Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
2014-07-24GUOQiangandWANGTao
GUO Qiang (郭 强) and WANG Tao (王 涛)
State Key Lab of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
GUO Qiang (郭 强) and WANG Tao (王 涛)**
State Key Lab of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
A sodium sulfate (Na2SO4)/silica (SiO2) composite was prepared as a shape-stabilized solid-liquid phase change material by a sol-gel procedure using Na2SiO3as the silica source. Na2SO4in the composite acts as a latent heat storage substance for solid-liquid phase change, while SiO2acts as a support material to provide structural strength and prevent leakage of melted Na2SO4. The microstructure and composition of the prepared composite were characterized by the N2adsorption, transmission electron microscope (TEM), scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction. The results show that the prepared Na2SO4/SiO2composite is a nanostructured hybrid of Na2SO4and SiO2without new substances produced during the phase change. The macroscopic shape of the Na2SO4/SiO2composite after the melting and freezing cycles does not change and there is no leakage of Na2SO4. Determined by differential scanning calorimeter (DSC) analysis, the values of phase change latent heat of melting and freezing of the prepared Na2SO4/SiO2(50%, by mass) composite are 82.3 kJ·kg−1and 83.7 kJ·kg−1, and temperatures of melting and freezing are 886.0 °C and 880.6 °C, respectively. Furthermore, the Na2SO4/SiO2composite maintains good thermal energy storage and release ability even after 100 cycles of melting and freezing. The satisfactory thermal storage performance renders this composite a versatile tool for high-temperature thermal energy storage.
sodium sulfate, silicon dioxide, phase change material, shape-stabilized, sol-gel method
1 INTRODUCTION
The technique of thermal energy storage is an effective way to improve the utilization of energy. In recent years, the interest for latent heat storage technology using phase-change materials (PCMs) increases, since they can store a large amount of thermal energy at a constant temperature due to their high fusion heat during phase transition [1-5]. The phase-change materials have been applied in many fields, such as solar energy storage, building energy saving, heat storage in space stations, and waste heat recovery and storage from industrial furnaces [6-9].
A shape-stabilized PCM composed of a phasechange substance and a carrier matrix is a kind of novel PCMs, with phase change properties lying between solidsolid and solid-liquid phase change. They keep solid state even though the phase-change substance change from solid to liquid, which is more convenient for applications than the solid-liquid system [10-13]. Among the composite PCMs available, inorganic salt/ceramic shape-stabilized PCMs for high-temperature (>300 °C) thermal energy storage has significant advantages: good chemical and thermal stability, good high-temperature resistance and high heat conductivity [14-19].
Na2SO4/SiO2composites are one kind of shapestabilized PCM with good heat storage performance at high temperatures [20-22]. As a phase-change substance, Na2SO4has a high latent heat of phase transition, excellent stability, and low vapor pressure. As a support material, SiO2, which will soften at 1400 °C, has good mechanical property, good thermal conductivity and thermal stability. Two main methods have been used for the preparation of Na2SO4/SiO2shape-stabilized phase change composites. In the infiltration method, the Na2SO4powder melts in an electric furnace and infiltrates into the porous SiO2matrix previously prepared with specific shape and size, and the Na2SO4/SiO2composite is formed when the furnace cools to room temperature [20]. In another method, a mixture of SiO2powder, Na2SO4, and an appropriate amount of an additive is pressed to form a short cylinder and sintered at high temperature [21]. In both methods, pure SiO2and Na2SO4are used as the raw materials, and a lot of energy is consumed for high-temperature sintering.
In this study, Na2SiO3is used as the silicon source for the preparation of shape-stabilized PCM Na2SO4/SiO2composites by a sol-gel process. The Na2SiO3solution reacts with sulfuric acid to form a silica gel, and Na2SO4is generated in situ in the sol-gel process. A Na2SO4/SiO2shape-stabilized PCM for thermal energy storage is prepared by embedding Na2SO4in the silica xerogel network structure. The composites are characterized with respect to chemical compatibility by Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). The thermal properties and thermal reliabilities of the composite PCMs are investigated using the differential scanning calorimeter (DSC) analysis technique. The morphology and microstructure are investigated by scanning electron microscope (SEM) and transmission electron microscope (TEM). The results demonstrate this composite has good properties and will be useful for the high-temperature thermal energy storage.
2 EXPERIMENTAL
2.1 Chemicals and materials
Sodium metasilicate nonahydrate (Na2SiO3·9H2O, AR), sodium form cation exchange resin (732) and Na2SO4(98%, AR) were bought from Beijing Modern Eastern Fine Chemicals (Beijing, China). Deionized water was prepared in our laboratory by electrodialysis.
2.2 Methods
Na2SiO3solution (0.5 mol·L−1) passed through the sodium form cation exchange resin to remove Na+until pH of the solution reached 7. The solution (pH=7) was stirred vigorously for 10 min after adding Na2SO4. The mixture was kept at 25 °C in a water bath to gelatinize for 24 h. The gel formed was dried in the oven at 100 °C for 48 h to produce a powder Na2SO4/SiO2composite. The composite was pressed to form circular shapes at 4 MPa for 2 min to study the shape stability of the composite. The maximum mass percentage of Na2SO4dispersed into the PCM composites was determined as 50%. There was no leakage of Na2SO4from the surface of the composite up to this mass ratio even when it melts.
2.3 Characterization
The composition and structure of the prepared samples were characterized by infrared (IR) spectroscopy (FTIR-8201, Shimadzu, Kyoto, Japan), X-ray diffraction (XRD; D8A, Bruker, Karlsruhe, Germany), N2adsorption analyzer (Autosorb-1-C Quantachrome, USA), high-resolution transmission electron microscopy (TEM; JEM2010, JEOL, Kyoto, Japan) and scanning electron microscopy (SEM; JSM7401, Shimadzu, Kyoto, Japan). Differential scanning calorimetry (DSC; STA409PC, Selb, Netzsch, Germany) measurements were conducted to determine the phase-transition enthalpy, phase-transition temperature, and thermal stability for the prepared Na2SO4/SiO2shape-stabilized PCM. is caused by the SO stretching vibration and a peak at 617 cm−1is caused by the S O symmetric stretch-
3 RESULTS AND DISCUSSION
3.1 IR analysis of Na2SO4/SiO2composite
The IR spectra of Na2SO4, SiO2, and the Na2SO4/SiO2[50% (by mass) Na2SO4] composite are shown in Fig. 1 (a). For Na2SO4, a peak at 1126 cm−1represents the SiOSi antisymmetric stretching vibration, and the peaks at 800 cm−1and 473 cm−1are caused by the SiO Si symmetric stretching and ing vibration [23]. For SiO2, the peak at 1049 cm−1bending vibrations. The peak at 3483 cm−1 in the IR spectrum of silica corresponds to the OH stretching vibration from the SiOH groups and adsorbed H2O in the silica [24]. Characteristic peaks at 1103 cm−1, 794 cm−1, 621 cm−1, and 486 cm−1are found in the IR spectrum of the composite PCM without significant new peaks. Fig. 1 (b) shows the IR spectrum of the supporting SiO2with removal of Na2SO4in the composite by washing and extracting, which is quite consistent with the IR spectrum of pure SiO2. Thus there is no residual Na2SO4in the supporting SiO2after washing and extracting Na2SO4. The IR spectral analysis indicates that the prepared composite PCM is a physical hybrid of Na2SO4and SiO2only.
3.2 XRD analysis of Na2SO4/SiO2composite
X-ray spectra of the Na2SO4/SiO2[50% (by mass) Na2SO4] composites after different heating and cooling cycles are shown in Fig. 2. Although they look different, only Na2SO4and SiO2are present in the composite by phase analysis. The difference in the X-ray spectra is mainly caused by crystal structure change of SiO2and Na2SO4during the melting and cooling cycle. The much stronger SiO2peaks are attributed to the change of SiO2in the composite PCM from an amorphous structure to a crystalline structure, while the change of Na2SO4peaks indicates the crystal transformation of Na2SO4after the heating-cooling process [25]. Formation of new compounds is not found during the process, demonstrating good chemical stability of this Na2SO4/SiO2composite.
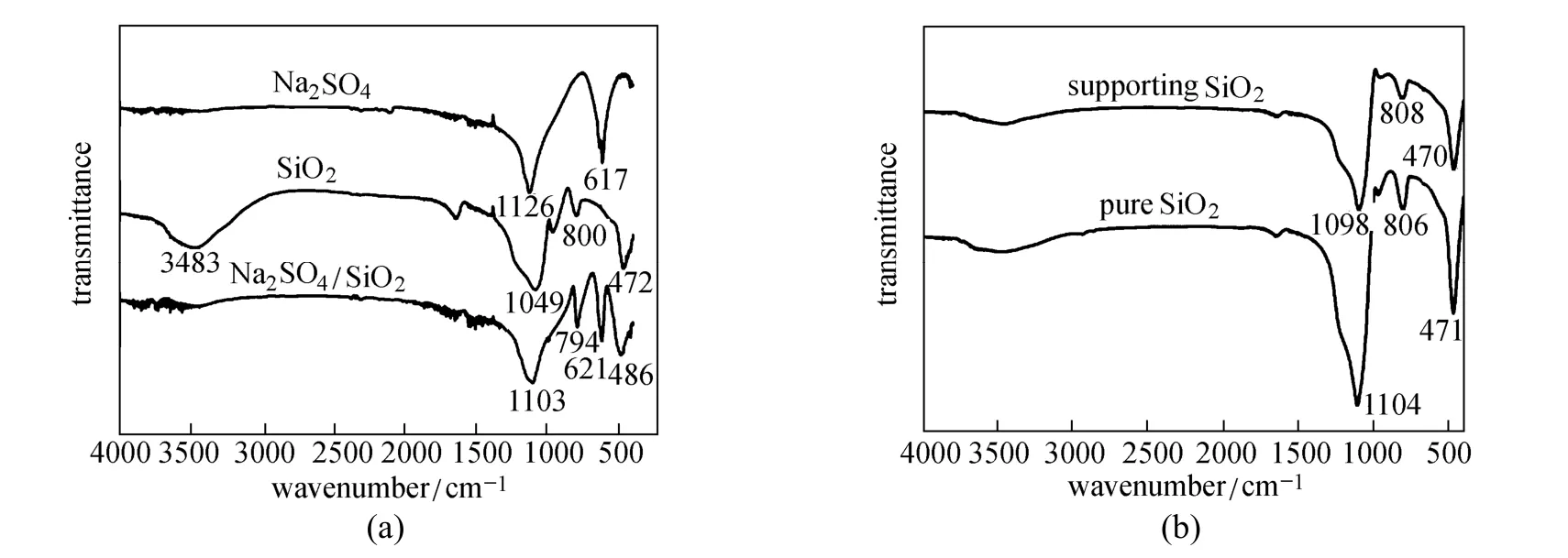
Figure 1 FTIR spectra for: Na2SO4, SiO2, and Na2SO4/SiO2composite (a), pure SiO2and supporting SiO2(b)
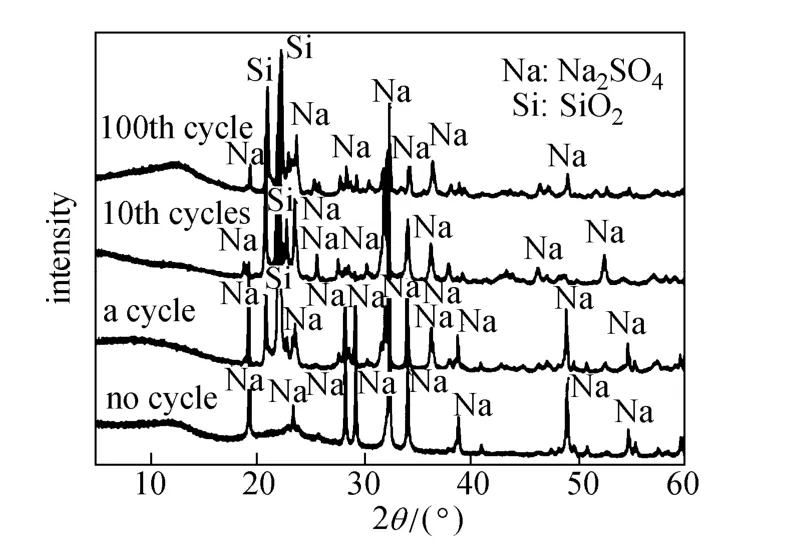
Figure 2 X-ray spectrum of Na2SO4/SiO2composite after different heating and cooling cycles
3.3 Morphology characterization of Na2SO4/SiO2composite
The macroscopic and external SEM photographs of the Na2SO4/SiO2[50% (by mass) Na2SO4] composite are shown in Fig. 3. Apart from a slight shrink after the first heating-cooling cycle, the macroscopic shape of the composite does not change after other cycles of melting and cooling and there is no leakage of Na2SO4in the composite [Fig. 3 (a)]. The average thermal expansion coefficient of the composite determined is 35×10−6°C−1at 800-900 °C, which is in the similar level as reported by Huang et al [25]. These results indicate that the Na2SO4/SiO2composite is well shape-stable. The surface SEM shows that SiO2and Na2SO4are distributed in a staggered way with scales less than 100 nm [Fig. 3 (b)]. After heating to 950 °C and cooling to room temperature, Na2SO4crystals are found in some small disjunctive regions (< 1 μm2) on the external surface of the sample [Fig. 3 (c)]. This phenomenon implies that some Na2SO4permeates through from the interior of the sample when it melts. However, the melted Na2SO4only penetrates and adheres to the micro-areas, so the solid shape of the sample is unchanged. There are a number of honeycomb-like holes on the surface of the sample after 10 heating-cooling cycles [Fig. 3 (d)], caused by the seepage of melted Na2SO4into the void of the SiO2matrix due to capillary force and surface tension. All these results show that the Na2SO4/SiO2composite is capable of maintaining its shape in the solid state without losing melted Na2SO4during the phase-change process.
3.4 Microstructure and pore size distribution analysis of Na2SO4/SiO2composite
TEM image of the Na2SO4/SiO2composite shows that the supporting SiO2in the composite forms a porous network structure with the pore size below 20 nm to provide strong supporting body for the composite (Fig. 4). Fig. 5 shows the pore size distributions of the composite [50% (by mass) Na2SO4] and the supporting SiO2after removal of Na2SO4. There is no collapse of SiO2framework after removal of Na2SO4by washing and extracting without stirring, because the nanoporous SiO2framework has enough mechanical strength. The range of pore size and pore volume are 2.8-7.5 nm and 0.261 cm3·g−1for the composite, respectively, and 3.6-16.3 nm and 0.819 cm3·g−1for the supporting SiO2. From larger pore sizeand pore volume of the supporting SiO2after removing Na2SO4, we conclude that Na2SO4in the prepared composite is coated in SiO2porous network structure.

Figure 3 Images of the Na2SO4/SiO2composite

Figure 4 The TEM images of the Na2SO4/SiO2composite

Figure 5 Isotherm N2adsorption-desorption curves and pore size distribution curves of Na2SO4/SiO2composite and SiO2support after removing Na2SO4
3.5 Thermal analysis of Na2SO4/SiO2composite
DSC curves of Na2SO4and the Na2SO4/SiO2composite at scanning rate of 20 °C·min−1are shown in Fig. 6. The melting heat and freezing latent heat are respectively 167.1 and 171.4 kJ·kg−1at its melting temperature of 888.7 °C and freezing temperature of 888.2 °C for pure Na2SO4. The phase-transition enthalpies of the prepared composite [50% (by mass) Na2SO4] are 82.3 kJ·kg−1at 886.0 °C for melting and 83.7 kJ·kg−1at 880.6 °C for freezing. The melting heat of both pure Na2SO4and the composite is less than its crystallization heat, possibly caused by crystal structure change of Na2SO4during freezing [25]. The melting point of Na2SO4confined in the network structure of the SiO2support is obviously lower than that of Na2SO4in the bulk state. This melting point depression is considered to be caused mainly by the small-size effect and the surface effect [26, 27].
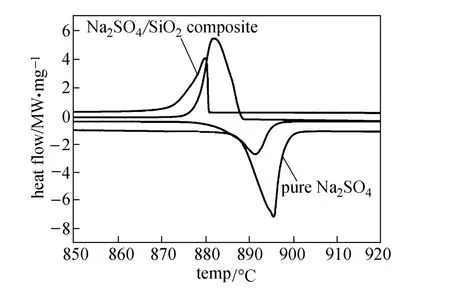
Figure 6 DSC curves of Na2SO4and Na2SO4/SiO2(50%, by mass) composite at scanning rate of 20 °C·min−1
To test the thermal cycling stability, we determined the phase change latent heats of the Na2SO4/SiO2composite (50%, by mass) after 100 cycles of heating and cooling between 750 °C and 950 °C at a rate of 20 °C·min−1. Fig. 7 shows that the melting latent heat of the composite is less than the crystallization latent heat. In addition, the phase change latent heats of the composite decreases with the increase of the number of thermal cycles, which may be caused by crystal structure change of Na2SO4[25, 28].
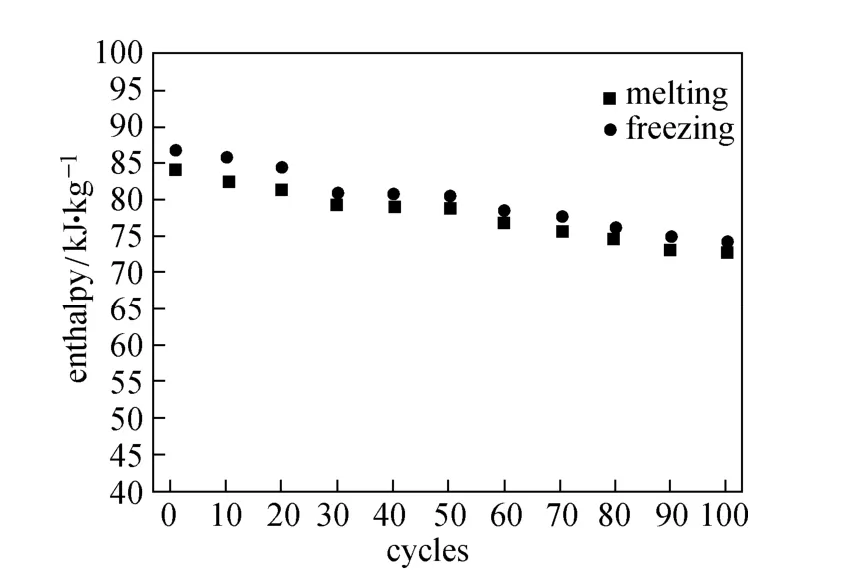
Figure 7 Phase change enthalpy of Na2SO4/SiO2(50%, by mass) composite after different cycles of melting and cooling
4 CONCLUSIONS
The Na2SO4/SiO2composite was prepared as the shape-stabilized phase-change material by sol-gel process. The composite [50% (by mass) Na2SO4] can maintain its solid shape without any leakage of Na2SO4after melting and freezing cycles. In the composite, Na2SO4is coated in SiO2porous network structure with average pore diameter less 20 nm. The phase change latent heats of melting and freezing of the prepared Na2SO4/SiO2composite are 82.3 and 83.7 kJ·kg−1, and temperatures are 886.0 °C and 880.6 °C, respectively. The Na2SO4/SiO2composite maintains good thermal energy storage and release ability after 100 cycles of meting and freezing. The thermal storage performance renders this composite a versatile tool for the high-temperature thermal energy storage.
REFERENCES
1 Zalba, B., Marin, J.M., Cabeza, L.F., Mehling, H., “Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications”, Appl. Therm. Eng., 23, 251-283 (2003).
2 Farid, M.M., Khudhair, A.M., Razack, S.AK., Al-Hallaj, S., “A review on phase change energy storage: Materials and applications”, Energ. Convers. Manag., 45, 1597-1615 (2004).
3 Sharma, S.D., Sagara, K., “Latent heat storage materials and systems: A review”, International Journal of Green Energy, 2, 1-56 (2005).
4 Regin, A.F., Solanki, S.C., Saini, J.S., “Heat transfer characteristics of thermal energy storage system using PCM capsules: A review”, Renew. Sust. Energ. Rev., 12, 2438-2458 (2008).
5 Raoux, S., “Phase change materials”, Ann. Rev. Mater. Res., 39, 25-48 (2009).
6 Kenisarin, M., Mahkamov, K., “Solar energy storage using phase change materials”, Renew. Sust. Energ. Rev., 11, 1913-1965 (2007).
7 Tyagi, V.V., Buddhi, D., “PCM thermal storage in buildings: A state of art”, Renew. Sust. Energ. Rev., 11, 1146-1166 (2007).
8 Mondal, S., “Phase change materials for smart textiles—An overview”, Appl. Therm. Eng., 28, 1536-1550 (2008).
9 Cabeza, L.F., Castell, A., Barreneche, C., Gracia, A., Fernandez, A.I.,“Materials used as PCM in thermal energy storage in buildings: A review”, Renew. Sust. Energ. Rev., 15, 1675-1695 (2011).
10 Feng, L.L., Zheng, J., Yang, H.Z., Guo, Y.L., Li, W., Li, X.G.,“Preparation and characterization of polyethylene glycol/active carbon composites as shape-stabilized phase change materials”, Sol. Energ. Mater. Sol. C., 95, 644-650 (2010).
11 Wang, Y., Xia, T.D., Zheng, H., Feng, H.X., “Stearic acid/silica fume composite as form-stable phase change material for thermal energy storage”, Energ. Buildings, 43, 2365-2370 (2011).
12 Ahmet, S., Kamil, K., “Thermal energy storage characteristics of myristic and stearic acids eutectic mixture for low temperature heating applications”, Chin. J. Chem. Eng., 14, 270-275 (2006).
13 Sun, D., Wang, L.J., Li, C.M., “Preparation and thermal properties of paraffin/expanded perlite composite as form-stable phase change material”, Materials Letters, 108, 247-249 (2013).
14 Petri, R.J., Ong, E.T., Claar, T.D., “High-temperature salt/ceramic thermal storage phase-change media”, In: Proceedings of the 18th Intersociety Energy Conversion Engineering Conference, American Institute of Chemical Engineers, Orlando, 1769-1774 (1983).
15 Notter, W., Hahne, E., “Thermal expansion models for polycrystalline salt-ceramics”, Thermochim Acta, 290, 93-100 (1997).
16 Kenisarin, M.M., “High-temperature phase change materials for thermal energy storage”, Renew. Sust. Energ. Rev., 14, 955-970 (2010).
17 Mao, A., Park, J.H., Han, G.Y., Seo, T., Kang, Y., “Heat transfer characteristics of high temperature molten salt for storage of thermal energy”, Korean J. Chem. Eng., 27, 1452-1457 (2010).
18 Zhao, C.Y., Wu, Z.G., “Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite”, Sol. Energ. Mater. Sol. C., 95, 636-643 (2011).
19 Wang, T., Mantha, D., Reddy, R.G., “Thermal stability of the eutectic composition in LiNO3-NaNO3-KNO3ternary system used for thermal energy storage”, Sol. Energ. Mater. Sol. C., 100, 162-168 (2012).
20 Notter, W., Lechner, T., Gro, U., Hahne, E., “Thermophysical properties of the composite ceramic-salt system (SiO2/Na2SO4)”, Thermochim Acta, 218, 455-463 (1993).
21 Huang, P., Guo, Y., Quirk, R.P., Ruan, J., Lotz, B., Thomas, E.L., Hsiao, B.S., Avila-Orta, C.A., Sics, I., Cheng, Z.D., “Comparison of poly(ethylene oxide) crystal orientations and crystallization behaviors in nano-confined cylinders constructed by a poly(ethylene oxide)-β-polystyrene diblock copolymer and a blend of poly(ethylene oxide)-b-polystyrene and polystyrene”, Polymer, 47, 5457-5466 (2006).
22 Shi, D.Z., Rapp, R.A., “The solubility of SiO2in fused Na2SO4at 900 °C”, J. Electrochem. Soc., 133, 849-850 (1986).
23 Qu, Q., Li, L., Bai, W., Yan, C.W., “Initial atmospheric corrosion of zinc in presence of Na2SO4and (NH4)2SO4”, T. Nonferr. Metal. Soc., 16, 887-891 (2006). (in Chinese)
24 Wang, W.L., Yang, X.X., Fang, Y.T., Ding, J., “Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid-liquid phase change materials”, Appl. Energ., 86, 170-174 (2009).
25 Huang, J., Zhang, R.Y., Wu, B., “Crystal forms transformation and thermal expansion property of polycrystalline Na2SO4/SiO2composite phase change energy storage materials”, J. Mater. Eng., 12, 16-20 (2006). (in Chinese)
26 Alba-Simionesco, C., Coasne, B., Dosseh, G., Dudziak, G., Gubbins, K.E., Radhakrishnan, R., Sliwinska-Bartkowiak, M., “Effects of confinement on freezing and melting”, J. Phys-condens. Mater., 18, 15-68 (2006).
27 Cheng, T., Charnaya, E.V., Podorozhkin, D.Y., Lee, M.K., Baryshnikov, S.V., “Ferroelectricity and gradual melting in NaNO2particles confined within porous alumina”, Physica. Status. Solidi. B., 246, 2346-2351 (2009).
28 Jiang, S., Ji, X., An, L., Jiang, B., “Crystallization behavior of PCL in hybrid confined environment”, Polymer, 42, 3901-3907 (2001).
MATERIALS AND PRODUCT ENGINEERING
Chinese Journal of Chemical Engineering, 22(3) 360—364 (2014)
10.1016/S1004-9541(14)60047-1
2012-06-14, accepted 2013-01-07.
*Supported by the National Natural Science Foundation of China (2107611).
**To whom correspondence should be addressed. E-mail: taowang@tsinghua.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Determination of Transport Properties of Dilute Binary Mixtures Containing Carbon Dioxide through Isotropic Pair Potential Energies
- Superparamagnetic Supported Catalyst H3PW12O40/γ-Fe2O3for Alkylation of Thiophene with Olefine*
- A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
- A Contraction-expansion Helical Mixer in the Laminar Regime*
- Effects of Shape and Quantity of Helical Baffle on the Shell-side Heat Transfer and Flow Performance of Heat Exchangers*
- Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor
