Adsorption characteristics of Pbfrom urban stormwater runoff by construction wastes
2014-09-06YangLiqiongWangJianlongZhangXiaoranCheWu
Yang Liqiong Wang Jianlong Zhang Xiaoran Che Wu
(1Key Laboratory of Urban Stormwater System and Water Environment of Ministry of Education, Beijing Universityof Civil Engineering and Architecture, Beijing 100044, China)(2Beijing Cooperative Innovation Research Center on Architectural Energy Saving and Emission Reduction, Beijing Universityof Civil Engineering and Architecture, Beijing 100044, China)(3Beijing Climate Change Response Research and Education Center, Beijing University of Civil Engineeringand Architecture, Beijing 100044, China)
Adsorption characteristics of Pbfrom urban stormwater runoff by construction wastes
Yang Liqiong1Wang Jianlong1Zhang Xiaoran2Che Wu3
(1Key Laboratory of Urban Stormwater System and Water Environment of Ministry of Education, Beijing Universityof Civil Engineering and Architecture, Beijing 100044, China)(2Beijing Cooperative Innovation Research Center on Architectural Energy Saving and Emission Reduction, Beijing Universityof Civil Engineering and Architecture, Beijing 100044, China)(3Beijing Climate Change Response Research and Education Center, Beijing University of Civil Engineeringand Architecture, Beijing 100044, China)
Construction wastes were selected as the adsorbents, and static and dynamic adsorption batch experiments were carried out to investigate the adsorption of Pb to construction wastes with different particle size gradations in the simulated stormwater runoff system. The experimental results show that the pseudo-second-order kinetics model can better characterize the adsorption process of Pb than the pseudo-first-order kinetics model. The adsorption equilibrium data can be well fitted by the Freundlich isotherm model. The construction wastes with different tested size gradations can greatly remove Pb from stormwater runoff and their average removal rate can reach up to 99%. The construction wastes with narrow size distribution can better remove Pb but with worse permeability than those with wide size distribution. The particle size gradation of construction wastes greatly influences the equilibrium time, rate and the capacity of Pb adsorption. The equilibrium adsorption rate and capacity are 18.1 μg/min and 5.5 μg/g, respectively, for the construction wastes with the size of 2.36 to 4.75 mm, which are the greatest among the different size gradations. The present study provides a scientific basis for effectively controlling Pb pollution from stormwater runoff and the construction wastes resource utilization.
stormwater runoff; heavy metal; construction waste; adsorption
With the rapid development of urbanization, increasing urban impervious areas interrupt stormwater infiltration channels and result in a sharp increase in stormwater runoff volume and peak flow. In addition, due to the human activities, atmospheric deposition and other factors, a large number of pollutants are accumulated and discharged into the municipal storm sewer by the stormwater runoff flushing, and as a consequence, enter the city receiving water bodies. With the increasing perfection of the city point pollution control, non-point pollution caused by stormwater runoff has become one of the important sources of watershed pollution. A large number of studies on urban stormwater runoff quality showed that heavy metals from stormwater runoff had become one of the important sources of surface water pollutants, where Pb is one of the highest pollution loads of heavy metals[1]. Vehicle brake emissions, tire wear, building sidings (weathering of paints and metal components) and atmospheric deposition are important sources of heavy metals[2]. Heavy metals are persistent pollutants which are widely distributed in the environment, and, thus, they are difficult to be treated due to their characteristics of persistency, wide-ranging and management difficulties. Unlike organic pollutants, heavy metals are difficult to be degraded in the environment and easy to be accumulated in the human body through the food chain and other ways and become toxic when reaching a certain concentration. The Pb in the environmental water can prevent the respiratory metabolism and photosynthesis of aquatic plants, thus affecting the quality and biomass of the plants and changing the population quantity of aquatic animals. Therefore, it becomes necessary to remove heavy metals from stormwater runoff to a feasible extent to improve the quality of water entering the local watershed in an appropriate treatment and management way.
In 1970s, researchers from the United States investigated heavy metal pollution and migration characteristics in urban stormwater runoff. From then on, some researchers from developed countries started to research on heavy metal pollution and its impact factors[3]. As one of the low impact development (LID) stormwater management measures, bioretention technology (also known as rain garden), which uses vegetable and media to hold and treat stormwater runoff at the source, can not only efficiently control water quality (the removal rate reaches up to 90%)[4], but also has an ecological function and landscape effects. In addition, bioretention technology can be widely used to control road green belt and open spaces stormwater runoff. Water quality is improved via permeation, soil filtering and adsorption, decomposition and biotransformation mechanisms in bioretention. Laboratory and pilot-scale bioretention box studies have proved that bioretention is an effective practice for improving the quality of urban stormwater runoff[5]. The type of bioretention media has a significant impact on the removal of heavy metals[6-10]. Therefore, to select an effective and economical bioretention medium is of great importance for the wide application of bioretention technology.
This study takes construction wastes as the research objects. Sorption of Pb to gradation construction wastes with different particle sizes in stormwater runoff is studied by static and dynamic adsorption experiments. The feasibility of the application of crushed construction wastes as bioretention media is discussed. This study can provide a scientific basis for effectively controlling the Pb pollution from stormwater runoff and the construction waste resource utilization.
1 Materials and Methods
1.1 Adsorbents and characterization
The construction wastes used in the present study are mainly composed of brick and mixed with a small amount of concrete. After crushing with a crusher, the construction wastes were sieved with a test sieve to four different particle size distributions of 2.36 to 4.75 mm, 4.75 to 10.0 mm, 2.36 to 10.0 mm and≤10.0 mm. The particles were soaked and washed several times with distilled water to remove surface residuals, and then they were dried in an oven at 105 ℃ for 24 h and stored in a desiccator. The surface area and porosity factor of construction wastes were 2.269 4 m2/g and 15%, respectively. The chemical oxide composition of the construction wastes was determined by the scanning wavelength dispersive X-ray fluorescence (XRF) using a Rigaku ZSX Primus Ⅱ spectrometer. BET surface area was measured by automatic adsorption using a NOVE 4200E instrument. The chemical composition of fresh construction wastes, reported as oxides, is shown in Tab.1. The main components of the construction wastes are oxide of silicon, calcium and aluminum, with the percentage content of 42.5%, 14.2% and 12.0%, respectively. These results are similar to the results reported by previous studies where XRF analyses of construction wastes were carried out[11-12]. In addition, there are some other components which are probably harmful to human health and environment, such as Cr and As. However, studies have shown that there is no adverse effect of percolate.
1.2 Adsorption experiment
Tab.1 Chemical composition and physical characteristics of construction waste

ConstituentMasspercentage/%ConstituentMasspercentage/%Na2O0.931 Fe2O34.750 MgO2.580 NiO0.0060Al2O312.00 CuO0.0042SiO242.50 ZnO0.0145P2O50.1460 As2O30.0029K2O2.330 Rb2O0.0103CaO14.20 SrO0.0517TiO20.6470 ZrO20.0352Cr2O30.0174 BaO0.0960MnO0.0939 Bi2O30.0096
The stock solution was prepared with a Pb standard solution with a concentration of 1 mg/μL. The permeability coefficients of construction wastes were measured by the hydrostatic head method (see Tab.2). The results show that the permeability coefficients decreased with the increasing particle size gradation of the construction wastes. The static and dynamic adsorption experiments were carried out in the present study.
Tab.2 Permeability coefficients of construction wastes with different size gradations

Sizegradation/mmk1/(m·s-1)k2/(m·s-1)≤10.01.29×10-51.19×10-52.36to10.01.56×10-31.50×10-32.36to4.751.98×10-31.88×10-34.75to10.06.54×10-36.22×10-3
1.2.1 Static adsorption experiment
100 g construction wastes with four different particle sizes were weighed and transferred into a series of 250 mL triangle conical flasks. After adding 150 mL stock solution, the flasks were shaken on a temperature-regulated orbital platform shaker at 80 r/min. Samples were analyzed at 1, 3, 5, 10, 20, 30, 40, 60, 90, 120, 180, 240 and 300 min intervals during shaking to study the influence of reaction time on the adsorption equilibrium and determine the optimum reaction time. Prior to analysis, 50 mL samples were collected and then subsequently filtered through 0.45 μm hydrophilic membranes to remove suspended solids, and the filtrates were stored in PVC bottles and were kept in the fridge at 4 ℃ for determination.
1.2.2 Dynamic adsorption experiment
The construction wastes with four different particle size distributions were added into experimental columns with several steps, and compacted with each step. The dynamic adsorption experiment was operated under a condition of continuous water input with a peristaltic pump at 4 mL/s. 50 mL samples were collected at the time intervals of 3, 5, 10, 20, 30, 60, 90, 180 and 300 min. The samples were filtered through 0.45 μm hydrophilic membranes to remove the suspended matters, and then stored in PVC bottles. The samples were kept in the fridge at 4 ℃ for further analysis. Bioretention columns dynamic simulation experimental set-up is shown in Fig.1.
1.3 Aqueous sample analysis
Samples were analyzed by the graphite furnace atomic absorption spectrophotometer method using a Hitachi Z-2010 Polarized Zeeman atomic absorption spectrophotometer (AAS). A calibration curve was made with the Pb standard solution of the National Standard Center for standard matter. 20.00 μg/L standard Pb solution was diluted by the standard solution from the National Standard Center with 2% nitric acid. The absorbance of the standard solution with the concentration of 0, 5.00, 10.00, 15.00, 20.00 μg/L was measured by the graphite furnace automatic dilution function. The correlation coefficient of the calibration curve was 0.999 2. The concentration of Pb in the supernatant after adsorption by construction wastes was measured. The amount of sorption was calculated based on the concentration of Pb in the solution before and after adsorption.
1.4 Adsorption isotherms
Under optimum adsorption equilibrium time, 5, 10, 20, 30, 50, 70 and 100 g of construction wastes with different particle size distributions were weighed to study the isothermal adsorption process. The adsorption capacity of the construction wastes is calculated as
(1)
whereQis the adsorption capacity per unit mass of construction wastes, mg/g;C0is the initial concentration of Pb in the aqueous solution, mg/L;Ctis the equilibrium concentration of the test solution, mg/L;Vis the volume of sample, L;mis the mass of construction wastes, g.
2 Results and Discussion
2.1 Characterization of construction wastes
The scanning electron microscopy (SEM) images for the surfaces of construction wastes before and after adsorption are shown in Fig.2. The surface of construction wastes becomes smoother after adsorption, which may be due to the deposition of Pb by physisorption, or due to a progressive change in surface mineralogy with time. The comparison of the component composition of construction wastes before and after adsorption (see Fig.3) shows a significant decrease of the element content in Al and Ca, while showing a significant increase in Mg and Si. The significant decrease in Ca content may be related to the loss of free lime (CaO), which results in high pH[13]. Therefore, the changes in pH before the discharge of the stormwater should be taken into account for practical applications.

(a)

(b)
Fig.2 SEM images of the construction wastes. (a) Before adsorption; (b) After adsorption

(a)

(b)Fig.3 Change of relative concentration of mineral elements. (a) C, N, O, etc.; (b) F, P, Cl, etc.
This result shows that there are no detrimental effects on human health or on the environment by application of construction wastes in stormwater runoff purification.
2.2 Adsorption isotherms
Under a constant temperature, sorption between solid and liquid is often described by the Langmuir and Freundlich isotherm model. The Langmuir adsorption isotherm (in linear form)[14]is given as
(2)
The Freundlich adsorption isotherm (in linear form)[8]is given as
(3)
whereKfis the Freundlich adsorption constant, andnis a constant.


Tab.3 Parameters of Langmuir and Freundlich equations

(a)

(b)

(c)

(d)
Fig.4 Sorption isotherms of Pb sorption to construction wastes with different particle size gradations fitted by the Freundlich model. (a) 2.36 to 4.75 mm; (b) 2.36 to 10.0 mm; (c) 4.75 to 10.0 mm; (d)≤10.0 mm
2.3 Adsorption kinetics
The adsorption of Pb from simulated stormwater runoff to the construction wastes can be analyzed by pseudo-first-order and pseudo-second-order rate expressions as applied by Liu et al[16]. The linear expression of the pseudo-first-order equation is given as[11]
(4)
whereQtis the amount of Pb adsorbed at timet, mg/g;k1is the equilibrium rate constant, which can be determined by a linear plot of log(Qe-Qt) vs.t.
Tien et al.[18-20]found that the adsorption kinetics of heavy metals was better fitted to a pseudo-second-order kinetic model. The linear expression is given as
(5)
wherek2is the rate constant at equilibrium. The adsorption kinetic fitting curves of Pb by construction wastes with different particle sizes are shown in Fig.5. The fitting kinetic equations are shown in Tab.4. The pseudo-second-order kinetic equation of Pb adsorption from the synthetic simulated runoff to the construction wastes fits the linear equation well (see Fig.5) and it is significantly better than the pseudo-first-order kinetics model (see Tab.4). Many studies at domestic and overseas on adsorption of heavy metals to saccharomyces cerevisiae[21], natural zeolite[22], blast furnace slag[23]and hazelnut shell[24]have shown that the data of sorption kinetics fit the pseudo-second-order kinetics model[25], which is consistent with the experimental results in the present study.
图2中箭头所指的界线之右侧,便是形成的这种生物鲕层。相比于高能鲕层,其特点较明显,①同心圈层厚度较大;②同心圈层数量较少;③同心圈层间界线较模糊,常呈弯曲状;④单层厚度不均匀,常有尖灭现象;⑤透明度较低,且不均匀,系含有机质所致。

Fig.5 Second-order equation for the adsorption of Pb by construction wastes
Tab.4 Parameters and correlation coefficients for the kinetic models

Sizedistribution/mmk2/(g·mg-1·min-1)R2Kineticmodel2.36to4.753099.500.9999Qt=0.0936t1+17.035t4.75to10.087.480.9996Qt=0.00113t1+0.315t2.36to10.0132.810.9987Qt=0.00155t1+0.453t
2.4 Effect of construction wastes with different particle size gradations on Pb removal
2.4.1 Adsorption capacity
The adsorption capacities of construction wastes with different particle sizes are shown in Fig.6. At the initial stage, the adsorption rate increases dramatically with the increase of time until reaching a plateau. The size distributions of adsorbent significantly influence the adsorption efficiency of heavy metals[26]. Construction wastes with sizes smaller than 10.0 mm reach equilibrium in the shortest time (about 1 h). The equilibrium adsorption capacities of construction wastes with particle sizes smaller than 10.0 mm, 2.36 to 10.0 mm, 2.36 to 4.75 mm and 4.75 to 10.0 mm for Pb sorption are 4.1, 3.4, 5.5 and 3.6 μg/g, respectively. The adsorption capacity of construction wastes with particle size of 2.36 to 4.75 mm is the largest (see Fig.6(a)), probably because of their larger specific surface area. Dynamic experimental results (see Fig.6(b)) show that the construction wastes with four different size distributions for Pb sorption reach equilibrium within 10 min. Their equilibrium adsorption capacities (i.e., 1.8, 2.1, 2.1 and 2.3 μg/g, respectively) are much smaller than the data derived from static experimental results. Therefore, the construction wastes have not reached adsorption saturation. The data show that construction wastes with 2.36 to 4.75 mm can be used as a bioretention media to remove Pb from stormwater runoff.

(a)

(b)
Fig.6 Changes in adsorption capacity of construction wastes with different particle sizes on Pb adsorption. (a) Static experiment; (b) Dynamic experiment
2.4.2 Adsorption rate
Changes in adsorption rates of construction wastes with different particle sizes on Pb adsorption are depicted in Fig.7. The adsorption rate equation is given as
(6)
whereνis the adsorption rate, mg/min;mtis the amount of Pb adsorbed within timet, mg; andtis the sampling interval time, min.

(a)

(b)
Fig.7 Changes in adsorption rates of construction wastes with different particle size gradations on Pb adsorption. (a) Static experiment; (b) Dynamic experiment
As shown in Fig.7(a), the adsorption rates of Pb adsorption to construction wastes with the size of 2.36 to 4.75 mm and smaller than 10.0 mm decrease rapidly. The adsorption rates of Pb adsorption to construction wastes with 2.36 to 10.0 mm first increase and then decrease, while construction wastes with 4.75 to 10.0 mm first decrease and then increase until reaching a plateau, which may be due to the unevenly distributed size grading of construction wastes in this study. The stable adsorption rates of Pb sorption to construction wastes with four particle sizes are 18.1, 1.4, 5.6 and 1.2 μg/min, respectively. The adsorption rates of construction wastes with different particle sizes in the dynamic adsorption process (see Fig.7(b)) decrease gradually. The adsorption rates are basically identical, and stable adsorption rates (i.e., 0.8, 0.6, 0.7 and 0.7 μg/min, respectively) are much smaller than the data derived by the static experimental data. With the increase of bioretention media depth, the Pb concentration of stormwater decreases and may be harmful to the environment.
2.4.3 Removal rate
The average removal rates of construction wastes with different particle sizes and their maximum values are summarized in Tab.5. The equation of removal rate is given as

(7)
Tab.5 Average removal rates of construction wastes with different particle sizes and their maximum values
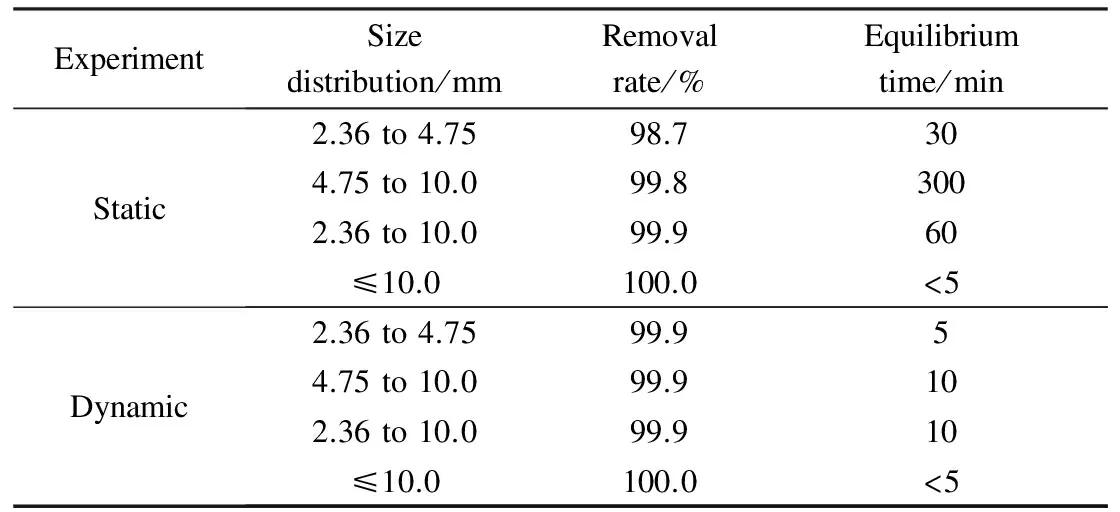
ExperimentSizedistribution/mmRemovalrate/%Equilibriumtime/minStatic2.36to4.7598.7304.75to10.099.83002.36to10.099.960≤10.0100.0<5Dynamic2.36to4.7599.954.75to10.099.9102.36to10.099.910≤10.0100.0<5
As can be seen from Tab.5, construction wastes with different particle sizes show a good purification effect on Pb removal from stormwater runoff and up to a 90% Pb removal rate is achieved. In general, particles with smaller size have larger specific surface area and better surface adsorption properties. As a consequence, the adsorption capacity is higher for construction wastes of smaller sizes than those of larger sizes[27]. Our conclusion is consistent with the general conclusions reported previously. The removal rates derived from dynamic experiments are higher than those from static experiments. At the initial stage of dynamic experiments, the removal rate of heavy metal increases rapidly with the increase of time. Construction wastes with four particle sizes reach equilibrium within 10 min, which indicates that the adsorption of Pb to construction wastes is a fast reaction process that can be completed in a short time. As a medium for bioretention facilities, construction wastes can be used to remove Pb from urban stormwater runoff due to their great adsorption capacities.
2.5 Influencing factors of adsorption
Numerous studies domestically and overseas showed that the impact factors of solid adsorption effects on Pb were mainly the adsorption time, the amount of adsorbent, the initial concentration and the pH value. With the increase of the adsorption time, the adsorption removal rate of the particulate matter for Pb in the solution gradually increases until reaching equilibrium where the removal rate does not change significantly[28]and may even decrease to a certain extent. The increase of adsorbent dosage may also increase the removal rate for Pb adsorption. However, the removal rate may decrease if too much adsorbent is used. Therefore, taking into account the cost, it is desirable to optimize the dosage of adsorbent. The pH of the solution is an important factor of Pb adsorption. The optimum adsorption pH values are different for different adsorbents[29-30]. Previous studies have showed that competition occurs between different heavy metal ions and adsorbents adsorb heavy metals selectively[31]. In practical applications, the design of parameters should be optimized based on the influencing factors of heavy metal removal.
3 Conclusions
1) Construction wastes with different particle sizes show a good effect on Pb adsorption, and their average removal rate reaches up to 99%. The removal efficiency of construction wastes increases with the increase of size grading, but the penetrating ability of construction wastes turns to worse. The adsorption removal effect of construction wastes with different sizes on Pb adsorption follows in the order:≤10.0 mm>2.36 to 4.75 mm>2.36 to 10.0 mm>4.75 to 10.0 mm.
2) The adsorption isotherm analysis shows that the adsorption of Pb to construction wastes adsorption fits well with the Freundlich isotherm model and the correlation coefficients are 0.976 8 to 0.996 3. The adsorption kinetic process is well described by the pseudo-second-order kinetic equation and the correlation coefficients are 0.999 6 to 0.999 9.
3) In the engineering projects, construction wastes can be applied as the adsorption media of bioretention facilities, constructed wetlands and other ecological measures for the removal of Pb from stormwater runoff. The present study provides a scientific basis for effectively controlling Pb pollution from stormwater runoff and the construction wastes resource utilization.
[1]Hu A B, Li Z F, Zhang S H, et al. Progress of the research of urban road rainwater runoff quality [J].WaterandWastewaterEngineering, 2010, 36(3): 123-127. (in Chinese)
[2]Davis A P, Shokouhian M, Ni S. Loadings of lead, copper, cadmium, and zinc in urban runoff from specific sources [J].Chemosphere, 2001, 44(5): 997-1009.
[3]Hu A B, Zhang S H, Chen J G. Progress on the improvement of urban stormwater runoff quality by bioretention [J].EnvironmentalPollutionandControl, 2011, 33(1): 74-82. (in Chinese)
[4]Sun X L, Davis A P. Heavy metal fates in laboratory bioretention systems [J].Chemosphere, 2007, 66(9): 1601-1609.
[5]Davis A P, Shokouhian M, Sharma H, et al. Laboratory study of biological retention for urban storm water management [J].WaterEnvironmentalResearch, 2001, 73(1): 5-14.
[6]Davis A P, Shokouhian M, Sharma H, et al. Water quality improvement through bioretention: lead, copper, and zinc removal [J].WaterEnvironmentalResearch, 2003, 75(1): 73-82.
[7]Pitchera S K, Sladea R C T, Ward N I. Heavy metal removal from motorway stormwater using zeolites [J].ScienceoftheTotalEnvironment, 2004, 334-335: 161-166.
[8]Modina H, Perssona K M, Andersson A, et al. Removal of metals from landfill leachate by sorption to activated carbon, bone meal and iron fines [J].JournalofHazardousMaterials, 2011, 189(3): 749-754.
[9]Guan Z Q, Jin C J, Ren J, et al. Adsorption characteristics of pinecone to Cu2+, Pb2+and Zn2+in wastewater [J].IndustrialWaterandWastewater, 2010, 41(4): 59-63. (in Chinese)
[10]Zhang Z L, Kuang Q, Jia X S. Study on the kinetics and thermodynamics of Pb2+, Cu2+, Cr3+, Cd2+, Ni2+adsorption onto peanut hull [J].EcologyandEnvironmentalSciences, 2010, 19(12): 2927-2977. (in Chinese)
[11]Wang X, Sun K W. Study on comprehensive utilization of crushed brick [J].ChinaResourcesComprehensiveUtilization, 2008, 26(10): 34-35. (in Chinese)
[12]Yuan Y, Sun K W. Primary discussion of mechanicalchemical activation on construction waste slag [J].ChinaResourcesComprehensiveUtilization, 2009, 27(1): 32-33. (in Chinese)
[13]Okochi N C, McMartin D W. Laboratory investigations of stormwater remediation via slag: effects of metals on phosphorus removal [J].JournalofHazardousMaterials, 2011, 187(1/2/3): 250-257.
[14]Aksu Z. Application of biosorption for the removal of organic pollutants: a review [J].ProcessBiochemistry, 2005, 40(3/4): 997-1026.

[16]Liu S Y, Gao J, Yang Y J, et al. Adsorption intrinsic kinetics and isotherms of lead ions on steel slag [J].JournalofHazardousMaterials, 2010, 173(1/2/3): 558-562.
[17]Zou W H, Chen Z Z, Han R P, et al. Removal of copper cation and lead cation from aqueous solution by manganese-oxide-coated-sand [J].ActaScientiaeCircumstantiae, 2005, 25(6): 779-784. (in Chinese)
[18]Tien C T, Huang C P. Formation of surface complexes between heavy metals and sludge particles[C]//TraceMetalsintheEnvironmental. 1.HeavyMetalsintheEnvironment. Amsterdam: Elsevier, 1991: 295-311.
[19]Li X M, Zheng W, Wang D B, et al. Removal of Pb (Ⅱ) from aqueous solutions by adsorption onto modified areca waste: kinetic and thermodynamic studies [J].Desalination, 2010, 258(1/2/3): 148-153.
[20]Motsi T, Rowson N A, Simmons M J H. Kinetic studies of the removal of heavy metals from acid mine drainage by natural zeolite [J].InternationalJournalofMineralProcessing, 2011, 101(1/2/3/4): 42-49.
[21]Chen C, Wang J L. Characteristics and kinetics of Zn2+biosorption by Saccharomyces cerevisiae [J].JournalofTsinghuaUniversity:ScienceandTechnology, 2006, 46(12): 2069-2072. (in Chinese)
[22]Shavandi M A, Haddadian Z, Ismail M H S, et al. Removal of Fe(Ⅲ), Mn(Ⅱ) and Zn(Ⅱ) from palm oil effluent (POME) by natural zeolite[J].JournaloftheTaiwanInstituteofChemicalEngineers, 2012, 43(5): 750-759.
[23]Nehrenheim E, Gustafsson J P. Kinetic sorption modeling of Cu, Ni, Zn, Pb and Cr ions to pine bark and blast furnace slag by using batch experiments [J].BioresourceTechnology, 2008, 99(6): 1571-1577.
[24]Bulut Y, Tez Z. Adsorption studies on ground shells of hazelnut and almond [J].JournalofHazardousMaterials, 2007, 149(1): 35-41.
[25]Ho Y S, McKay G. Pseudo-second order model for sorption processes [J].ProcessBiochem, 1999, 34: 451-465.
[26]Li Y H, Zhu Y Q, Zhao Y M, et al. Different morphologies of carbon nanotubes effect on the lead removal from aqueous solution [J].DiamondandRelatedMaterials, 2006, 15: 90-94.
[27]Wang W H, Wong M H, Leharne S, et al. Fractionation and biotoxicity of heavy metals in urban dusts collected from Hong Kong and London [J].EnvironmentalGeochemistryandHealth, 1998, 20(4): 185-198.
[28]Chen H Y, Yang Y J, Tan X D, et al. Preparation of activated charcoal from pyrolusite-added sewage sludge and adsorption of lead ion in wastewater [J].ChineseJournalofEnvironmentalEngineering, 2010, 4(11): 2473-2478. (in Chinese)
[29]Fu R J, Xue W P, Ma C, et al. Adsorption of Cu2+and Ni2+by active carbon of peanut shell [J].JournalofDalianPolytechnicUniversity, 2009, 28(3): 200-203. (in Chinese)
[30]Rozada F, Otero M, Moran A, et al. Adsorption of heavy metals onto sewage sludge-derived materials [J].BioresourceTechnology, 2008, 99(14): 6332-6338.
[31]Liu Y, Xiao D, Guo L H, et al. Adsorption of metal ions on natural vermiculite [J].JournalofSichuanUniversity:EngineeringScienceEdition, 2006, 38(3): 92-96. (in Chinese)
建筑垃圾对城市雨水径流中Pb的吸附特性
杨丽琼1王建龙1张晓然2车 伍3
(1北京建筑大学城市雨水系统与水环境省部共建教育部重点实验室,北京 100044) (2北京建筑大学北京建筑节能减排关键技术协同创新中心,北京 100044) (3北京建筑大学北京应对气候变化研究和人才培养基地,北京 100044)
以砖混建筑垃圾为研究对象,采用人工模拟雨水,通过静态和动态吸附实验研究了不同粒径粒级建筑垃圾对雨水径流中Pb的吸附效果.实验结果表明:准二级动力学模型比准一级动力学模型能更好地描述建筑垃圾对Pb的吸附过程;Freundlich等温模型能较好地拟合其等温吸附过程;不同粒径粒级建筑垃圾均对雨水径流中的Pb具有较好的净化效果,去除率高达99%,粒径粒级越小,对Pb的净化效果越好,但其渗透性能越差;建筑垃圾的粒径粒级对Pb的吸附平衡时间、吸附速率和吸附量具有重要影响,粒径2.36~4.75 mm的建筑垃圾对Pb的平衡吸附速率和平衡吸附量最大,分别为18.1 μg/min和5.5 μg/g.上述研究结果为城市雨水径流中Pb污染的有效控制以及建筑垃圾资源化提供了科学依据.
雨水径流;重金属;建筑垃圾;吸附
TU993.1
s:The National Natural Science Foundation of China(No.51208022), the National Science and Technology Major Project of China (No.2011ZX07301-004-01).
:Yang Liqiong, Wang Jianlong, Zhang Xiaoran, et al. Adsorption characteristics of Pb from urban stormwater runoff by construction wastes[J].Journal of Southeast University (English Edition),2014,30(2):212-219.
10.3969/j.issn.1003-7985.2014.02.014
10.3969/j.issn.1003-7985.2014.02.014
Received 2013-10-15.
Biographies:Yang Liqiong(1988—), female, graduate; Wang Jianlong(corresponding author), male, doctor, associate professor, wjl_xt@163.com.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Prediction on effectiveness of road sweepingfor highway runoff pollution control
- A preliminary sustainability assessment of innovativerainwater harvesting for residential properties in the UK
- Approach to estimating non-point pollutant load removal rates based on water environmental capacity: a case study in Shenzhen
- Potential contributions to Beijing’s water supply from reuse of storm- and greywater
- Characteristics of Hg pollution in urban stormwater runoff in Nanjing city, China
- Rainwater harvesting in the challenge of droughts and climate change in semi-arid Brazil
