苏丹红I号在纳米金/碳球修饰硼掺杂金刚石电极上的电化学行为
2014-06-09王一涛李西营陈蔚萍
刘 勇,王一涛,李西营,陈蔚萍
(河南大学化学化工学院,河南开封475004)
苏丹红I号在纳米金/碳球修饰硼掺杂金刚石电极上的电化学行为
刘 勇*,王一涛,李西营,陈蔚萍
(河南大学化学化工学院,河南开封475004)
采用纳米金/碳球(Au/CS)复合物修饰硼掺杂金刚石(BDD)电极,研究了苏丹红Ⅰ号在Au/CS修饰BDD电极上的电化学行为,并据此建立了实际样品中的苏丹红Ⅰ号的测定方法.结果表明,与裸BDD电极相比,苏丹红Ⅰ号在Au/CS修饰BDD电极上的氧化峰电流由0.24μA增加到0.83μA,峰电位由0.809 V负移到0.743 V.在最优测试条件下,苏丹红Ⅰ号浓度与其峰电流在4~100μmol/L范围内呈线性关系,线性方程为Ip=0.011 26c+0.116(R2=0.999),检出限为8.33μmol/L.采用本方法对实际样品中的苏丹红Ⅰ号进行测定,测定结果及平均回收率均优于BDD电极法.
纳米金/碳球;硼掺杂金刚石电极;苏丹红Ⅰ号;电化学行为
苏丹红Ⅰ号(1-苯基偶氮-2-萘酚)是一种用于油脂、汽油、溶剂和鞋油等的增色添加剂,被国际癌症研究机构(ⅠARC)列为致癌物,一直被禁止用于食品生产和加工中[1].由于苏丹红Ⅰ号检测技术不够成熟,检测起来费时费力,给许多不法商贩可乘之机.因此,为保障居民饮食安全,建立一种快速、简便的苏丹红Ⅰ号的检测方法迫在眉睫.目前,检测苏丹红的方法主要有高效液相色谱法[2-3]、液-质联用法[4-5]、电化学方法[6-9]等,其中电化学方法因其仪器简单、灵敏度高、重现性好、分析成本低且易于操作而备受关注.
利用纳米材料对电极进行修饰,可提高电极的导电性、加快电子转移及增强灵敏度,近年来一些纳米材料已被广泛应用于修饰电极,并取得了良好的效果[6-9].金纳米粒子能够增加电极的导电性,促进电子转移,已广泛应用于电化学传感器中[10].碳球具有较强的导电性[11-14]和较好的化学稳定性.结合两种材料的优势,本文通过化学方法合成Au/CS复合物,用于修饰硼掺杂金刚石(BDD)电极,研究了苏丹红Ⅰ号在Au/ CS-BDD和BDD上的电化学行为.
1 实验部分
1.1 仪器和试剂
CHⅠ-660D电化学工作站(上海辰华仪器有限公司).AL-104型分析天平;PHS-3C型酸度计;KQ-100E型数字超声波清洗器;电化学实验用三电极系统,BDD和Au/CS-BDD为工作电极,铂(Pt)为对电极,Ag/ AgCl(饱和KCl)为参比电极.本文中所有电位均是相对于Ag/AgCl电极.苏丹红Ⅰ号购自阿拉丁试剂公司,乙醇、硫酸钠、氯化钾、氢氧化钠、磷酸氢二钠、磷酸二氢钾等试剂均为分析纯,实验用水为二次蒸馏水.
1.2 修饰电极的制备
首先采用Stöber法制备碳球[11],然后将BDD电极用无水乙醇、超纯水超声清洗数次.根据文献[10]制备Au/CS复合材料,然后用盐酸羟胺还原法生长纳米金.取4 mg Au/CS加入10 m L超纯水中,开启磁力搅拌使其均匀分散在水溶液中.用移液枪取适量的Au/CS溶液滴加在处理过的BDD电极表面,在室温下干燥即可得到Au/CS-BDD修饰电极.
1.3 实际样品中的苏丹红I号的检测
选用番茄酱作为实际样品,取番茄酱0.5 g,用乙醇溶解、过滤,取10 m L滤液移至25 m L容量瓶,加入2.5 m L浓度为0.01 mol/L的苏丹红Ⅰ号母液,用乙醇定容至刻度线.按所建立的方法操作测定回收率,根据建立的标准曲线方程可以计算相应的苏丹红Ⅰ号含量.
2 结果和讨论
2.1 BDD和Au/CS-BDD电极的电化学性质
图1为BDD电极和Au/CS-BDD电极在含5 mmol/L[Fe(CN)6]3-溶液(含0.1 mol/L KCl)中的循环伏安曲线.从图中可以看出,Au/CS-BDD电极的氧化峰电流为39.05μA,高于BDD电极的(24.85μA).△E p(氧化峰和还原峰电位差)从0.659 V降为0.347 V,表明Au/CS具有较高的电催化活性,可促进电极表面的电子转移,提高电极的导电性,加快反应速率.
2.2 苏丹红1号在不同电极上的电化学行为
图2为由苏丹红Ⅰ号在BDD和Au/CS-BDD电极上的差分脉冲伏安曲线.从图中可以看出,经Au/CS修饰后,苏丹红Ⅰ号的氧化峰电流由0.24μA增强到0.83μA,峰电位由0.809 m V负移到0.743 m V,说明Au/CS修饰后的电极对苏丹红Ⅰ号具有更高的电催化活性,能够促进电子的转移.

图1 BDD(a)和Au/CS-BDD(b)电极在5 mmol/L[Fe(CN)6]3-溶液(含有0.1 mol/L KCl)中的循环伏安曲线,扫描速率为50 m V/sFig.1 Cyclic voltammetry of 5 mmol/L[Fe(CN)6]3-(0.1 mol/L KCl)aqueous solution at BDD(a)and Au/CS-BDD(b)electrodes,scan rate:50 m V/S
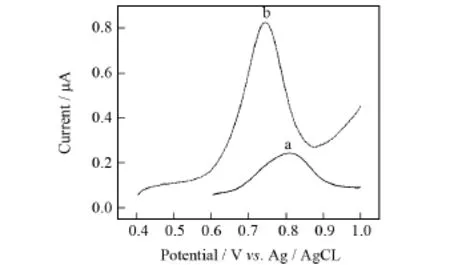
图2 苏丹红Ⅰ号标液(浓度5×10-4mol/L,p H=4.0)在BDD电极(a)和Au/CS-BDD电极(b)上的差分脉冲伏安图Fig.2 Differential pulse voltammetry of Sundan redⅠ(5×10-4mol/L,p H=4.0)at BDD(a) and Au/CS-BDD(b)electrodes
2.3 不同p H缓冲液对电极响应的影响
图3为不同p H磷酸盐(PBS)缓冲液对Au/CS-BDD电极响应的影响.从图中可以看出,随着p H的逐渐增大,峰电流的变化趋势是先增大然后减小,当p H为4.0时,峰电流最大,电极催化效率最高,故在本实验中缓冲液最佳p H取为4.0.
2.4 修饰量对电极响应的影响
图4为不同Au/CS纳米材料修饰量对峰电流的影响,在含有5×10-4mol/L苏丹红Ⅰ号的PBS缓冲液中(p H=4.0),随着修饰量的增加,电化学信号明显增强,氧化峰的峰电流也随之增加,在修饰量为5μL时峰电流达到最大.但随着修饰量的继续增大,电极表面的涂层厚度明显增加,电极响应会变得迟缓,信号反而不好,且背景电流太大,峰电流下降,故Au/CS纳米材料最佳修饰量为5μL.

图3 不同p H的PBS缓冲液和峰电流的关系Fig.3 Relationship between peak currents and p H value of PBS aqueous solution

图4 不同修饰量和峰电流的关系Fig.4 Relationship between peak currents and different modified quality
2.5 BDD电极和Au/CS-BDD电极上标准曲线的建立
利用微分脉冲伏安法分别在BDD电极和Au/CS-BDD电极上对不同浓度的苏丹红Ⅰ号PBS溶液进行定量测定,测试结果如图5和图6所示.从两图中均可以看出,氧化峰电流随着检测物浓度的增大而升高,对于BDD电极,在7~100μmol/L的浓度范围内峰电流与苏丹红Ⅰ号的浓度呈线性关系,线性方程为Ip=0.003 79c+0.016 76(R2=0.999),检出限为47.03μmol/L.Au/CS-BDD电极上峰电流与苏丹红Ⅰ的浓度的线性范围为4~100μmol/L,线性方程为Ip=0.011 26c+0.116(R2=0.999),检出限是8.33μmol/ L.

图5 BDD电极上苏丹红Ⅰ号的浓度与峰电流的关系Fig.5 Relationship between peak currents and Sudan redⅠconcentrations at BDD electrode
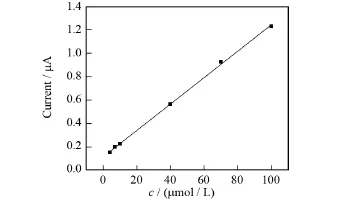
图6 Au/CS-BDD电极上苏丹红Ⅰ号浓度和峰电流的关系Fig.6 Relationship between peak currents and Sudan redⅠconcentrations at Au/CS-BDD electrode
2.6 BDD和Au/CS-BDD对实际样品中苏丹红I号的测定
表1为含有苏丹红Ⅰ号实际样品的检测结果.从表中可以看出,在相同的实验条件下,加标量为10 μmol/L时,BDD的平均回收率为65.65%,Au/CS-BDD的平均回收率为78.9%;加标量为100μmol/L时,测得BDD的平均回收率为90.24%,Au/CS-BDD的平均回收率为101.5%.说明Au/CS-BDD电极上的测定结果及平均回收率均优于BDD电极,更适合用于实际样品的检测,显示出较大的应用潜力.

表1 两种电极上样品中苏丹红Ⅰ号的测定结果和回收率Table1 Recovery ratios of methyl parathion in practical samples
3 结论
本文设计了Au/CS修饰BDD电极,并考察了苏丹红Ⅰ号在该修饰电极上的电化学行为.研究结果表明,与BDD电极相比,苏丹红Ⅰ号在Au/CS-BDD电极上的峰电流显著增加,峰电位负移,显示出经Au/CS修饰的电极对苏丹红Ⅰ号具有较好的催化作用.采用该方法测定番茄酱实际样品中苏丹红Ⅰ号含量,Au/ CS-BDD电极测定结果及平均回收率均优于BDD电极.该检测方法操作简单,灵敏高、检出限低,显示出较强的实用价值.
[1]STⅠBOROVA M,MARTⅠNEK V,RYDLOVA H,et al.SudanⅠis a potential carcinogen for humans:evidence for its metabolic activation and detoxication by human recombinant cytochrome P450 1A1 and liver microsomes[J].Cancer Res, 2002,62:5678-5684.
[2]ERTAS E,OZER H,ALASALVAR C.A rapid HPLC method for determination of sudanⅠdyes and para red in red chili pepper[J].Food Chem,2007,105:756-760.
[3]王明月,桂卫星,袁宏球.HPLC法测定咸蛋、皮蛋中四种苏丹红染料[J].食品科学,2009,30(2):193-195.
[4]SUN Hanwen,WANG Fengchi,AⅠLianfeng.Determination of banned 10 azo-dyes in hot chili products by gel permeation chromatography-liquid chromatography-electrospray ionization-tandem mass spectrometry[J].J Chromatogr A,2007, 1164:120-128.
[5]赵珊,张晶,丁晓静,等.凝胶净化/超高效液相色谱电喷雾质谱法检测调味油中11种禁用偶氮染料及罗丹明[J].分析测试学报,2012,31(4):448-452.
[6]DU Meiju,HAN Xiaogang,ZHOU Zihao,et al.Determination of SudanⅠin hot chili powder by using an activated glassy carbon electrode[J].Food Chem,2007,105:883-888.
[7]GAN Tian,LⅠKai,WU Kangbing.Multi-wall carbon nanotube-based electrochemical sensor for sensitive determination of SudanⅠ[J].Sensor Actuat B-Chem,2008,132:134-139.
[8]高愿军,张永峰,许光日.利用3-噻吩丙二酸修饰玻碳电极快速检测苏丹红[J].食品科学,2010,31(16):233-236.
[9]LⅠN Huogang,LⅠGang,WU Kangbing.Electrochemical determination of sudanⅠusing montmorillonite calcium modified carbon paste electrode[J].Food Chem,2008,107:531-536.
[10]NARANG J,CHAUHAN N,PUNDⅠR C S.A non-enzymatic sensor for hydrogen peroxide based on polyaniline,multiwalled carbon nanotubes and gold nanoparticles modified Au electrode[J].Analyst,2011,136:4460-4466.
[11]LⅠU Jian,QⅠAO Shizhang,LⅠU Hao,et al.Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres[J].Angew ChemⅠnt Ed,2011,50:5947-5951.
[12]LⅠYing,LⅠTingting,YAO Meng,et al.Metal-free nitrogen-doped hollow carbon spheres synthesized by thermal treatment of poly(o-phenylenediamine)for oxygen reduction reaction in direct methanol fuel cell applications[J].J Mater Chem,2012,22:10911-10917.
[13]YAN Zaoxue,MENG Hui,SHEN Kaipei,et al.Effect of the templates on the synthesis of hollow carbon materials as electrocatalyst supports for direct alcohol fuel cells[J].Ⅰnt J Hydrogen Energ,2012,37:4728-4736.
[14]SHEN Kaipei,YAN Zaoxue,MENG Hui,et al.Synthesis of Pd on porous hollow carbon spheres as an electrocatalyst for alcohol electrooxidation[J].RSC Adv,2011,1:191-198.
Electrochemical behavior of Sudan red I on nanoscale gold/carbon sphere modified boron-doped diamond electrode
LⅠU Yong*,WANG Yitao,LⅠXiying,CHEN Weiping
(College of Chemistry and Chemical Engineering,Henan University,Kaifeng 475004,Henan,China)
Au nanoparticles/carbon sphere(Au/CS)composite was adopted to modify borondoped diamond(BDD)electrode.The electrochemical behavior of Sudan redⅠon Au/CS-modified BDD electrode was investigated,and the method for testing Sudan redⅠin practical samples was established accordingly.Results indicate that,as compared with bare BDD electrode, the oxidation peak current of Sudan redⅠon Au/CS-modified BDD electrode increases from 0.24μA to 0.83μA,and the oxidation peak potential negatively shifts from 0.809 V to 0.743 V.Under the optimized condition,the peak current is proportional to Sudan redⅠconcentration in the range of 4-100μmol/L,the linear equation is Ip=0.011 26 c+0.116(R2=0.999),and the detection limit is 8.33μmol/L.The present method can be used to detect Sudan redⅠin practical samples with better detection results and average recovery than those of BDD electrode method.
Au nanoparticles/carbon spheres;BDD electrode;Sudan redⅠ;electrochemical behavior
O 657.1
A
1008-1011(2014)02-0124-04
2013-12-18.
刘勇(1979-),男,讲师,研究方向为催化反应工程.*
,E-mail:liuyong@henu.edu.cn.
