Metal cation crosslinking of TiO2-alginate hybrid gels
2014-09-06MengJunliWuMinDingWenLiYingZhangJinNiHenmei
Meng Junli Wu Min Ding Wen Li Ying Zhang Jin Ni Henmei
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
Metal cation crosslinking of TiO2-alginate hybrid gels
Meng Junli Wu Min Ding Wen Li Ying Zhang Jin Ni Henmei
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
In order to improve the substrate diffusion properties and stability of an immobilized enzyme, alginate microgels modified with TiO2nanoparticles were employed as the enzyme immobilizing support. Ionotropic gelation was applied for the preparation of hybrid gels, while Ca2+, Ce3+, Ni2+, Cu2+and Fe3+were employed as the crosslinkers. Papain was selected as the model enzyme. UV-Vis spectroscopy was employed to investigate the activity of papain to evaluate kinetics and stability. Analysis results show that the highest affinity, the lowest Michaelis-Menten constant (Km=11.0 mg/mL) and the highest stability are obtained when using Cu2+as the crosslinker. The effect of the mass ratio of TiO2to papain on the stability and leakage of papain is also investigated, and the results show that 10∶1 (TiO2∶papain) is optimal because the proper use of TiO2can reduce enzyme leakage and ensure enzyme stability. Preparing Cu/alginate/TiO2hybrid gels via ionotropic gelation can provide a satisfactory diffusion capability and enzyme stability.
cationic crosslinker;diffusion;hybrid gel;ionotropic gelation; Michaelis-Menten constant;titanium dioxide modified alginate
Nanoparticles are found in an increasing number of applications in biological materials because of their interesting properties and behaviors[1]. Their large specific surface area allows them to immobilize enzymes and retain enzymatic activities under moderate conditions in a state of high-leveled dispersion[2-3]. Nanosized TiO2is an ideal enzyme support, ascribed to its strong bioadhesive properties, high enzyme loading capacity and activity, and efficient substrate diffusion[4].
Ionic biopolymers such as alginate and pectin undergo ionotropic gelation and precipitation to form microspheres, in the presence of divalent cations such as Ca2+, Cu2+, Zn2+, and trivalent cations such as Al3+. The mechanism of ionotropic gelation is an electrostatic force between the carboxylic acid groups and cations[5]. Ion-crosslinked alginate microgels are suitable enzyme supports, because of the relatively mild/inert aqueous environment within their matrix, and high gel porosity for substrate and product diffusion[6]. A properly structured alginate matrix can decrease steric hindrance, and provide a network to accommodate conformational change during enzyme reactions. Structures with larger pores allow rapid diffusion, fast enzyme reactions and high enzyme activity. However, enzyme leakage from porous alginate matrices can reach 30% to 80%[7-8].
The current study aims to improve the substrate diffusion properties and immobilized enzyme stability of alginate microgels. Nanosized TiO2-modified alginate was used as an immobilizing support. Ca2+, Ce3+, Ni2+, Cu2+and Fe3+were used as crosslinkers for ionotropic gelation, and papain was used as a model enzyme. The effect of crosslinkers on enzyme kinetics and stability was investigated. The effect of the mass ratio of TiO2to papain in relative enzyme activity was optimized for Cu/alginate/TiO2hybrid gel-immobilized papain.
1 Materials and Methods
1.1 Materials
The nanoparticles of TiO2(14 nm) with a surface area of 280 m2/g was prepared in the laboratory. Enzyme, papain, cysteine and sodium alginate were purchased from the Chinese Medicine Group Co., Ltd. Calcium chloride, cerous nitrate, nickel chloride, copper chloride and ferric chloride were bought from Shanghai Hua Jing Biological High-Tech Co., Ltd. All reagents were used as received.
1.2 Preparation of alginate/TiO2microsphere hybrid gel-immobilized papain
TiO2nanoparticles (0.2 g) were ultrasonically dispersed in 2 mL of 0.1 mol/L tris (hydroxymethyl) aminomethane (Tris) buffer (pH 7.5) for 20 min. Thereafter, 1 mL of 20 mg/mL papain was added and shaken for 30 min. After a total of 5 mL of alginate was added, the mixture was stirred for 30 min, and then poured into 10 mL of crosslinker solution and aged at room temperature for 1 h to yield the immobilized enzyme microgels. Polymer chains were crosslinked by exchanging gluronic acid Na+with Ca2+, Ce3+, Ni2+, Cu2+and Fe3+. The structure formed was called an egg-box as depicted in Fig.1. As a result, gel networks were formed through the crosslinking of several different chains. Immobilized enzyme microgels were rinsed with pH 7.5 Tris buffer, dried at room temperature for 6 h and stored at 4 ℃ until use.
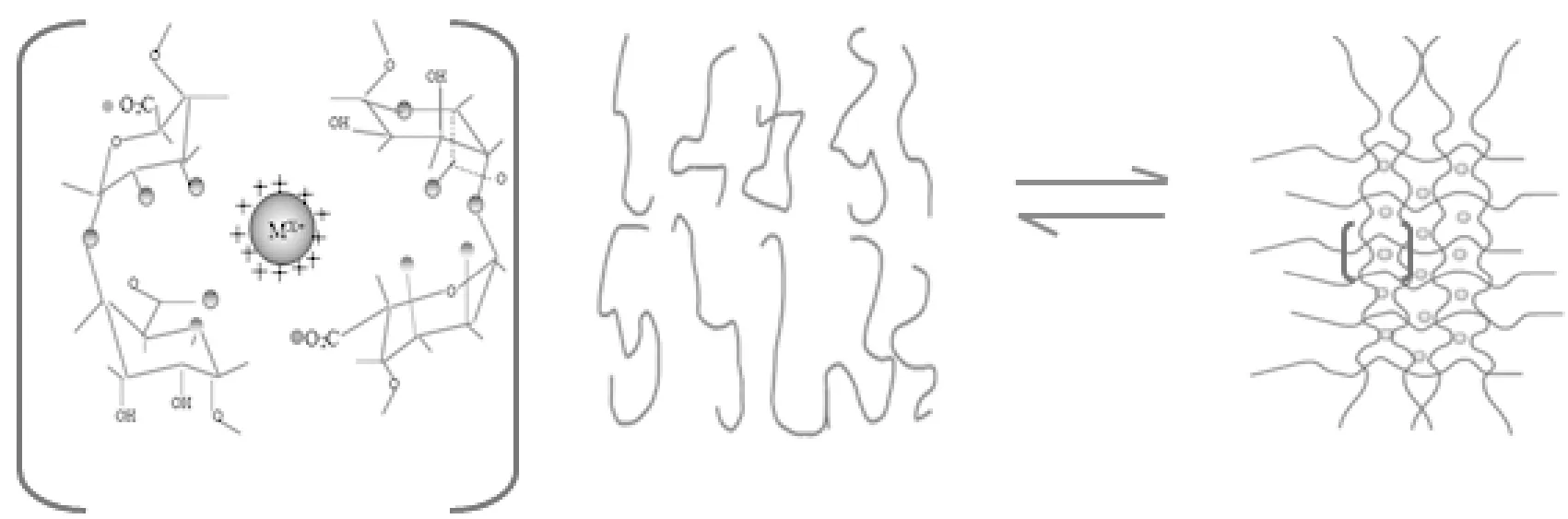
Fig.1 Egg-box association of alginate matrix undergoing ionotropic gelation procedure in the presence of multivalent cations
1.3 Assays of immobilized enzyme activity
Papain activity was assayed using a casein substrate. The hydrolysis of casein catalyzed by papain released tyrosine, whose presence was detected by the UV absorbance at 275 nm. An appropriate amount of microgels was added to 3 mL of enzyme activator containing 0.005 mol/L cysteine and 0.002 mol/L EDTA. The resultant solution was incubated at 37 ℃ for 10 min. After adding 10 mL of 10 mg/mL casein (preheated to 37 ℃ in a water bath), the solution was incubated at 37 ℃ for 10 min. 10 mL of 5% quality fraction trichloroacetic acid was added to terminate the reaction[9]. After centrifuging at circa 4 000 g for 10 min, the serum was measured by using a UV/VIS spectrophotometer (Metertek SP-830, Metertech Inc., Taiwan, China). One unit of enzyme activity (U, active unit) was defined as the amount of enzyme liberating 1 μg of tyrosine per minute under the assay conditions. The residual activity and relative activity of the immobilized enzyme were calculated by[3]
Residual activity=
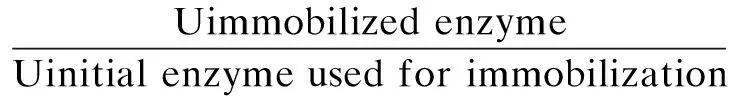
(1)
Relative enzyme activity=
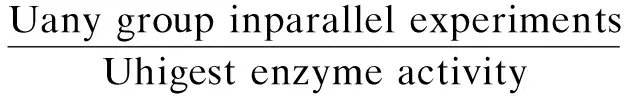
(2)
1.4 Microgel catalytic kinetics
The maximum reaction velocityVmaxand Michaelis-Menten constantKmwere determined at pH 7.5 and 37 ℃. The casein substrate concentration was varied from 2.0 to 10.0 mg/mL. The papain reaction rateVwas estimated from the Michaelis-Menten equation, and parameters for the equation were estimated using the linearization method of Lineweaver-Burk[10].
1.5 Determination of leakage from metal/alginate/TiO2hybrid gels
The enzyme leakage from alginate/TiO2hybrid gels was evaluated. Immobilized microgels in 10.0 mL of Tris buffer were shocked at 37 ℃ for 6 h. The mixture was centrifuged at approximately 4 000 g, and the clear supernatant was collected. The mass of papain was determined spectrophotometrically (275 nm) at 37 ℃. Microgel leakage is calculated by
Percentage of leakage of the microspheres=

2 Results and Discussion
2.1 Preparation of immobilized enzyme microgels
Crosslinking with the different cations yielded well-dispersed microgels of alginate/papain. Alginate/TiO2/papain hybrid microgels all had an average diameter of 2.5 mm. The microgels were immersed in saturated calcium chloride to investigate their density and the effect of the crosslinker. Microgels ionotropic-gelated by the five different cations possessed different alginate network structures, particularly with respect to surface characteristics and the pore structure, as shown in Fig.2. It was observed that the density of the two microgels crosslinked with Cu2+and Ca2+, respectively, which is less than that of the saturated CaCl2solution. It is suggested that their porosity and specific surface areas were different[11]. Lower density microgels exist on the top of the saturated calcium chloride, and the higher density ones at the bottom. This implies that the diffusion properties of porous hybrid microgels can be controlled by the ionotropic gelation conditions.
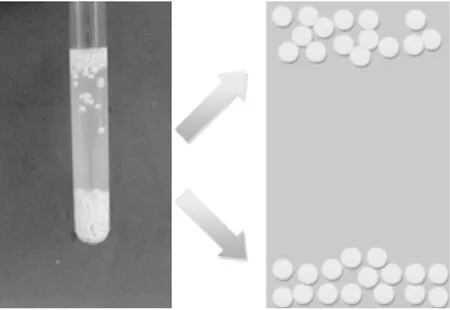
Fig.2 Different density properties of immobilized enzyme microspheres affected by cross-linking agent in saturated calcium chloride solution
2.2 Kinetics of catalytic hydrolysis
Kinetic parameters were obtained by using the Lineweaver-Burk double reciprocal model:
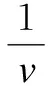
Microgel porosity greatly depended upon the type of crosslinker and its concentration. In the immobilized enzyme reaction, the substrate had to be transported from the reaction solution to the outer surface of gel matrix, and then to the inner matrix. Accordingly, the resulting diffusion and mass transfer properties were important parameters in the ionotropic gelation procedure.
The same amount of free and immobilized enzyme applied to 10 mL Tris buffer solution (0.1 mol/L, pH 7.5) at 37 ℃ for 10 min was 20 mg. The substrate concentration of casein varied between 2.0 and 10.0 mg/mL. Each point represented the average value of three independent measurements.
TheKmvalues of free (not shown) and immobilized papain on Ca2+, Ce3+, Ni2+, Cu2+and Fe3+crosslinked microgels (shown in Fig.3 and Tab.1) were 7.3, 19.3, 24.3, 20.5, 11.0 and 27.7 mg/mL, respectively. LowerKmvalues for immobilized papain indicated a higher affinity to the substrate, and correspondingly, a higher catalytic activity at a lower substrate concentration. The substrate affinity of the microgels decreased after ionotropic gelation, likely because of a lower accessibility of the substrate to the enzyme’s active site. At the same ionic concentration, Cu/alginate/TiO2hybrid gel microspheres exhibited the highest affinity. Such characteristics depended on the crosslinker affinity[9]. Cu2+has a stronger affinity with the carboxyl group than other divalent ions, which improved the permeability of the matrix by exchanging more Na+within the alginate. This benefited the rapid diffusion of the substrate and product[12]. Multivalent ions have a high ionic strength, which restrained the adsorption of the enzyme in the matrix, leading to low affinity[9].
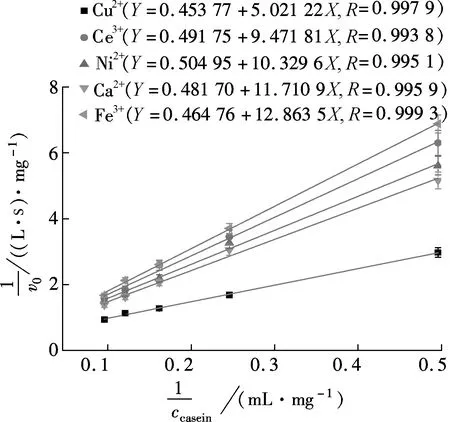
Fig.3 Lineweaver-Burk plot for immobilized enzyme with different cations as crosslinking agent

Tab.1 Summary of kinetic parameters for hydrolysis of casein catalyzed by papain
Kmof the immobilized enzyme was likely predominantly affected by steric hindrance at the enzyme active site, electrostatic forces from ionized functional groups and diffusion limits of substrates within the porous gel.
In summary, the hybrid gel microstructure can be reasonably controlled through the ionotropic gelation conditions. An appropriate pore structure facilitated access of the substrate to the enzyme active site, and afforded a high catalytic efficiency with lowKmvalues.
2.3 Stability of immobilized papain in microgel
The operational stability test were carried out for 5 batch reactions in which the immobilized microspheres were shaken with casein (10 mg/mL) at 37 ℃ for 6 min, and their relative activity were determined by taking the activity of the first batch as a reference(100 %). The activities of free enzyme after one cycle of reaction are shown in Fig.4. Immobilized enzyme activity was measured by the method in Section 1.3. It is clear that papain immobilized in microgels crosslinked with Fe3+exhibited a considerable decrease in activity. Only 30 % of its initial activity was retained after one cycle of the reaction. In contrast, the activity of papain immobilized in the microgels crosslinked with Cu2+, Ce3+, Ni2+and Ca2+, respectively, exhibited a gradual reduction with the increasing cycles of reaction. As shown in Fig.4, correspondingly, the retained activities were 68 %, 54 %, 56 % and 58 % of initial activity after five cycles of reaction, respectively. The gradual decrease in activity was attributed to enzyme leakage[13].
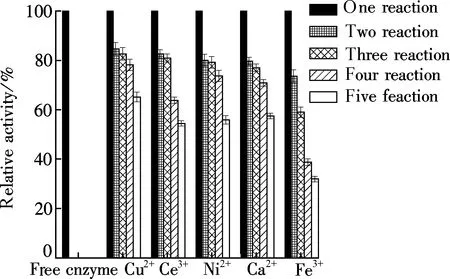
Fig.4 Operational stability of immobilized enzymes gelated by different crosslinking agents
The differing microstructures and gel rigidities of papain immobilized microgels were predominantly ascribed to the varying affinities of metal ions and ionic strength[9]. Cu/alginate/TiO2hybrid gels exhibited the strongest affinity and lowest leakage, ensuring a high activity of the immobilized papain. Changes in ionic strength during ionotropic gelation generally impacted profoundly on the matrix structure. The high ionic charge of Ce3+and Fe3+severely reduced the electrostatic interaction and affinity between the metal ions, papain and alginate; thus, the stability of papain immobilized in microgels was lower.
2.4 Effect of mass ratio of TiO2to papain on the relative activity of immobilized enzyme
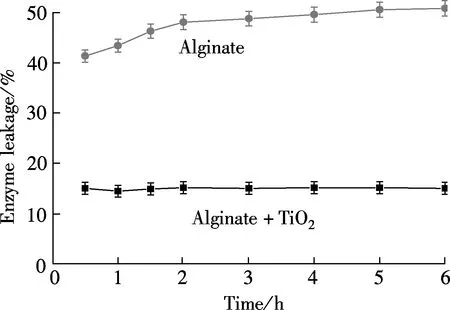
The porosity of the alginate matrices results in potential enzyme leakage. Cu/alginate/TiO2hybrid gels appeared an attractive support material for overcoming this problem. Increasing the mass ratio of TiO2to papain from 5∶1 to 17.5∶1 for Cu/alginate/TiO2hybrid gels resulted in a gradual increase in relative enzyme activity until 10∶1 was reached. After that, as shown in Fig.5(a), further increasing the ratio led the relative enzyme activity to decrease. Enzyme activity related to the amount of adsorbed enzyme. Therefore, the highest activity at 10∶1 was attributed to adsorption saturation. An appropriate use of nanosized TiO2can ensure enzyme stability and reduce leakage[14]. The effect of TiO2on enzyme leakage is shown in Fig.5(b). The determination of leakage from metal/alginate/TiO2hybrid gels was measured by the method of section 1.5. Enzyme leakage from the hybrid gel matrix changed with time. There was about 41% to 51% leakage from the alginate gel, while the hybrid gel exhibited a leakage of approximately 14% to 15%. This indicated that TiO2-modified alginate hybrid gel strongly adsorbed the enzyme, preventing leakage and improving stability.
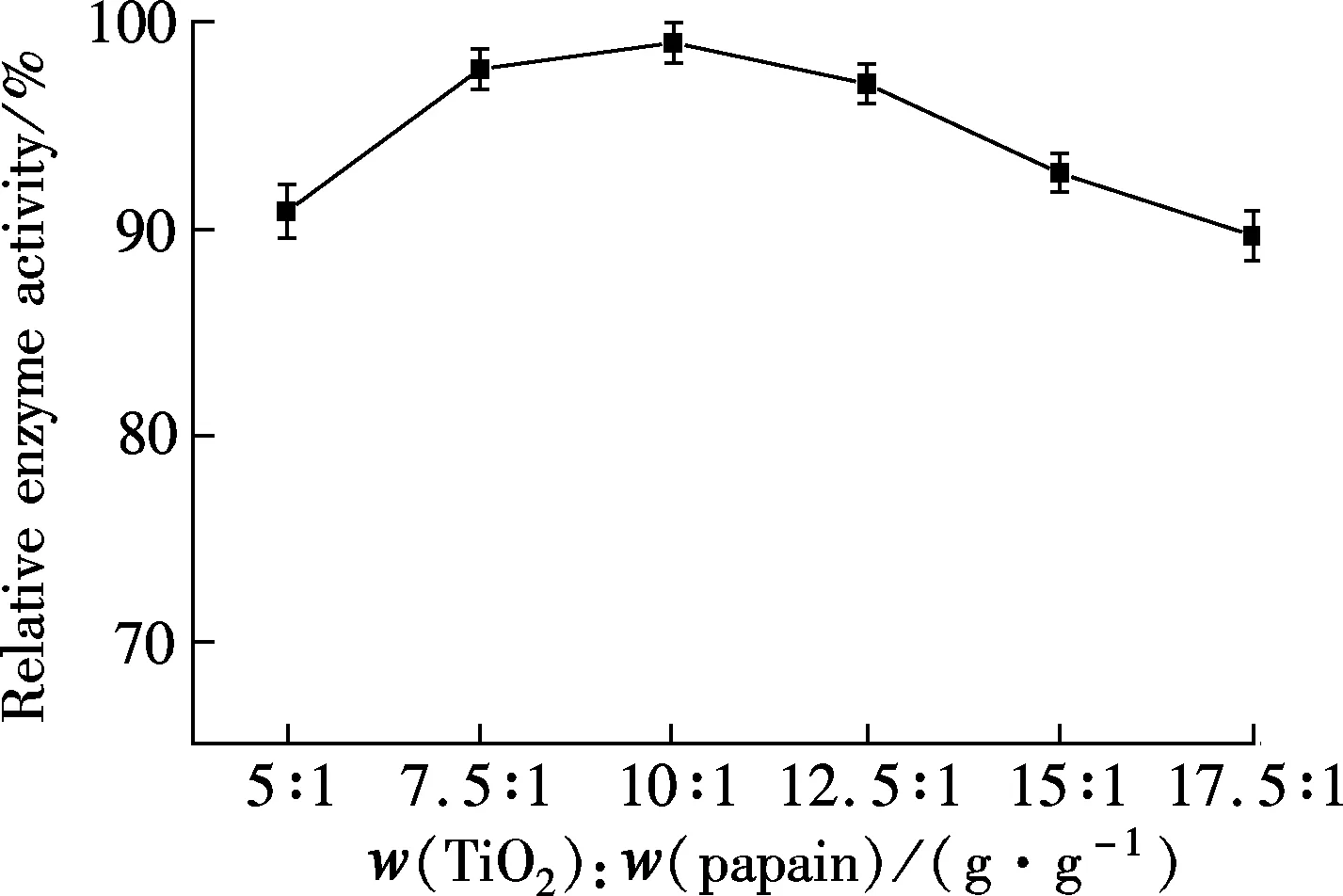
(a)
(b)
3 Conclusion
Ionotropic gelation induced by Ca2+, Ce3+, Ni2+, Cu2+and Fe3+can improve the diffusion properties and stability of immobilized papain in TiO2-alginate hybrid gels. The hydrolysis of casein catalyzed by papain immobilized in Cu2+crosslinked microgels exhibited the smallestKmof 11.0 mg/mL, and thus the highest stability. The optimal ratio of nanosized TiO2to papain for the best performance of Cu/alginate/TiO2hybrid gels was 10∶1. Nanosized TiO2in microgels help to prevent leakage of the immobilized papain. Metal ions are effective in regulating the internal pore structure of alginate matrices, and ensuring high rates of diffusion.
[1]Tao C N, Li G, Wang Y, et al. Enzymatic reporting of peste des petits ruminants virus genes ligating two specific probes on nanoparticles [J].BiotechnologyLetters, 2013, 35(4):613-618.
[2]Li Y H, Liang H, Sun L W, et al. Nanoparticle-tethered NAD with in situ cofactor regeneration [J].BiotechnologyLetters, 2013, 35(6):915-919.
[3]Wu M, He Q, Shao Q F, et al. Preparation and characterization of monodispersed microfloccules of TiO2nanoparticles with immobilized multienzymes [J].ACSAppliedMaterials&Interfaces, 2011, 3(9):3300-3307.
[4]Xu Z, Liu X W, Ma Y S, et al. Interaction of nano-TiO2with lysozyme: insights into the enzyme toxicity of nanosized particles [J].EnvironmentalScienceandPollutionResearch, 2010, 17(3):798-806.
[5]Nayak A K, Das B, Maji R. Calcium alginate/gum Arabic beads containing glibenclamide: development and in vitro characterization [J].InternationalJournalofBiologicalMacromolecules, 2012, 51(5):1070-1078.
[6]Maritz J, Krieg H M, Yeates C A, et al. Calcium alginate entrapment of the yeast Rhodosporidium toruloides for the kinetic resolution of 1,2-epoxyoctane [J].BiotechnologyLetters, 2003, 25(20):1775-1781.
[7]Kierstan M, Bucke C. The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels [J].BiotechnologyandBioengineering, 1977, 19(3):387-397.
[8]Ghanem A, Ghaly A. Immobilization of glucose oxidase in chitosan gel beads [J].JournalofAppliedPolymerScience, 2004, 91(2):861-866.
[9]He Z Y, Christopher B W, Zhou Y T, et al. Papain adsorption on chitosan-coated nylon-based immobilized metal ion (Cu2+, Ni2+, Zn2+, Co2+) affinity membranes [J].SeparationScienceandTechnology, 2010, 45(4):525-534.
[10]Xue Y, Wu C Y, Branford-White C J, et al. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity [J].JournalofMolecularCatalysisB:Enzymatic, 2010, 63(3/4):188-193.
[11]Thu B, Bruheim P, Espevik T, et al. Alginate polycation microcapsules: I. interaction between alginate and polycation [J].Biomoterials, 1996, 17(10):1031-1040.
[12]Phetsom J, Khammuang S, Suwannawong P, et al. Copper-alginate encapsulation of crude laccase from Lentinus polychrous Lev. and their effectiveness in synthetic dyes decolorizations [J].JournalofBiologicalSciences, 2009, 9(6):573-583.
[13]Kuo C H, Liu Y C, Chang C M J, et al. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4nanoparticles [J].CarbohydratePolymers, 2012, 87(4):2538-2545.
[14]Zheng D, Wang N, Wang X, et al. Effects of the interaction of TiO2nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity [J].JournalofHazardousMaterials, 2012, 199-200:426-432.
金属阳离子交联TiO2-海藻酸杂化凝胶
孟俊丽 吴 敏 丁 稳 李 颖 张 进 倪恨美
(东南大学化学化工学院,南京211189)
为了改善海藻酸钠固定化酶微球的扩散性与酶活稳定性,以纳米二氧化钛修饰海藻酸为固定化酶载体,Ca2+, Ce3+, Ni2+, Cu2+和 Fe3+为杂化凝胶的阳离子交联剂,木瓜蛋白酶为模型酶,制备固定化酶微球.通过UV-Vis光谱检测酶活,考察了5种交联剂对固定化酶的动力学和稳定性的影响,结果表明:当Cu2+为交联剂时,固定化酶具有最高的亲和性、最低的米氏常数(Km=11.0 mg/mL)和最高 的稳定性.研究了TiO2与木瓜蛋白酶的质量比对固定化酶稳定性以及酶蛋白泄漏的影响,结果显示纳米二氧化钛与木瓜蛋白酶的质量比为10∶1时,制备的固定化酶微球性能最好,因为二氧化钛能有效阻止固定化酶的泄漏.通过离子胶凝制备Cu/海藻酸/TiO2杂化凝胶可获得较好的扩散能力和酶活稳定性.
阳离子交联剂;扩散;杂化凝胶;离子胶凝;米氏常数;二氧化钛修饰海藻酸
Q814.2
Received 2014-04-27.
Biographies:Meng Junli (1986—), female, graduate; Wu Min (corresponding author), female, doctor, professor, seuwumin@seu.edu.cn.
s:The National Natural Science Foundation of China (No.21005016), the Foundation of Educational Commission of Jiangsu Province (No.JHB2011-2).
:Meng Junli, Wu Min, Ding Wen, et al. Metal cation crosslinking of TiO2-alginate hybrid gels[J].Journal of Southeast University (English Edition),2014,30(4):526-530.
10.3969/j.issn.1003-7985.2014.04.021
10.3969/j.issn.1003-7985.2014.04.021
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Simulation of urban affordable housing land-use evolution based on CA-MAS model
- Dynamical chiral symmetry breaking in QED3
- A decision model of optimal production reliability and warranty length in an imperfect production system
- One-pot facile synthesis of highly photoluminescent graphene quantum dots with oxygen-rich groups
- Modeling household car ownership using ordered logistic regression model
- An automatic identification algorithm for freeway bottleneckbased on loop detector data
