Sep. 2010)
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA. 2007. How strongly can forest management influence soil carbon sequestration? Geoderma, 137: 253−268.
Jiang YL, Zhou GS. 2002. Carbon balance of Larix gmelinii forest and impacts of management practices. Acta Phytoecologica Sinica, 26(3): 317−322. (In Chinese with English abstract)
Johnson MG, Kern JS. 2003. Quantifying the organic carbon held in forested soils of the United States and Puerto Rico. In: Kimble JM, Heath LS, Birdsey RA, Lal R (Eds.), The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect. Boca Raton, FL: CRC Press, pp. 47−72.
Johnson D, Todd D, Tolbert V. 2003. Change in ecosystem carbon and nitrogen in a Loblolly pine plantation over the first 18 years. Soil Science Society America Journal, 67: 1594−1601.
Jobbagy EG, Jackson RB. 2002. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications, 10(2): 423−436.
John L, Sharon AB, Susan EZ, Deeya G, Rebecca R, Adrien CF, Robert BJ, Elizabeth AS, William HS. 2008. Soil carbon sequestration in a pine forest after 9 years of atmospheric CO2enrichment. Global Change Biology, 14: 1−13.
King JS, Giardina CP, Pregitzer KS, Friend AL. 2007. Biomass partitioning in red pine (Pinus resinosa) along a chronosequence in the Upper Peninsula of Michigan. Can J For Res, 37(1): 93−106.
Lal R. 1999. Soil management and restoration for C sequestration to mitigate the accelerated greenhouse effects. Progress in Environmental Science, 1(4): 307−326.
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S, Grelle A, Hari P, Jarvis PG, Kolari P, Kowalski AS, Lankreijer H, Law BE, Lindroth A, Loustau D, Manca G, Moncrieff JB, Rayment M, Tedeschi V, Valentini R, Grace J. 2007. The human footprint in the carbon cycle of temperate and boreal forests. Nature, 447: 849−851.
Man XL, Liu B, Li Y. 2010. Distribution characteristics of organic carbon, nitrogen and phosphorus in the soils of herbaceous peat swamps in the Xiaoxing’an Mountains. Journal of Beijing Forestry University, 32(6): 48−53. (In Chinese with English abstract)
Meelis SMScF. 2009. Carbon dynamics of boreal mixed woods in central Canada. Lakehead University.
Mund M, Kummetz E, Hein M, Bauer GA, Schulze ED. 2002. Growth and carbon stocks of a spruce in central Europe. Forest Ecol Manag, 171: 275−296.
Oscar JC, Russell MW, Kenneth GM. 2004. Carbon monitoring costs and their effect on incentives to sequester carbon through forestry. Mitigation and Adaptation Strategies for Global Change, 154: 273−293.
Paul K, Polglase P, Nyakuengama J, Khanna P. 2002. Change in soil carbon following afforestation. Forest Ecol Manag, 168: 241−257.
Pibumrung P, Gajaseni N, Popan A. 2008. Profiles of carbon stocks in forest, reforestation and agricultural land, Northern Thailand. Journal of Forestry Research, 19(1): 11−18.
Pregitzer KS. 2003. Carbon cycling in forest ecosystems with an emphasis on belowground processes. In: The potential of U.S. forest soils to sequester carbon and mitigate the greenhouse effect. CRC Press, Boca Raton, Fla. pp. 93−107
Richardson J, Björheden R, Hakkila P, Lowe AT, Smith CT (Eds.). 2002. Bioenergy from Sustainable Forestry: Guiding Principles and Practices. Kluwer Academic, Dordrecht, The Netherlands.
Romanya J, Cortina J, Falloon P, Coleman K, Smith P. 2000. Modeling changes in soil organic matter after planting fast growing Pinus radiata on Mediterranean agricultural soils. Eur J Soil Sci, 51: 627−641.
Robert CM, Douglas GF. 1991. A review of the role of temperate forests in the global CO2balance. Journal of the Air and Waste Management Association, 41(6): 798−807.
Sang WG, Su HX, Chen LZ. 2002. Coupling biomass and energy in warm temperate deciduous broad-leaved Oak (Quercus liao tungensis) forest ecosystem. Acta Phytoecologica Sinica, 26(S1): 88−92. (In Chinese with English abstract)
Schwarze R, Niles JO, Olander J. 2002. Understanding and managing leakage in forest-based green house gas-mitigation projects. Philos Trans Math Phys Eng Sci, 360(1797): 1685−1704.
Shen ZK, Lu SP, Ai XR. 2005. Study on biomass and productivity of Larix Kaempferi Plantation. Journal of Hubei Institute for Nationalities, 23(3):289−292. (In Chinese with English abstract)
Silver WL, Kueppers LM, Lugo AE, Ostertag R, Matzek V. 2004. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecological Applications, 14(4): 1115−1127.
Skog KE, Pingoud K, Smith JE. 2004. A method countries can use to estimate changes in carbon stored in harvested wood products and the uncertainty of such estimates. Environmental Management, 33(S1): 65−73.
Smith JE, Heath LS. 2002. A model of forest floor carbon mass for United States forest types. USDA Forest Service, Northeastern Research Station, NE-RP-722, Newtown Square, PA
Smith JE, Heath LS, Skog KE, Birdsey RA. 2006. Methods for calculating forest ecosystem and harvested carbon with standard estimates for forest types of the United States. Gen. Tech. Rep. NE-343. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station. 216 p
Smith JE, Heath LS. 2008. Carbon stocks and stock changes in U.S. forests. In: U.S. Department of Agriculture. U.S. Agriculture and Forestry Greenhouse Gas Inventory: 1990-2005. Technical Bulletin No. 1921. Washington, DC: Office of the Chief Economist: 65-80, C1-C7
Stinson G, Kurz WA, Smyth CE, Neilson ET, Dymond CC, Metsaranta JM, Boisvenue C, Rampley GJ, Li Q, White TM, Blain D. 2011. An inventory-based analysis of Canada's managed forest carbon dynamics, 1990 to 2008. Global Change Biology, 17: 2227−2244.
Susan T. 2000. Age of soil organic matter and soil respiration: radiocarbon constraints on belowground C dynamics. Ecological Applications, 10: 399−410.
Susan T, Enir SDC, Daniel CN, Plinio BDC, Luiz AM, David R, Teresa R, Whendee S. 2006. Dynamics of fine root carbon in Amazonian tropical ecosystems and the contribution of roots to soil respiration. Global Change Biology, 12: 217−229.
Tobias K, Pontus O, Oleh C, Matthias B, Katarzyna O, Curtis EW, Richard AH, Patrick H, William SK, Volker CR. 2010. Post-Soviet cropland abandonment, forest recovery, and carbon sequestration in western Ukraine. Global Change Biology, 17(3): 1335−1349.
Tolunay D. 2009. Carbon concentrations of tree components, forest floor and understorey in young Pinus sylvestris stands in north-western Turkey, Scandinavian Journal of Forest Research, 24(5): 394−402.
UNFCCC. 2011. Land Use, Land-use Change and Forestry, Draft Decision /CMP.6 Available at: http://unfccc.int/files/meetings/cop16/application/pdf/cop16lulucf.pdf)
USDA Forest Service. 2007. Forest Inventory and Analysis National Core Field Guide, version 4.0. Available at: http://www.fia.fs.fed.us/ library/field -guides-methods-proc (28 Apr. 2008)
USDA Forest Service. 2011. Forest inventory and analysis national core field guide; Phase 3 field guide, Down Woody Materials. Version 5.0. St. Paul, MN. Available at http://fia.fs.fed.us/library/ field-guides- methods-proc/docs/ (18 Oct. 2011)
USDA Forest Service, North Central Research Station, St. Paul, MN. US EPA. 2005. Inventory of U.S. Greenhouse gas emissions and sinks: 1990-2003. EPA 430-R-05-003. Available at http://yosemite.epa.gov/oar/globalwarming.nsf/content/ResourceCenterP ublicationsGHGEmissionsUSEmissionsInventory2005.html (12 Nov. 2011)
U.S. Environmental Protection Agency, Office of Atmospheric Programs, Greenhouse Gas Mitigation Potential in U.S. forestry and Agriculture, EPA 430-R-05-006, Washington, DC, November 2005, Table 2-1, http://www.epa.gov/sequestration/pdf/greenhousegas2005.pdf
Vesterdal L, Ritter E, Gundersen P. 2002. Change in soil organic carbon following afforestation of former arable land. Forest Ecol Manag, 169: 137−147.
Wang CM, Shao B, Wang RN. 2010a. Carbon sequestration potential of ecosystem of two main tree species in Northeast China. Acta Ecologica Sinica, 30(7): 1764−1772. (In Chinese with English abstract).
Wang YG, Li Y, Ye XH, Chu Y, Wang XP. 2010b. Profile storage of organic/inorganic carbon in soil: From forest to desert. Science of the Total Environment, 408: 1925−1931.
Woodall CW, Monleon VJ. 2008. Sampling protocol, estimation, and analysis procedures for the down woody materials indicator of the FIA program. Gen. Tech. Rep. NRS-22. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. 68 p
Woodall CW, Heath LS, Smith JE. 2008. National inventories of down and dead woody material forest carbon stocks in the United States: Challenges and opportunities. Forest Ecol Manag, 256: 221−228.
Woodall CW, Williams MS. 2005. Sampling, estimation, and analysis procedures for the down woody materials indicator. Gen Tech Rep, NC-256.
Woodbury PB, Smith JE, Heath LS. 2007. Carbon sequestration in the U.S. forest sector from 1990 to 2010. Forest Ecol Manag, 241: 14−27.
Woodbury PB, Heath LS, Smith JE. 2006. Land use change effects on forest carbon cycling throughout the southern USA. J Environ Qual, 35(4): 1348−1363.
Wu G, Feng ZW. 1995. Study on the biomass of Larix SPP. Forest community in the frigid-temperate zone and the temperate zone of China. Journal of norhteast forestry university, 23(1): 95−101. (In Chinese with English abstract)
Wu QB, Wang XK, Duan XN, Deng LB, Lu F, Ouyang ZY, Feng ZW. 2008. Carbon sequestration and its potential by forest ecosystems in China. Acta Ecologica Sinica, 28(2): 517−524. (In Chinese with English abstract)
Zerva A, Ball T, Smith KA, Mencuccini M. 2005. Soil carbon dynamics in a Sitka spruce (Picea sitchensis (Bong.) Carr.) chronosequence on a peaty gley. For Ecol Manage, 205: 227−240.
Zhang QZ, Wang CK. 2009. Carbon concentration variability of 10 Chinese temperate tree species. Forest Ecol Manag, 258(5): 722−727.
Zheng DL, Heath LS, Ducey MJ, Smith JE. 2011 Carbon changes in conterminous US forests associated with growth and major disturbances: 1992-2001. Environmental Research Letters, 6(1): 1−10.
Zhou GY, Liu SG, Li Z, Zhang DQ, Tang XL, Zhou CY, Yan JH, Mo JM. 2006. Old-growth forests can accumulate carbon in soils. Science, 314: 14−17.
Zhou YR, Yu ZL, Zhao SD. 2000. Carbon storage and budget of major Chinese forest types. Acta Phytoecologica Sinica, 24(5): 518−522 (In Chinese with English abstract).
DOI 10.1007/s11676-014-0523-5
Project funding: This research was supported by the Special Public Interest Research and Industry Fund of Forestry (No. 200904003-1), Project of Forestry Science and Technology Research (No. 2012-07), and the Importation of Foreign Advanced Agricultural Science and Technology Program (2008-4-48).
The online version is available at http://www.springerlink.com
Wei MA, Yan-hong LIU (), Yu-jun SUN
The Key Laboratory of Silviculture and Conservation of the Ministry of Education, College of Forestry, Beijing Forestry University, Beijing, China. Email: liuyh@bjfu.edu.cn
Jason Grabosky
New Jersey Agricultural Experiment Station, Rutgers University, New Jersey, USA.
Corresponding editor: Hu Yanbo
杂志排行
Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Biomass accumulation and nutrient uptake of 16 riparian woody plant species in Northeast China
- Cloning and sequence analysis of nine novel MYB genes in Taxodiaceae plants
- Genetic and morphological variation in natural teak (Tectona grandis) populations of the Western Ghats in Southern India
- Improved salt tolerance of Populus davidiana × P. bolleana overexpressed LEA from Tamarix androssowii
- Growth performance of Avicennia officinalis L. and the effect of spacing on growth and yield of trees planted in the Western coastal belt of Bangladesh
