上海地区单中心住院儿童难治性肺炎支原体肺炎相关因素分析
2014-08-10陆爱珍杨皓伟王传凯钱莉玲张晓波王立波
陆爱珍 杨皓伟 王传凯 钱莉玲 张晓波 王立波
·论著·
上海地区单中心住院儿童难治性肺炎支原体肺炎相关因素分析
陆爱珍1杨皓伟1王传凯 钱莉玲 张晓波 王立波
目的 分析普通肺炎支原体肺炎(MPP)进展为难治性MPP的相关因素,为早期识别难治性MPP提供参考。方法 采集2012年9月至2013年8月复旦大学附属儿科医院住院的MPP且排除了其他常见病毒和细菌感染的病例,分为难治性MPP组和普通MPP组。复习文献收集与难治性MPP相关的指标,采集入院次日相关实验室检查指标、入院3 d内采集胸部X线和CT资料;以单因素和多因素分析进展为难治性MPP的相关因素。结果 653例MPP患儿进入分析,占同期收治肺炎患儿的51.9%(653/1 257例)。难治性MPP组300例,男171例;普通MPP组353例,男221例。①单因素分析显示,难治性MPP组年龄显著高于普通MPP组,(66.8 ± 37.5)vs(51.4 ± 34.4)月龄,>3岁的比例也显著高于普通MPP组(234/300vs224/353,P<0.01)。难治性MPP组发热时间和住院天数均显著高于普通MPP组(P均<0.01);CK、LDH、HBDH、ALT、AST、CRP、PCT和IL-6水平难治性MPP组均显著高于普通MPP组;难治性MPP组肺渗出面积评分显著高于普通MPP组,(1.95±1.12)vs(1.55±0.97),P<0.01。②选择单因素分析后有统计学意义的临床、实验室和影像学指标行逐步Logistic回归分析,发热天数(OR=1.954,95%CI:1.403~2.722)、血清LDH水平(OR=1.009,95%CI:1.001~1.018)和肺渗出面积评分(OR=2.422,95%CI:1.111~5.279)是难治性MPP的独立相关因素。结论 肺炎支原体已成为社区获得性肺炎住院患儿的主要病原体,难治性MPP病例常发生于3岁以上儿童。疾病早期存在持续高热、肺部渗出面积大、血清LDH水平增高是进展为难治性MPP的独立相关因素。
肺炎支原体; 难治性肺炎支原体肺炎; 相关因素; 儿童
肺炎支原体(Mp)是引起人类非典型性肺炎和许多呼吸道疾病的病原体之一。Mp呈全球性分布,肺炎支原体肺炎(MPP)每2~6年可引起一次全球暴发流行[1],人群普遍易感。在社区获得性肺炎(CAP)中10%~30%由Mp引起[2]。一项CAP的流行病学调查结果显示Mp的感染率已超过肺炎链球菌,成为成人CAP首要致病菌[3]。尽管Mp感染大部分是自限性疾病,仅有部分感染患儿需要住院治疗[1],但近年来Mp感染的危重病例不断增多,危重病例造成的后遗症如肺不张、支气管扩张、闭塞性细支气管炎、肺间质纤维化、肺蛋白沉积症、脑炎并发症和慢性肾病等严重损害了患儿的生活质量。在临床上如能早期识别Mp感染难治性病例,并给予早期干预可显著降低Mp感染的后遗症,提高危重患儿生活质量。目前国内相关难治性MPP危险因素的分析多为回顾性研究,相关因素报告差异较大和观察时间不统一,特别是影像学测量偏倚较大,结果偏倚的可能性较大。
1 方法
1.1 研究设计 复习文献尽可能收集难治性MPP潜在的相关因素,采集在复旦大学附属儿科医院(我院)住院且次日Mp检测阳性,并排除其他常见病原感染的肺炎患儿,统一截取入院次日采集的相关实验室和入院3 d内影像学指标,分为难治性MPP组和普通MPP组,以单因素和多因素分析进展为难治性MPP的相关因素。
1.2 诊断标准 难治性MPP诊断标准[4]:①符合MPP的诊断;②经大环内酯类抗菌药物正规治疗7 d及以上,临床症状加重、仍持续发热、肺部影像学所见加重者。
1.3 纳入标准 2012年9月至2013年8月在我院住院的MPP患儿,并符合以下条件:①入院次日Mp检测阳性,血清Mp-IgM阳性(滴度>1∶320)或鼻咽分泌物Mp-DNA阳性;②入院次日鼻咽分泌物细菌培养和其他病原学检查(呼吸道合胞病毒、腺病毒、副流感病毒、流感病毒、偏肺病毒、沙眼衣原体DNA、肺炎支原体DNA)阴性;③入院次日外周血EB病毒和血培养阴性。
1.4 排除标准 ①合并慢性基础性疾病,如先天性心脏病、慢性肾炎、肾病综合征、自身免疫性疾病和免疫缺陷病;②近期使用免疫抑制剂者(如糖皮质激素、雷公藤多苷等)。
1.5 Mp检测方法 Mp-IgM 采用SeroMPTMIgM 检测试剂盒(酶联免疫法),按照说明书操作进行检测;Mp-DNA检测采用肺炎支原体核酸扩增荧光检测试剂盒(上海申友生物有限公司)进行检测, >2 500 copies·mL-1为阳性结果。
1.6 相关因素的采集和定义 复习文献[5~11]共总结20余项与难治性MPP相关因素,编制病例登记表,逐项截取上述相关因素,20项相关因素包括①性别、年龄、入我院前发热天数、住院天数;②入院3 d内的胸部X线和(或)CT影像学资料,根据MPP的影像学特点分为:渗出、间质病变、肺门淋巴结改变、大叶性肺实变和肺不张、胸腔积液和肺坏死,对于渗出病变部位采用右上肺、右中肺、右下肺、左上肺,左中肺和左下肺进行描述,渗出面积采用评分法[12]进行描述,每个部位面积评分为1分;③肺外发现:住院过程中观察到的Mp感染相关的皮疹(除外药疹),浅表淋巴结肿大(查体检出的既往未被发现的淋巴结肿大),肝功能损害(病程中B超证实伴肝功能指标异常),脑病(住院期间精神状态改变,谵妄或意识模糊);④病原负荷量:入院次日清晨鼻咽分泌物标本Mp-DNA和血清Mp-IgM;⑤入院次日清晨实验室指标:血常规,CRP,ALT,AST,心肌酶谱(CK, CK-MB, LDH, HBDH),PCT,IL-6和ESR。
1.7 质量控制 ①我院呼吸科病房入院患儿临床常规为入院次日完成相关实验室检查(鼻咽分泌物、血清Mp-IgM等),入院3 d内完成影像学检查;②在本文病例收集结束后,统一调阅相关病例影像学资料,由我院放射科1名影像学主治医生在不知晓患儿临床信息的情况下阅片和评估。
2 结果
2.1 一般情况 653例MPP患儿进入分析(图1),占同期收治的肺炎患儿的51.9%(653/1 257)。难治性MPP组300例,男171例;普通MPP组353例,男221例。两组性别构成比差异无统计学意义(P=0.15)。
2.2 难治性MPP组相关因素的单因素分析 表1显示,难治性MPP组年龄显著高于普通MPP组,(66.8 ± 37.5)vs (51.4 ± 34.4)月龄,>3岁的比例也显著高于普通MPP组(234/300vs224/353,P<0.01)。难治性MPP组发热时间和住院天数均显著高于普通MPP组(P均<0.01)。肺外发现,两组肝功能损害发生率差异有统计学意义(8/353vs33/300,P<0.01),皮疹、淋巴结肿大的发生率差异无统计学意义。难治性MPP组IgM≥1∶1 280比例和Mp-DNA也显著高于普通MPP组(P均<0.01) 。 难治性MPP组的CK、LDH、HBDH、ALT、AST、PLT、CRP、PCT和IL-6水平均显著高于普通MPP组。
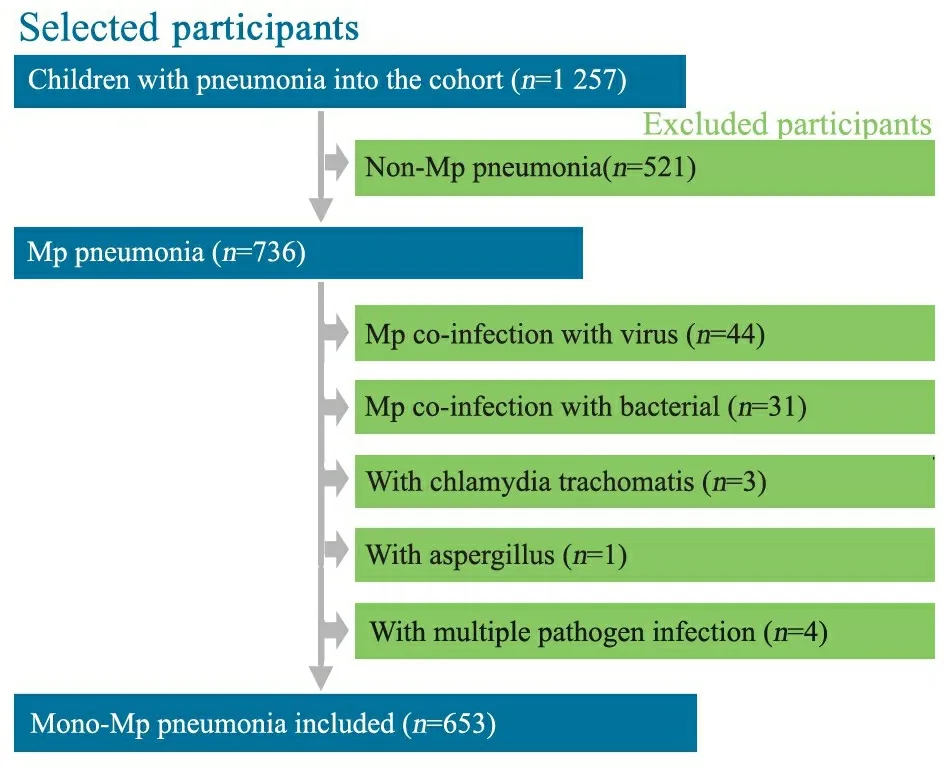
图1 病例纳入和排除流程图
Fig 1 Flow chart of inclusion and exclusion of patients
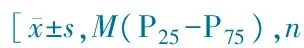

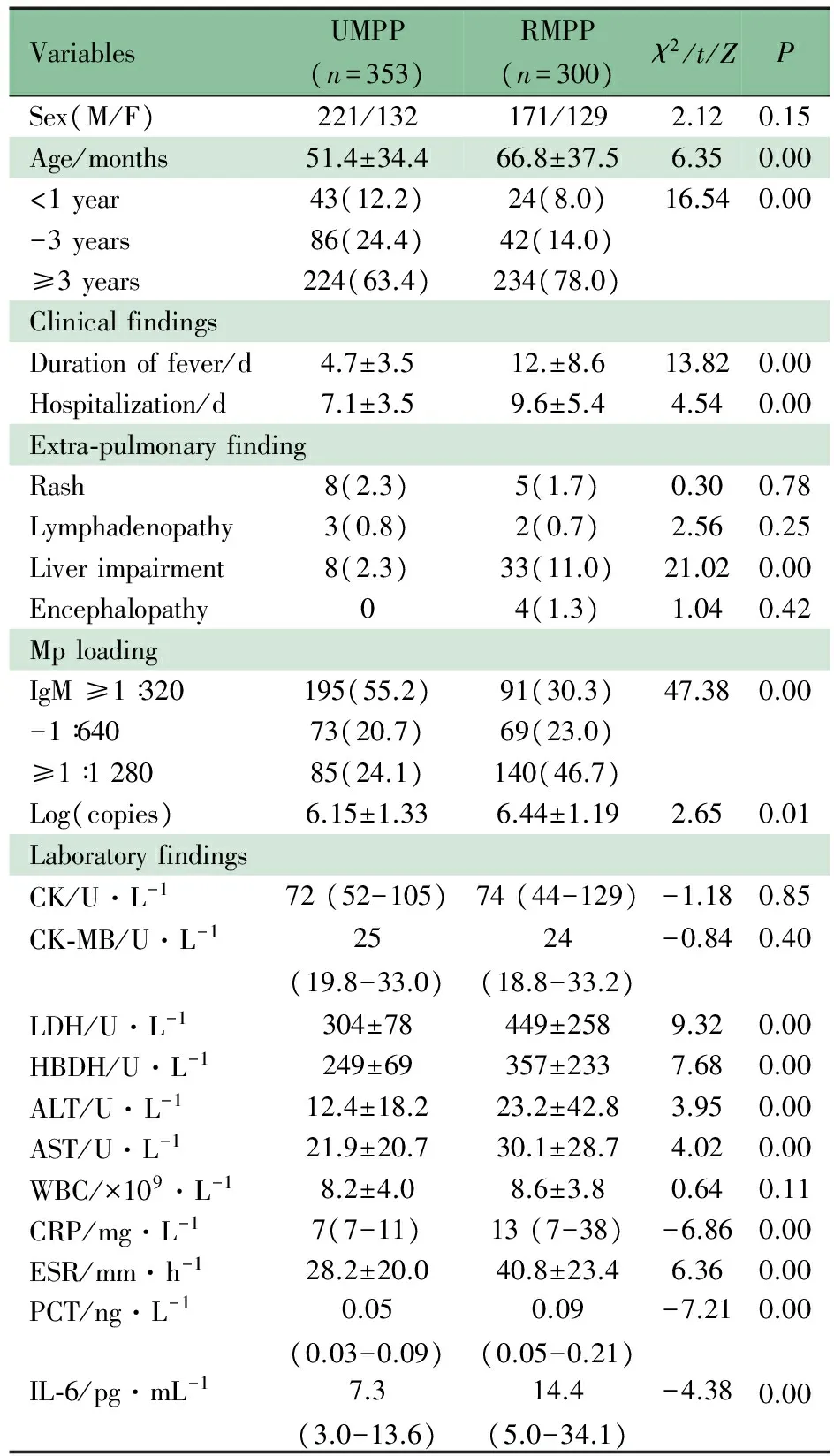
VariablesUMPP(n=353)RMPP(n=300)χ2/t/ZPSex(M/F)221/132171/1292.120.15Age/months51.4±34.466.8±37.56.350.00<1year43(12.2)24(8.0)16.540.00-3years86(24.4)42(14.0)≥3years224(63.4)234(78.0)ClinicalfindingsDurationoffever/d4.7±3.512.±8.613.820.00Hospitalization/d7.1±3.59.6±5.44.540.00Extra⁃pulmonaryfindingRash8(2.3)5(1.7)0.300.78Lymphadenopathy3(0.8)2(0.7)2.560.25Liverimpairment8(2.3)33(11.0)21.020.00Encephalopathy04(1.3)1.040.42MploadingIgM≥1∶320195(55.2)91(30.3)47.380.00-1∶64073(20.7)69(23.0)≥1∶128085(24.1)140(46.7)Log(copies)6.15±1.336.44±1.192.650.01LaboratoryfindingsCK/U·L-172(52-105)74(44-129)-1.180.85CK⁃MB/U·L-125(19.8-33.0)24(18.8-33.2)-0.840.40LDH/U·L-1304±78449±2589.320.00HBDH/U·L-1249±69357±2337.680.00ALT/U·L-112.4±18.223.2±42.83.950.00AST/U·L-121.9±20.730.1±28.74.020.00WBC/×109·L-18.2±4.08.6±3.80.640.11CRP/mg·L-17(7-11)13(7-38)-6.860.00ESR/mm·h-128.2±20.040.8±23.46.360.00PCT/ng·L-10.05(0.03-0.09)0.09(0.05-0.21)-7.210.00IL⁃6/pg·mL-17.3(3.0-13.6)14.4(5.0-34.1)-4.380.00
Notes RMPP:refractorymycoplasmapneumoniaepneumonia; UMPP: usualmycoplasmapneumoniaepneumonia; M/F: male/female
483例MPP患儿入我院3 d内行胸部X线和(或)CT检查,其中普通MPP组242例,难治性MPP组241例。两组均以渗出性病变为主,均为右下肺渗出最多(分别为56.8%和44.2%),其次是左下肺(分别为49.0%和41.3%)。难治性MPP组肺渗出面积评分显著高于普通MPP组(1.95±1.12)vs(1.55±0.97),P<0.01。两组肺间质病变的发生率分别为34.4%和47.9%,难治性MPP组观察到大叶性肺实变不张38例,中等量以上的胸腔积液 56例,坏死性肺炎3例(表2)。
表2 难治性MPP组和普通MPP组影像学特征比较[n(%)]
Tab 2 Comparison of characteristics of chest images of subjects with RMPP and UMPP[n(%)]

VariablesUMPP(n=242)RMPP(n=241)χ2PLocationofinfiltrationRight⁃up73(30.2)79(32.8)0.380.54Right⁃mid37(15.3)60(24.9)6.940.01Right⁃down107(44.2)137(56.8)7.710.01Left⁃up45(18.6)47(19.5)0.060.81Left⁃mid15(6.2)30(12.4)5.580.02Left⁃down100(41.3)118(49.0)2.840.09Infiltrationarea1.55±0.971.95±1.124.010.00Interstitialchanges116(47.9)83(34.4)9.070.00Pulmonaryhilarlymph⁃nodesenlargement9(3.7)16(6.6)2.010.15Lobarlungconsolidationandatelectasis038(15.8)41.30.00Pleuraleffusion056(23.2)63.50.00Pulmonarygangrene03(1.2)3.020.08
Notes RMPP:refractorymycoplasmapneumoniaepneumonia; UMPP: usualmycoplasmapneumoniaepneumonia
2.3 难治性MPP相关因素的Logistic回归 选择单因素分析后有统计学意义的指标(临床、实验室和影像学)行逐步Logistic回归分析,发现发热天数(OR=1.954,95%CI:1.403~2.722,P=0.000)、血清LDH水平(OR=1.009,95%CI:1.001~1.018,P=0.029)和肺渗出面积评分(OR=2.422,95%CI:1.111~5.279,P=0.026)是难治性MPP的独立相关因素。
3 讨论
本研究采集MPP病例行难治性MPP的相关因素分析,在设计和实施时充分考虑了研究中可能的偏倚和混杂因素,主要体现在:①我院呼吸科临床常规为入院次日行鼻咽分泌物细菌培养和其他病原学检查,并行血培养,较好的保证了纳入的病例为单一Mp感染的患儿;②本文观察的实验室检查指标均在入院次日采集,对早期判断MPP病情的严重程度有重要意义;③仅采集入院3 d内的影像学检查资料,且在研究结束后由放射科专人盲法读片,降低了可能的测量偏倚。因此本文结果可以较好的描述难治性MPP的相关因素。
本研究发现,因单一MPP住院的患儿占全年肺炎住院患儿的51.9%,是我院住院肺炎患儿的首要病原。近年来,Mp的感染率全球范围内呈增高趋势,尤其是亚洲地区Mp感染发生率和重症肺炎发生率远高于欧美[13~19]。一项亚洲范围内的流行病学调查显示,Mp感染引起的CAP占12.2%[16]。一项儿童呼吸道感染的调查显示Mp在呼吸道感染中占19.1%[15]。本研究显示,单一MPP患儿,男童构成比高于女童,与中国杭州地区的调查结果相似[20],但也有研究显示MPP无性别差异[21]。同时,本研究发现难治性MPP组>3岁患儿占78%,与既往的研究结果相似[20,22]。可能原因与3岁以上儿童入托后容易感染Mp,且大年龄儿童由于免疫系统发育相对完善,Mp感染后易于激发过强的免疫反应,造成免疫损伤。
本文难治性MPP组发热时间和住院天数显著高于普通MPP组,皮疹、淋巴结肿大两组分布差异无统计学意义,难治性MPP组肝功能损害情况较普通MPP组严重。值得一提的是本文难治性MPP组有4例合并脑病。Mp感染的肺外表现可累及全身任何一个器官[23~28],引起急性期损伤甚至死亡,部分患儿可能会留下后遗症[29,30]。Mp感染肺外表现的机制与支原体触发过强的免疫反应有关,另外,近年来提出的血管炎性/闭塞性损伤可能是Mp感染肺外表现的重要机制[31]。其他实验室指标的单因素分析显示,Mp-IgM、Mp-DNA与难治性MPP有一定关联,提示MPP严重程度与病原载量呈明显的量效关系;此外,LDH、HBDH水平与难治性MPP组有一定的相关性,LDH是糖酵解途径中一种重要的酶,被认为是肺间质性疾病中的重要血清学指标[32,33],HBDH是LDH活性的间接反映,两者水平的增高提示有发展为难治性Mp肺炎的趋向,炎症相关指标在难治性MPP组均呈显著高于普通MPP组的趋势,提示在难治性MPP的早期,强烈的炎症反应可能参与了MPP病情进展。Logistic 回归分析仅筛选出LDH为相关因素,考虑可能由于上述炎症因子之间存在一定的生物学相关性,因此未进入最终的回归模型。
普通MPP组和难治性MPP组肺渗出部分均以右下叶最多,其次是左下叶,与文献报道较为一致[34];难治性MPP组的肺渗出面积评分显著高于普通MPP组,两组累及间质的病例均较多(34.4%vs47.9%)。有研究显示[35],MPP的影像学表现早期为支气管壁的增厚、肺门淋巴结增大、片状模糊影和节段实变,难治性MPP可出现大叶实变不张、胸腔积液和肺坏死。本文病例资料显示,难治性MPP组中大叶性肺实变不张、胸腔积液、肺坏死和肺门淋巴结增大的比例较普通MPP组显著增多,与文献[35]报道一致。另外,该研究提示早期的胸部CT形态学特征如气管壁的增厚和小叶结节样改变对是否进展为难治性MPP无预测作用[35]。而关于早期炎症渗出面积与难治性MPP的相关性研究甚少,本文单因素分析发现肺渗出面积评分与进展为难治性MPP相关,进一步Logistic 回归显示肺渗出面积评分是难治性MPP的独立相关因素。
本研究的不足与局限性:儿童无法完全配合,仅取鼻咽部分泌物,其检测结果不能完全取代肺内分泌物的病原情况。
综上所述,Mp已成为CAP住院患儿的主要病原体,重症病例常发生于3岁以上儿童。疾病早期存在持续高热、肺部渗出面积大、血清LDH增高是进展为难治性MPP的独立相关因素,应早期积极干预,预防并发症的发生。
[1]Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther, 2008,6(4):509-521
[2]Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev, 2004,17(4):697-728
[3]Liu YN(刘又宁), Zhao TM, Yao WZ, et al. Prevalence of atypical pathogens in adult patients with community-acquired pneumonia in Beijing. Chin J Tubero Respir Dis(中华结核和呼吸杂志),2004,27(1):27-30
[4]The Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association(中华医学会儿科学分会呼吸学组). Guidelines for management of childhood community acquired pneumonia(for trial implementation) (Ⅰ). Chin J Pediatr(中华儿科杂志), 2013, 51(10):745-752
[5]Yu ZX(俞珍惜),Liu XY,Jiang ZF. Analysis of relevant factors of severe mycoplasma pneumoniae pneumonia in acute phase in children.J Appl Clin Pediatr(实用儿科临床杂志),2011,26(4):246-248
[6]Zhao SY(赵顺英), Ma Y, Zhang GF, et al. Clinical analysis of 11 cases of severe mycoplasma pneumonia. Chinese Journal of Practical Pediatrics(中国实用儿科杂志),2003,18(7 ):414-416
[7]Sun J(孙静),Ma HG, Qi BS. 儿童呼吸道感染检测降钙素原、肺炎衣原体抗体IgM 和肺炎支原体抗体IgM 的临床意义观察. Chinese Pediatrics Of Integrated Traditional And Western Medicine(中国中西医结合儿科学),2014,6 (1):47-48
[8]Meng SS(孟珊珊), Zhang HL. Extrapulmonary manifestations and pathogenesis of Mycoplasma pneumoniae infection in children. Int J Pediatr(国际儿科学杂志),2013,40(1): 14-18
[9]Qu J,Gu L, Wu J, Dong J,et al. Accuracy of IgM antibody testing, FQ-PCR and culture in laboratory diagnosis of acute infection by Mycoplasmapneumoniae in adults and adolescents with community-acquired pneumonia. BMC Infect Dis.2013 ,13:172
[10]Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007 ,56(Pt 12):1625-1629
[11]Izumikawa K, Izumikawa K, Takazono T, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother.2014, 20(3):181-185
[12]Liu DY(刘东宇). Imaging observation of Tanreqing injection in treatment of severe pneumonia. Journal of Liaoning University of TCM(辽宁中医药大学学报), 2013,15 (2): 173-174
[13]Eshaghi A, Memari N, Tang P, et al. Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010-2011. Emerg Infect Dis, 2013,19(9): 1525-1527
[14]Yamada M, Buller R, Bledsoe S, et al. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J, 2012,31(4):409-400
[15]Zhao H, Li S, Cao L, et al. Surveillance of Mycoplasma pneumoniae infection among children in Beijing from 2007 to 2012. Chin Med J (Engl), 2014,127(7):1244-1248
[16]Ngeow YF, Suwanjutha S, Chantarojanasriri T, et al. An Asian study on the prevalence of atypical respiratory pathogens in community-acquired pneumonia. Int J Infect Dis, 2005,9(3):144-153
[17]Daxboeck F, Eisl B, Burghuber C, et al. Fatal Mycoplasma pneumoniae pneumonia in a previously healthy 18-year-old girl. Wien Klin Wochenschr, 2007,119(11-12):379-384
[18]Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol, 2007,56(Pt 12):1625-1629
[19]Izumikawa K, Takazono T, Kosai K, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother, 2014,20(3):181-185
[20]Xu YC, Zhu LJ, Xu D, et al. Epidemiological characteristics and meteorological factors of childhood Mycoplasma pneumoniae pneumonia in Hangzhou. World J Pediatr, 2011,7(3):240-244
[21]Kung CM, Wang HL. Seroprevalence of Mycobacterium pneumoniae in healthy adolescents in Taiwan. Jpn J Infect Dis, 2007,60(6):352-354
[22]Klement E, Talkington DF, Wasserzug O, et al. Identification of risk factors for infection in an outbreak of Mycoplasma pneumoniae respiratory tract disease. Clin Infect Dis, 2006,43(10):1239-1245
[23]Zhou Y, Zhang Y, Sheng Y, et al. More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother, 2014,58(2):1034-1038
[24]Wanat KA, Castelo-Soccio L, Rubin AI, et al. Recurrent Stevens-Johnson syndrome secondary to Mycoplasma pneumoniae infection. Cutis, 2014,93(4):E7-8
[25]Vujic I, Shroff A, Grzelka M, et al. Mycoplasma pneumoniae-associated mucositis - case report and systematic review of literature. J Eur Acad Dermatol Venereol, 2014
[26]Rock N, Belli D, Bajwa N. Erythema bullous multiforme: a complication of Mycoplasma pneumoniae infection. J Pediatr, 2014,164(2):421
[27]Tay CG, Fong CY, Ong LC. Transient Parkinsonism Following Mycoplasma pneumoniae Infection With Normal Brain Magnetic Resonance Imaging (MRI). J Child Neurol, 2013, 29(12):193-195
[28]Miyashita N, Kawai Y, Akaike H, et al. Atelectasis caused by macrolide-resistant Mycoplasma pneumoniae pneumonia in an adult patient. J Infect Chemother, 2013,19(6):1161-1166
[29]Santiago-Burruchaga M, Zalacain-Jorge R, Alvarez-Martinez J, et al. Hereditary pulmonary alveolar proteinosis. Could it be triggered by Mycoplasma pneumoniae pneumonia? Respir Med, 2013,107(1):134-138
[30]Lee M, Joo IS, Lee SJ, et al. Multiple cerebral arterial occlusions related to Mycoplasma pneumoniae infection. Neurol Sci, 2013,34(4):565-568
[31]Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother, 2010,16(3):162-169
[32]Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev, 2004,25(2):121-132
[33]Nakajima M, Kawahara Y, Yoshida K, et al. Serum KL-6 as a possible marker for amiodarone-induced pulmonary toxicity. Intern Med, 2000,39(12):1097-1100
[34]Wang SH(王恒申). 105例儿童支原体肺炎胸部X线影像学特点分析. Clinical Focus(临床荟萃),2010,25(15):1329-1330
[35]Miyashita N, Sugiu T, Kawai Y, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging, 2009,9:7
(本文编辑:丁俊杰)
The associated factors of refractory Mycoplasma pneumoniae pneumonia in hospitalized children in a mono-center in Shanghai
LUAi-zhen1,YANGHao-wei1,WANGChuan-kai,QIANLi-ling,ZHANGXiao-bo,WANGLi-bo
(DepartmentofRespiratory,Children′sHospitalofFudanUniversity,Shanghai, 201102,China; 1hasequalcontributiontothisstudy)
Corresponding Author:ZHANG Xiao-bo,E-mail: zhangxiaobo0307@163.com; WANG Li-bo,E-mail:wanglbc@163.com
ObjectiveTo analyze the associated factors of refractoryMycoplasmapneumoniae(Mp) pneumonia.MethodsThe cases of Mono-Mp pneumonia admitted in Children′s Hospital of Fudan University from September 2012 to August 2013 were collected, and divided into two groups, refractory Mp pneumonia(RMPP) and usual Mp pneumonia(UMPP) . Laboratory outcomes collected by literature review were performed on the next day of admission. Chest X-ray and/or CT data were also collected in the first 3 days after admission. The laboratory outcomes and chest images were analyzed for the associated factors of refractory Mp pneumonia by univariate and multivariate logistic regression analysis.Results653 cases of Mono-Mp pneumonia were collected, covered 51.7% (653/1257) of the total number of pneumonia. There were 300 cases in refractory pneumonia group, 171 boys and 129 girls. And there were 353 cases in normal pneumonia group, 221 boys and 132 girls. ①Univariate analysis showed the age in RMPP group was significantly higher than that of UMPP group, (66.8 ± 37.5)vs(51.4 ± 34.4) months,P<0.01; the proportion of cases over 3 years old in RMPP group was also significantly higher than that in UMPP group (234/300vs224/353,P<0.01); the hospitalized days and febrile days in RMPP group were significantly higher than those in UMPP group (P<0.01, respectively); laboratory outcomes in RMPP group, CK, LDH, HBDH, ALT, AST, CRP, PCT, IL-6, were significantly higher than those in UMPP group (P<0.01, respectively); infiltration area of chest images in RMPP was significantly greater than that in UMPP, (1.95 ± 1.12)vs(1.55 ± 0.97),P<0.01. ② Those significant outcomes in univariate analysis were chosen to perform stepwise logistic regression, and the analysis showed that febrile days (OR=1.954,95%CI:1.403-2.722), serum LDH level (OR=1.009,95%CI:1.001-1.018), and infiltration area of chest images (OR=2.422,95%CI:1.111-5.279) were the independent associated factors of RMPP. ConclusionMp became a major pathogen of hospitalized children with CAP. Severe cases often occurred in children over 3 years old. The persistent fever, the large area of infiltration in chest images, high level of serum LDH in the early stage suggested that it may progress into severe pneumonia.
Mycoplasmapneumoniae; Refractory Mycoplasma pneumoniae pneumonia; Associated factors; Children
复旦大学附属儿科医院呼吸科 上海,201102;1 共同第一作者
张晓波,E-mail: zhangxiaobo0307@163.com;王立波,E-mail:wanglbc@163.com
10.3969/j.issn.1673-5501.2014.06.003
2014-07-03
2014-11-27)
