Seasonal Distribution of Bioaerosols in the Coastal Region of Qingdao
2014-05-02QIJianhuaSHAOQianXUWenbingGAODongmeiandJINChuan
QI Jianhua, SHAO Qian, XU Wenbing, GAO Dongmei, and JIN Chuan
Laboratory of Marine Environment and Ecology of MOE, Ocean University of China, Qingdao 266100, P. R. China
Seasonal Distribution of Bioaerosols in the Coastal Region of Qingdao
QI Jianhua*, SHAO Qian, XU Wenbing, GAO Dongmei, and JIN Chuan
Laboratory of Marine Environment and Ecology of MOE, Ocean University of China, Qingdao 266100, P. R. China
Bioaerosols were collected by using a six-stage bioaerosols sampler from September 2007 to August 2008 in the coastal region of Qingdao, China. The terrestrial and marine microbes (including bacteria and fungi) were analyzed in order to understand the distribution features of bioaerosols. The results show that the average monthly concentrations of terrestrial bacteria, marine bacteria, terrestrial fungi and marine fungi are in the ranges of 80–615 CFU m−3, 91–468 CFU m−3, 76–647 CFU m−3and 231–1959 CFU m−3, respectively. The concentrations of terrestrial bacteria, marine bacteria, terrestrial fungi, marine fungi and total microbes are the highest in each microbial category during fall, high in spring, and the lowest in the summer and winter. The bacterial particles are coarse in spring, autumn and winter. The sizes of fungal particle present the log-normal distribution in all the seasons.
bioaerosols; bacteria; fungi; seasonal distribution; size distribution
1 Introduction
Bioaerosols are airborne particles or large molecules that either carry living organisms or are released from living organisms (e.g., bacteria, fungi, virus, pollen, cell debris, and biofilms) (Ariya and Amyot, 2004; Grinshpun and Clark, 2005). Most bioaerosols originate from natural sources, such as soils, lakes, animals and humans (Li and Kendrick, 1994). They are considered to be related to air pollution and the spread of human, animal and plant disease epidemics (Bush and Portnoy, 2001; Renet al., 2001; Sheltonet al., 2002). Other researches show that bioaerosols can act as ice nuclei (IN) and cloud condensation nuclei (CCN) (Baueret al., 2003; Franc and Demott, 1998). Bioaerosols have an indirect effect global climate change and atmospheric processes (Qi and Gao, 2006). International bioaerosol research has developed rapidly over the last two decades and gained additional interest.
Many studies on the temporal and spatial distribution of bioaerosols in the environment have been carried out. The concentrations of airborne bacteria in Montreal of Canada (Kelly and Pady, 1954) and in Mexico (Kelly and Pady, 1954; Vlodavets and Mats, 1958) are found to be higher in summer than in spring and winter due to regional climatic conditions. Jones and Cookson (1983) reported that the highest concentration of bacteria occurred in late summer and the lowest in winter in the Washington, D.C., suburban area, due to stronger solar radiation, lower relative humidity and higher temperatures. Higher concentrations of bacteria were also found in summer compared to other seasons in Saudi Arabia Shoubra because of higher temperatures in summer (Lightart and Shaffer, 1995). Higher bacterial concentrations have been observed in summer and fall in Beijing, China (Fanget al., 2007). The size distribution of bacteria has also been studied in many regions. The majority of bacteria found is associated with particles that have an aerodynamic diameter greater than 3 μm. Of the viable bacterial aerosol particles that have been observed, approximately 30% to 50% of them fall into the category of largest particle (>7.0 µm aerodynamic diameter) in midsummer atmosphere at isolated locations in the high chaparral; in addition, the categories of all particle size show their minimum bacterial concentration at noon with the lowest concentrations in the smaller size categories (<2.1 µm aerodynamic diameter) (Lighthart and Shaffer, 1995).
The distribution nature of airborne fungi in the environment has also been studied. Jones and Cookson (1983) observed that the highest concentrations occur in summer and fall, with lower concentrations in the winter in suburban area of Washington. Sheltonet al.(2002) observed higher concentrations of fungi in fall and summer and lower concentrations in winter and spring in many regions of the USA; they found the highest fungal concentrations in the Southwest, Far West and Southeast of the country. The culturable fungal concentrations in Beijing are higher in summer and autumn and lower in spring and winter (Fanget al., 2005). Furthermore, the culturable fungi in Beijing show a log-normal distribution with thelargest proportion in stage 4 (2.0–3.5 µm) and the smallest in stage 6 (<1.0 µm).
The information presented above shows that bioaerosols have different temporal and spatial distribution patterns in different regions due to meteorological conditions, geographic locations, as well as different types of media and sampling methods used by the researchers (Adhikariet al., 2004). However, the global information of bioaerosols, especially for coastal bioaerosols, is very limited. Many researchers (Klassen and Roberge, 1999; Moorthyet al., 1998; Chowet al., 2000; Posfaiet al., 2003; Alleret al., 2005) found that marine aerosols containing microorganisms could be transported for hundreds of kilometers from their origins. Therefore bioaerosols have special compositions and distributions because they are affected by both terrestrial and marine sources in the coastal region. Griffinet al.(2007) found that the microorganism in bioaerosols are routinely impacted by the dust from North Africa in the coastal region of Erdemli (Turkey) and observed a statistically significant correlation between the presence of dust and the increase in culturable microorganism. Gruberet al.(1998) reported that the origin of the airmasses has an obvious influence on bioaerosol particles, and that the concentration of particle is higher when airmasses has a continental source rather than the North Sea source. Hoet al.(2005) found that most fungal categories peaked during the warmer months (from May to July) in Hualien, facing the Pacific Ocean, and that the associations between ambient fungi and environmental parameters are complex. The seasonal variations of concentration of total (cultural and non-cultural) microbes, cultural fungi and bacteria are different because each microbial category has its suitable environmental conditions in the coastal region of Qingdao (Liet al., 2011). Some studies (Chen, 2003; Liuet al., 2008; Xuet al., 2011) found that there are more marine microorganisms than terrestrial microorganisms in the Qingdao coastal region in fall. Currently, how the ocean affects the composition and concentration of bioaerosol is unknown. Detailed research is needed to determine the impact mechanism of ocean on bioaerosol.
In this study, the bioaerosol samples were continuously collected by using a six-stage bioaerosols sampler from September 2007 to August 2008. The terrestrial and marine microbes were analyzed to determine the impact of ocean on the composition and distribution of microbes. Besides, the statistical distribution characteristics of the terrestrial and marine microbes are discussed.
2 Materials and Methods
2.1 Sampling Sites
The sampling site is the coast of Qingdao, China (Fig.1). The sampler was mounted and secured 1.5 m above the roof of an office building, in the downtown campus of Ocean University of China (36˚6΄N, 120˚19΄E, height of the building about 7.0 m), about l.0 km from shore. There are many trees and greenbelts around the sampling site, accounting for 50% of the total area. Additionally, there are some residents living in the neighbourhood, but their vehicles have little influence. The meteorological parameters (temperature, relative humnidity, wind direction, wind velocity) were obtained from Qingdao Meteorological Administration.

Fig.1 Location of the sampling site.
2.2 Sampling Methods
A six-stage culturable microorganism FA-1 bioaerosols sampler (Applied Technical Institute of Liaoyang, China) was used to isolate culturable bacteria and fungi from the atmosphere. Each stage includes a plate with 400 holes of uniform diameter through which air is drawn at 28.3 L·min−1to collect samples on Petri dishes containing agar media. Airborne particles are separated into six fractions. The aerodynamic cut-size diameters of the six stages are listed in Table 1. Sampling was conducted from September 2007 to August 2008, except March 2008. The bacterial and fungal samples were collected for 8 min and 5 min, respectively, in the morning (8:00–9:00), at noon (12:00–13:00) and in the afternoon (16:00–17:00) at ten days intervals during the observational period. Sample collection was taken on the 5th, 15th and 25th of each month. The collection would be altered to an earlier or later date when it’s raining.

Table1 Characteristics of FA-I sampler
Bioaerosols samples were collected on 9.0 cm glass plates, in which 27 mL of culture medium was added after sterilization. Some organic compounds, such as peptone and beef extract, were used as carbon source for bacteria growth. However, glucose is used as carbon source for fungus. Therefore, bacteria and fungus would grow primarily on respective culture medium. The culture medium for terrestrial microorganism was prepared with distilled water, and the medium for marine microorganism was prepared with seawater. Then the terrestrial andmarine microorganisms were isolated due to the different osmotic pressures of the selected culture medium.
One group of bacteria were cultured by a beef extract peptone medium (3 g beef extract, 10 g peptone, 5 g NaCl, 16 g agar, 1 L distilled H2O, pH 7.2–7.6) in a constant temperature cultivator for 48 h at 37℃ (Shenet al., 1999). We denote this group of bacteria as terrestrial bacteria for discussion purposes. The marine bacteria were cultured in a 2216E solid medium (5 g peptone, 1 g yeast extract, 0.1 g FePO4, 15 g agar, 400 mL distilled H2O and 600 mL pure seawater) for 48 h at 28℃ (Zobell, 1946). One group of non-halotolerant fungi were cultured in Martin medium (10 g glucose, 5 g peptone, 1 g KDP (Potassium Dihydrogen Phosphate), 0.5 g MgSO4•7H2O, 15 g agar, 1 L distilled H2O, 3.3 mL Rose Bengal (1%), 3 mL streptomycin (1%)) for 72 h at 27℃ (Shenet al., 1999). We denote this group of fungi as terrestrial fungi for discussion purposes. And the marine fungi were cultured in a common marine fungi medium (10 g glucose, 2 g peptone, 1 g yeast extract, 15 g agar, 400 mL distilled H2O and 600 mL aging seawater) for 72 h at 25℃ (Lin and Zhou, 2003). We are referring to marine microorganisms that are halotolerant microorganisms cultured in salty agar plates prepared with seawater, as different from to microorganisms from the ocean. The colonies in the glass plate of each stage were counted after culture.
2.3 Calculation Methods
The bioaerosols microbe concentrations and microbial percentages at each stage were calculated using the following formulas.
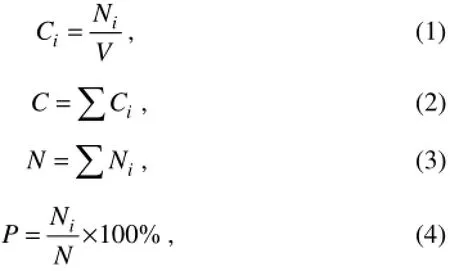
where,
Ci– Concentration of microorganisms in each stage of the sampler (CFU m−3);
Ni– The counted colonies on the glass plates from each stage of the sampler (CFU);
V– Volume of the collected air for the sample (m−3);
C– The sum of concentration of microorganisms in each stages of the sampler (CFU m−3);
N– The sum of colonies on the glass plates from each stages (CFU);
P– The ratio of colonies from each stage to sum of colonies (%).
2.4 Statistical Analyses
SPSS 19.0 (Trial Version) were applied to analyze the experimental data. ANOVA (Analysis of Variance) was conducted to validate the significance difference of bioaerosols concentration of different particle sizes or in different seasons, andPvalues of <0.01 were considered as significant difference. Pearson correlation analysis was used to correlate the bioaerosols concentrations with meteorological data.
3 Results and Discussion
3.1 Seasonal Distribution of Bioaerosols Microorganisms
3.1.1 Seasonal distribution of bioaerosols bacteria
Here bacterial concentrations in aerosol particles show the significant monthly variation in the coastal region of Qingdao (Fig.2). The monthly concentrations of terrestrial bacteria and marine bacteria are in the range of 80–615 CFU m−3and 91–468 CFU m−3, respectively. The average concentrations of terrestrial and marine bacteria are 258 CFU m−3and 217 CFU m−3. The monthly concentrations of terrestrial and marine bacteria show similar variations in trend, and the concentrations of terrestrial bacteria are significantly higher than that of marine bacteria (**P<0.01). From September 2007 to December 2007, the bacteria concentrations decrease gradually. Concentrations remain low in January and February but spike in April 2008. The bacteria concentrations decrease to the lowest value in May 2008 and increase somewhat during June to August 2008. The bacterial concentrations in aerosol particles show seasonal variation in the coastal region of Qingdao (Table 2). The average concentrations of terrestrial and marine bacteria are higher in fall (September to November) and spring (March to May) and lower in summer (June to August) and winter (December to February). The terrestrial and marine bacterial concentrations during fall are 436 CFU m−3and 327 CFU m−3, respectively, which is higher than during spring when concentrations are 347 CFU m−3and 280 CFU m−3for terrestrial and marine bacteria. The concentrations of terrestrial and marine bacteria in summer are 140 CFU m−3and 152 CFU m−3, respectively, which is close to the concentrations in winter.

Fig.2 The monthly variation of bacterial concentrations in the coastal region of Qingdao from September 2007 to August 2008.
This study shows that high concentrations occur in spring and fall, which is consistent with Li (1997) and Liet al.(2011). Ouyanget al.(2006) reported that both airborne bacteria and fungi reach maximum levels in spring in Guangzhou, China. Fanget al.(2007) found that high bacterial concentrations are observed in fall and summer in Beijing, China. Firstly, we speculate that seasonal variation of bacteria is mainly caused by climatic condition. In order to know the effect of the meteorological parameters on microbes, we analyzed the correlations between concentration and meteorological parameters (Table 3). We found that relative humidity (RH) is the most important meteorological factor for the terrestrial and marine bacteria, which have Pearson correlation coefficients of −0.24 (*P<0.01) and −0.26 (*P<0.01), respectively. Liet al.(2011) also found that the airborne bacteria in Qingdao would be more likely to propagate and grow at a relatively low RH. The release of microorganism from the object surface into atmosphere needs to overcome the adsorptive force. When RH rises, the adsorptive force will increase due to hydration between H2O and polar hydrophilic groups on the microorganism surface. It would be difficult for the adsorbed microorganism to be released into atmosphere (Maieret al., 2010). Therefore, the higher RH conditions will be adverse for aerosol release. Compared to winter and summer, the seasonal relative humidityRHaveis low in spring and fall, with a value of 57% and 58%. The seasonal average temperatureTaveof spring and fall is between 14℃ and 20℃. The meteorological conditions in spring and fall are favourable for microorganism to propagate and grow. In addition, we believe the wind velocity is not a key factor in concentration of airborne microbes in Qingdao, because we did not find a correlation of microbes with wind velocity.
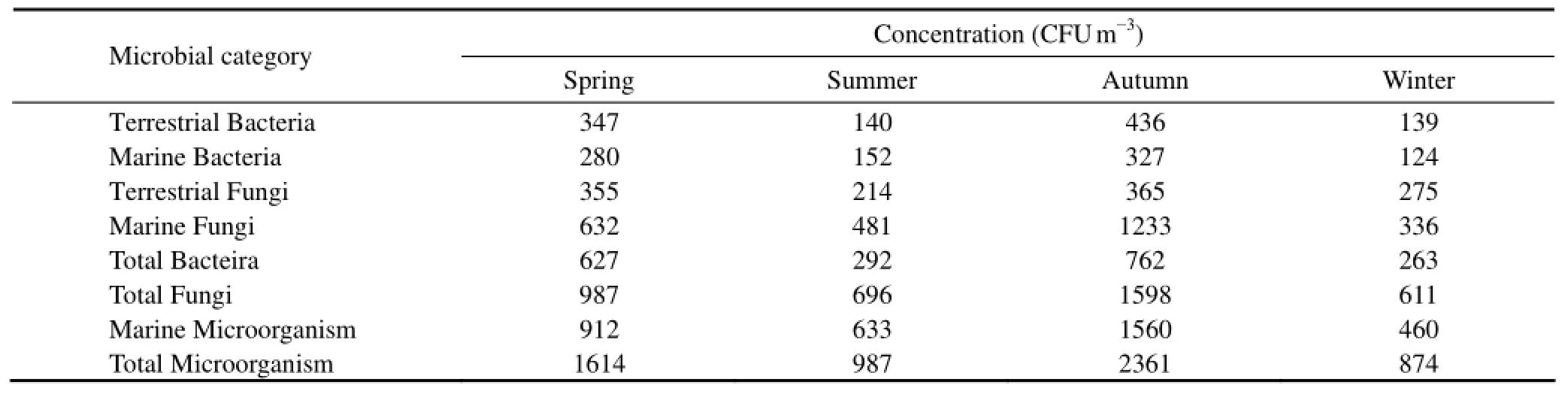
Table 2 Seasonal concentration (CFU m−3) of bioaerosols for each microbial category in samples from the Qingdao coastal region
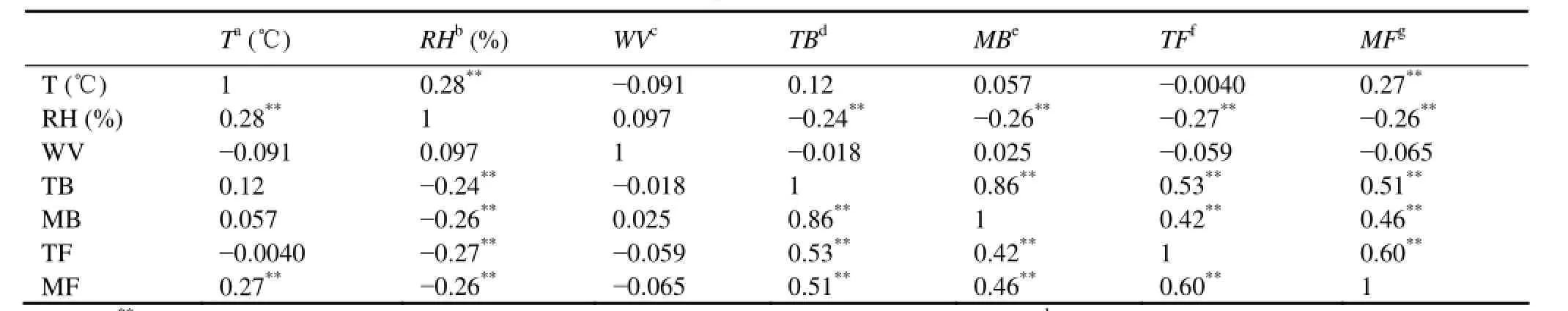
Table 3 Pearson correlation coefficients between concentrations of bioaerosols and meteorological factors during sampling (n=169)
Secondly, there are many fallen leaves and microbes released from the decomposition of leaves in fall. Therefore, high concentrations of bacteria are observed in fall. Thirdly, a dust storm occurred during sampling on 25 April 2008. Compared to the non-dust sample on 15 April 2008, the concentration of terrestrial and marine bacteria increased by 27 and 47 times due to this dust event. Many studies (Hoet al., 2005; Leeet al., 2009; Griffinet al., 2007) reported that Asian dust storms transport microorganisms in long distances. Liet al.(2011) found that the concentrations of culturable airborne microbes during the dust storms increase by 220%. Therefore, dust weather can increase the concentration of microbes in the bioaerosols in spring.
Other studies (Matthias-Maseret al., 2000; Vlodavets and Mats, 1958; Kelly and Pady, 1954) indicated that higher concentrations of bioaerosols bacteria are discovered in summer. The different distribution characteristics of bioaerosols in different areas are observed because sources and micro-environments of viable microorganisms are different (Bovalliuset al., 1978; Blomquist, 1994). There is stronger ultraviolet radiation and high relativein summer in Qingdao. As mentioned previously, a comparatively high RH would inhibit growth of airborne microbes (De Goffauet al., 2009); Liet al.(2011) also found low concentration of microbes correlated with high RH. Therefore, the lowest concentration of bacteria is observed in summer due to high RH and strong ultraviolet radiation. Most airborne microbes survive at a relatively high temperature and are inhibited at low temperature (Maieret al., 2010; Fanget al., 2004; Liet al., 2011). When air temperatures arevery low in wintercentration of airborne bacteria shows low values.
Besides, according to meteorological data of Qingdao, there were more rainy days and heavy rainfall in May 2008 than in the corresponding period of other years; the daily samples of May 15th, 2008 were collected just after the rain. Rain can clean out aerosol particles, on which bacteria mainly adhere to (Lighthartet al., 1993). And we found that the concentrations of terrestrial bacteria and marine bacteria decreased from 84 CFU m−3to 43 CFU m−3for terrestrial bacteria and from 177 CFU m−3to 38 CFU m−3for marine bacteria. As a result, the lowest concentration of bacteria was observed in May 2008.
In summary, the seasonal distribution characteristics of bacteria are determined by meteorological condition, such as temperature, RH, dust event and rain. Bioaerosols have different temporal and spatial distributions in different regions due to meteorological conditions, source and geographic locations.
The proportion of terrestrial bacteria to total bacteria was in the range of 42.0%–59.9%, with an average proportion of 52.5%. Marine microbes contribute slightly less to total bacteria than the terrestrial bacteria in the coastal region of Qingdao, which is confirmed by other studies (Chen, 2003; Liuet al., 2008).
3.1.2 Seasonal distribution of bioaerosols fungi
Fungi concentrations in aerosol particles also showed significant monthly variation in the coastal region of the Yellow Sea (Fig.3). The concentrations of marine fungi are higher than that of the terrestrial one and they showed a different monthly variation than terrestrial fungi (**P< 0.01). The monthly concentrations of terrestrial and marine fungi varied in the range of 76–647 CFU m−3and 231–1959 CFU m−3, respectively. Similar to bacteria, the concentration of fungi decreased after September 2007 and showed a higher value in April 2008. The lowest concentrations were found in June 2008 for terrestrial fungi and in February 2008 for marine fungi. Fungal concentrations in bioaerosol particles presented seasonal variations in the coastal region of Qingdao (Table 2). The average concentration of terrestrial and marine fungi was higher in fall and spring and lower in summer and winter.
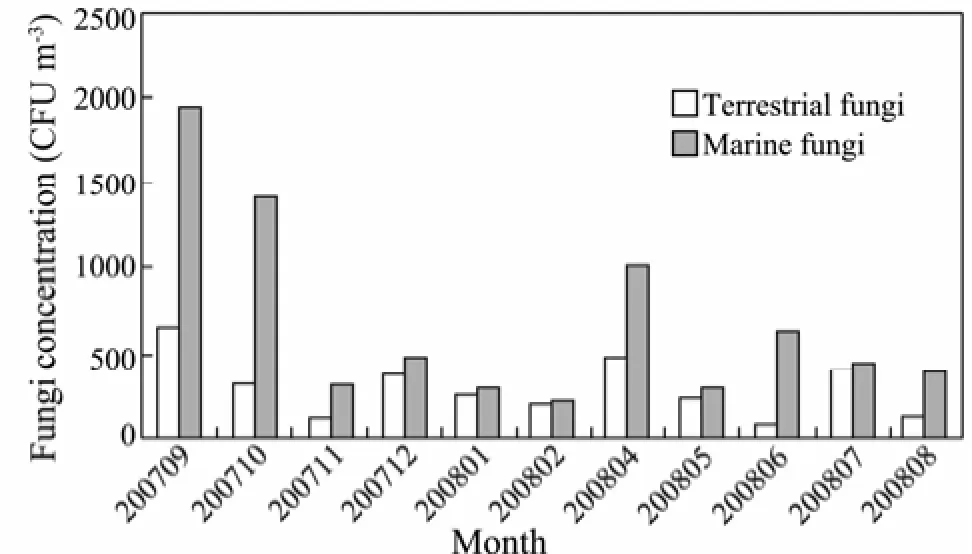
Fig.3 The monthly variation of fungal concentrations in the coastal region of Qingdao from September 2007 to August 2008.
In fall, the terrestrial and marine fungal concentrations were 365 CFU m−3and 1233 CFU m−3,respectively. In summer, the concentrations were 214 CFU m−3and 481 CFU m−3for terrestrial and marine fungi, respectively.
This study observed high concentrations in spring and fall, which is similar to Hu (2005) and Liet al.(2011). Adhikariet al.(2006) also reported that the concentration of total fungi is high in autumn and that the level of pollen is highest in spring in Ohio, USA. Table 3 shows that RH has a significant influence on terrestrial and marine fungi. The Pearson correlation coefficients was −0.27 (*P<0.01) and −0.26 (*P<0.01) for terrestrial and marine fungi. In general, airborne fungi concentration is higher in relatively low RH and vice versa. As mentioned previously, low RH in spring and fall is suitable for microorganism viability. In addition, temperature has a good correlation with the concentration of marine fungi. The fungi are abundant in a relatively high temperature and vice versa. Some studies (Maieret al., 2010; Fanget al., 2004) found that in a certain range of temperatures, airborne fungi could survive at a relatively high temperature and be inhibited at a low temperature. In this study, we found high concentration in fall (Tave=20℃) and low concentration in winter (Tave=−0.3℃) for marine fungi. In a coastal region, bioaerosol concentration is also affected by the ocean besides the influence of meteorological condition. Most terrestrial fungi originate from terrestrial natural sources, such as soils, lakes, animals and humans. They are prevailing under the south and southeast wind in summer (from ocean to land) and under the north and northwest wind in winter (from land to ocean). Accordingly, the terrestrial fungi present a lower value in summer and the marine fungi show a lower value in winter.
However, other studies (Jones and Cookson, 1983; Sheltonet al., 2002; Fanget al., 2005) indicate that the highest concentrations of bioaerosols fungi are seen in summer or fall. We think the distribution difference is mainly caused by the different climate conditions in different study areas. According to our study, the airborne fungi in Qingdao would be inhibited at high RH. The average of relative humidity is 82% in summer in Qingdao. There are frequent rains and strong ultraviolet radiation in summer. Therefore, it is the unfavorable season for culture microbe. As a result, the minimum concentrations of terrestrial and marine fungi are observed during the summer season. On the other hand, the dust event in spring has great effect on fungi concentration in Qingdao. Similar to bacteria, the dust storm occurring on 25 April 2008 increased the concentration of fungi greatly. Compared to the non-dust sample, the concentration of terrestrial and marine fungi increased by 5 and 19 times due to this dust event. Many studies (Griffinet al., 2007; Kellogget al., 2004; Limet al., 2011; Liet al., 2011) found that there is a correlation between dust and the increase in fungi. Therefore, high concentration of fungi was observed in spring in our study.
In short, the seasonal variation of fungi in coastal region is determined by the source and meteorological conditions, including temperature, RH, dust event and winddirection.
In our study, the proportion of terrestrial fungi to total fungi varied from 10.6% to 48.3%, with a monthly average proportion of 33.3%. A similar result was also found in studies by Chen (2003), Liuet al.(2008) and Liet al.(2011). We found that the concentrations of terrestrial and marine bacteria are also dependent on the wind direction. Some studies (Marks and Jankowska, 1997; Klassen and Roberge, 1999; Moorthyet al., 1998; Chowet al., 2000) suggested that marine aerosols are source of marine microorganisms entering into the atmosphere and could be transported downwind for hundreds of kilometers. Therefore, we think that the fungi concentration and proportion might be related with wind direction.
3.1.3 Seasonal distribution of bioaerosols microorganism
Total microbial concentrations in aerosol particles show clear monthly variations in the coastal region of Qingdao (Fig.4). The ranges of total bacteria, total fungi, total marine microbes and total microbes were 171–1083 CFU m−3, 426–2606 CFU m−3, 358–2421 CFU m−3and 705–3676 CFU m−3, respectively. The highest concentrations of total bacteria, total fungi, total marine microbes and total microbes appeared in September 2007, with values of 2606 CFU m−3, 1255 CFU m−3, 2421 CFU m−3and 3676 CFU m−3, respectively (**P<0.01). However, comparing with other urban areas, such as Taiwan, Beijing and Daegu (Huanget al., 2002; Fanget al., 2005; Jo and Seo, 2005), the microbial concentration is at least one order of magnitude lower in the coastal region of Qingdao, except that the total fungal concentration was higher in September 2007.

Fig.4 The monthly variation of total microbial concentrations in the coastal region of Qingdao from September 2007 to August 2008.
The total microbial concentrations in aerosol particles clearly show seasonal variation in the coastal region of the Yellow Sea (Table 2). The average concentrations of total bacteria, total fungi, total marine microbes and total microbes have similar distribution trends in each microbial category, in the order of: fall>spring> summer>winter. This is because the temperature is very low (T=−0.3℃) in winter, which is not the ideal environment for microbes growth.
In our study, the proportions of marine fungi to total marine microbes ranged from 69.3% to 79.1%, with an average of 74.3%. The fungi obviously contributed the largest portion to the total marine microbes in the coastal region of Qingdao. The proportion of total fungi to total microbes was in the range of 61.2%–70.4% with an average of 67.3%, and the proportions of total marine microbes to total microbes varied from 52.8%–66.1%, with an average of 59.9%. It was also found that total fungi contributed much more to the total microbes than total bacteria did and the total marine microbes contributed more to total microbes than any other species of microbes. From the results presented in this study, it is clear that the ocean has a great impact on bioaerosols composition because the ocean is a source of marine microbes.
3.2 Size Distribution of Bioaerosols Microorganisms
3.2.1 Size distribution of bioaerosols bacteria
The monthly distributions of particle size of terrestrial and marine bacteria are shown in Figs.5 and 6. The monthly bacterial particle size from September 2007 to August 2008 showed a skew distribution, which was also found by other studies (Fanget al., 2004; Liuet al., 2008). The highest proportion of bacteria was found in stage 1 (>7.0 µm), which was in the range of 27.0%–37.3% for the terrestrial bacteria and 26.9%–37.7% for the marine bacteria (**P<0.01), and the lowest proportion in stage 6 (0.65–1.1 µm), which was in the range of 1.1%–4.5% for the terrestrial bacteria and 0.6%–6.4% for the marine bacteria (**P<0.01). Similar results were also found by Lighthart and Shaffer (1995), with the exception for marine bacteria in June 2008. The terrestrial and marine bacteria mainly existed in coarse particles larger than 2.1 µm in this study, with an average proportion of coarse particle to total of 88.3% and 92.0% for terrestrial and marine bacteria, respectively.
The seasonal distributions of particle size of terrestrial and marine bacteria both show a skew distribution in spring, autumn and winter, but the marine bacteria particle size presents a peak in stage 4 (2.1–3.3 µm), accounting for 31.3% in summer. This study found that marine bacteria particles of diameters between 3.3–4.7 µm were in high concentration; and the proportion of 3.3–4.7 µm particles to the total increased from 23.7% in May to 28.8% in June. The studies on size distribution of aerosol in the coastal region of the Yellow Sea showed that the coarse particles contribute more to the total due to the increase of sea-salt aerosol in summer (Yuet al., 2007; Wang and Hu, 2001). Therefore, we think that the increase in sea-salt aerosol is probably due to the winds from the ocean in summer. For the marine bacteria, this study found that there were higher proportions in stage 1 (>7.0 µm) and stage 3 (3.3–4.7 µm), especially in spring and summer. Except for the terrestrial bacteria in summer, the highest proportions of bacteria were detected in the stage 1 (>7.0 µm), which were in the range of 27.3%–33.6% for the terrestrial bacteria and 26.1%–36.4% for the marine bacteria (**P<0.01); the lowest one was in thestage 6 (0.65–1.1 µm), which ranged 0–3.5% for the terrestrial bacteria and 1.1%–4% for the marine bacteria(**P<0.01). The terrestrial and marine bacteria mainly existed with coarse particles larger than 2.1 µm, and the average proportions of coarse particles to the total were 88.2% and 92.5% for terrestrial and marine bacteria, respectively. In the four seasons, the highest proportions of the terrestrial and marine bacteria were 91.9% and 93.1% in summer, respectively, and the lowest one was 85.0% in spring and 87.4% in winter.

Fig.5 The monthly distribution of terrestrial bacterial particle size in the coastal region of Qingdao from September 2007 to August 2008.

Fig.6 The monthly distribution of marine bacteria particle size in the coastal region of Qingdao from September 2007 to August 2008.
3.2.2 Size distribution of bioaerosols fungi
The monthly distributions of particle size of terrestrial and marine fungi are shown in Figs.7 and 8. The monthly fungi particle size from September 2007 to August 2008 showed a log-normal distribution for all the seasons, but also showed a skew distribution in December 2007, which accounted for 44.7% and 46.3% in stage 5 (1.1–2.1 µm) for terrestrial and marine fungi, respectively. The fungi concentrations in the size range 2.1–3.3 µm had the highest proportions, 27.1%–47.4% for the terrestrial fungi and 27.8%–51.3% for the marine fungi (**P<0.01). The fungi concentration in the size range 0.65–1.1 µm had the lowest contribution to the total, with proportions of 0–1.8% for the terrestrial fungi and 0–3.6% for the marine fungi (**P<0.01). Gliksonet al.(1995) found that the majority of aerosol particles adhering to fungi ranges from 2 µm to 10 µm in particle size in Queensland, Australia. Fanget al.(2005) reported that the highest fungal levels are found with the particle size ranging between 2.0–3.5 µm while the lowest level are found with particle size less than 1.0 µm in Beijing, China. But Meklinet al.(2002) found that the proportion of particles with the size 1.1–2.1 µm to the total is the highest for all sampling stages. This suggests that the fungal spores have different sources in different environments (Reponenet al., 1992), and that the size distributions are affected by meteorological factors, such as temperature and moisture (Meklinet al., 2002).
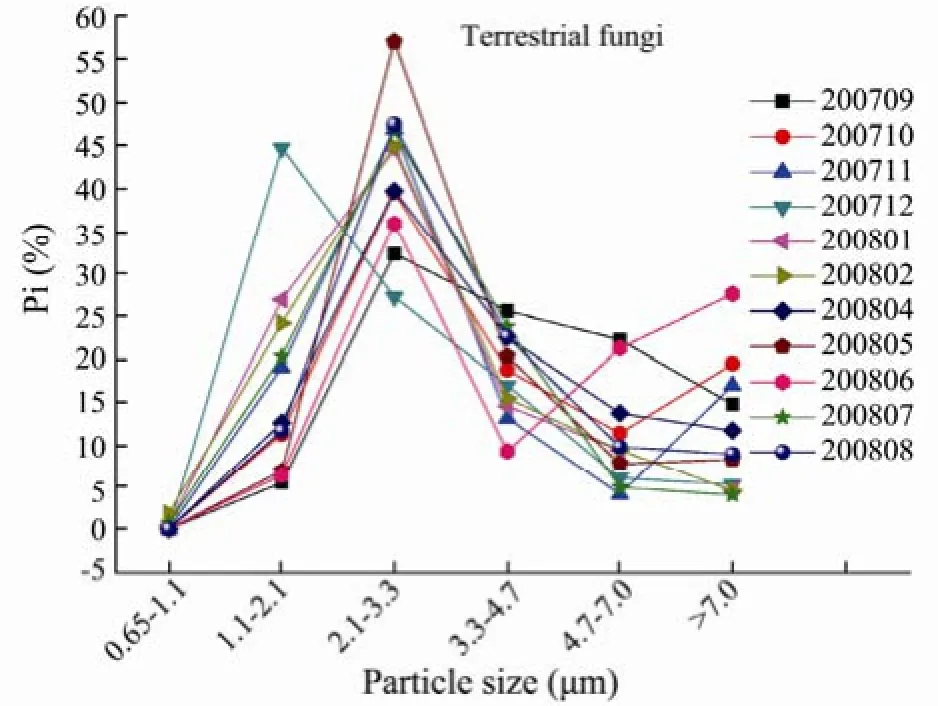
Fig.7 The monthly distribution of terrestrial fungal particle size in the coastal region of Qingdao from September 2007 to August 2008.
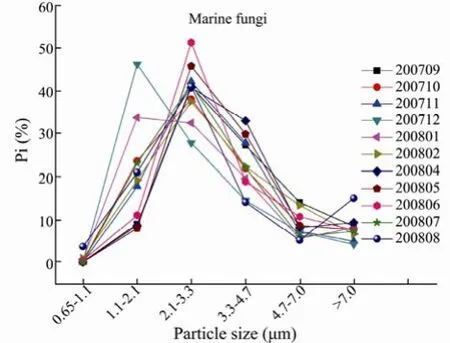
Fig.8 The monthly distribution of marine fungal particle size in the coastal region of Qingdao from September 2007 to August 2008.
In the coastal region of Qingdao, this study found that the terrestrial and marine fungi mainly exist in coarse particles larger than 2.1 µm for every month, with averageproportions 82.6% and 79.3%, respectively. These fungal particles show log-normal distributions, as also found by other studies (Fang et al., 2005; Meklin et al., 2002; Liu et al., 2008). In addition, the terrestrial and marine fungi particles were found primarily to exist in coarse particles larger than 2.1 µm (larger than 80%), which is similar to other studies (Lighthat and Shaffer, 1995; Fang et al., 2004; Reponen et al., 1992).
The seasonal distributions of particle size of terrestrial and marine fungi are log-normal. The fungal concentrations in 2.1–3.3 µm have the highest proportions 36.9%–47.5% for the terrestrial fungi and 35.4%–44.1% for the marine fungi (**P<0.01). The fungal concentrations in 0.65–1.1 µm have the lowest contributions to the total, with proportions of 0–0.8% for the terrestrial fungi and 0–1.6% for the marine fungi (**P<0.01). The terrestrial and marine fungi mainly exist in coarse particles larger than 2.1 µm, with average ratios of coarse particle to the total of 80.3% and 80.5% for terrestrial and marine fungi, respectively. For all the seasons, the terrestrial and marine fungi with coarse particles contribute the most in spring, with proportions of 89.9% and 91.9%, respectively, and contribute the least in winter with proportions of 65.2% and 68.2%, respectively.
The terrestrial and marine fungi mainly exist in coarse particles larger than 2.1 µm. The coarse particles account for 89.9%, 80.2%, 85.8%, 65.2% of the terrestrial fungi and 91.9%, 80.0%, 81.9%, 68.2% of the marine fungi in spring, summer, fall and winter, respectively, with the smallest proportions in winter.
4 Conclusions
In this study, observations of bioaerosols microbes in outdoor environments were conducted in the coastal region of Qingdao with the following results:
1) The average concentrations of all kinds of microbes are highest in fall, high in spring, and lower in summer and winter. Except for the terrestrial fungi, the lowest concentrations of the other microbes are in winter.
2) The size of bacteria particles is dominated by coarse particles in spring, autumn and winter, but sizes of the terrestrial bacteria particle present a log-normal distribution in summer. The size of fungal particle presents a lognormal distribution for all the seasons. The microbes mainly exist in coarse particles larger than 2.1 µm, with average ratios of coarse particles to the total larger than 80%.
3) Marine bacteria contribute slightly smaller proportions than those by other kind of bacteria, with an average proportion of 46.7%. Marine fungi contribute significantly more to total fungi than by terrestrial fungi, with an average proportion of 66.3%. Fungi clearly contribute more to the total marine microbes than by bacteria, with an average proportion of 74.3%. The total fungi contribute much more to the total microbes than by total bacteria, with an average proportion of 67.3%, and the total marine microbes contribute more to the total microbes than by any other microbes, with an average proportion of 59.9%. The ocean has a significant impact on bioaerosols formation.
Acknowledgements
This project was supported by NSFC under Grant No. 40705047 and Natural Science Foundation of Shandong Province (No. ZR2012DM003).
Adhikari, A., Sen, M. M., Gupta-Bhattacharya, S., and Chanda, S., 2004. Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: A 2-year study at five outdoor sampling stations. Science of the Total Environment, 326: 123-141.
Adhikari, A., Reponen, T., Grinshpun, S. A., Martuzevicius, D., and LeMasters, G., 2006. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: A two-year study. Environmental Pollution, 140: 16-28.
Aller, J. Y., Kuznetsova, M. R., Jahns, C. J., and Kemp, P. F., 2005. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. Aerosol Science, 36: 801-812.
Ariya, P. A., and Amyot, M., 2004. New directions: The role of bioaerosols in atmospheric chemistry and physics. Atmospheric Environment, 38: 1231-1232.
Bauer, H., Giebl, H., Hitzenberger, R., Kasper-Giebl, A., Reischl, G., Zibuschka, F., and Puxbaum, H., 2003. Airborne bacteria as cloud condensation nuclei. Journal of Geophysical Research, 108, 4658, DOI: 10.1029/2003JD003545.
Blomquist, G., 1994, Sampling of biological particles. Analyst, 119: 53-56.
Bovallius, A., Bucht, B., Roffey, R., and Anas, P., 1978. Three years investigation of the natural airborne bacterial flora at four localities in Sweden. Applied and Environmental Microbiol, 35: 847-852.
Bush, R. K., and Portnoy, J. M., 2001. The role and abatement of fungal allergens in allergic diseases. Journal of Allergy and Clinical Immunology, 107: 430-440.
Chen, H. W., 2003. Determination on condition of airborne microbes above Qingdao. Shandong Science, 16 (1): 9-13 (in Chinese).
Chow, J. C., Watson, J. G., Green, M. C., Lowenthal, D. H., and Bates, B., 2000. Cross-border transport and spatial variability of suspended particles in Mexicali and California’s Imperial Valley. Atmospheric Environment, 34: 1833-1843.
De Goffau, M. C., Yang, X., Van Dijl, J. M., and Harmsen, H. J. M., 2009. Bacterial pleomorphism and competition in a relative humidity gradient. Environmental Microbiology, 11: 809-822.
Fang, Z. G., Ouyang, Z. Y., Hu, L. F., Lin, X. Q., and Wang, X. K., 2004. Granularity distribution of airborne microbes in summer in Beijing. Environmental Science, 25 (6): 2-6 (in Chinese).
Fang, Z. G., Ouyang, Z. Y., Hu, L. F., Wang, X. K., Zheng, H., and Lin, X. Q., 2005. Culturable airborne fungi in outdoor environments in Beijing, China. Science of the Total Environment, 350: 47-58.
Fang, Z. G., Ouyang, Z. Y., Zheng, H., Wang, X. K., and Hu, L. F., 2007. Culturable airborne bacteria in outdoor environments in Beijing, China. Microbial Ecology, 54 (3): 487-496.
Franc, G. D., and Demott, P. J., 1998. Cloud activation characteristics of airborne Erwinia carotovora cells. Journal of Applied Meteorology, 37: 1293-1300.
Glikson, M., Rutherford, S., Simpson, R. W., Mitchell, C. A., and Yago, A., 1995. Microscopic and submicron componentsof atmospheric particulate matter during high asthma periods in Brisbane, Queensland, Australia. Atmospheric Environment, 29 (4): 549-562.
Griffin, D. W., Kubilay, N., Kocak, M., Gray, M. A., Borden, T. C., and Shinn, E. A., 2007. Airborne desert dust and aeromicrobiology over the Turkish Mediterranean coastline. Atmospheric Environment, 41: 4050-4062.
Grinshpun, S. A., and Clark, J. M., 2005. Measurement and characterization of bioaerosols. Journal of Aerosol Science, 36: 553-555.
Gruber, S., Matthias-Maser, S., Brinkmannz, J., and Jaenicke, R., 1998. Vertical distribution of biological aerosol particles above the north sea. Journal of Aerosol Science, 29 (SI): S771-772.
Ho, H. M., Rao, C. Y., Hsu, H. H., Chiu, Y. H., Liu, C. M., and Chao, H. J., 2005. Characteristics and determinants of ambient fungal spores in Hualien, Taiwan. Atmospheric Environment, 39: 5839-5850.
Huang, C. Y., Lee, C. C., Li, F. C., Ma, Y. P., and Su, H. J. J., 2002. The seasonal distribution of bioaerosols in municipal landfill sites: A 3-yr study. Atmospheric Environment, 36: 4385-4395.
Hu, L. F., 2005. Study of airborne fungal community composition and dynamics in Beijing urban ecosystem. Master thesis, Hunan Agricultural University, 4-6 (in Chinese).
Jones, B., and Cookson, J., 1983. Natural atmospheric microbiological condition in a typical suburban area. Applied and Environmental Microbiology, 45: 919-934.
Jo, W. K., and Seo, Y. J., 2005. Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools, and homes. Chemosphere, 61: 1570-1579.
Kellogg, C. A., Griffin, D. W., Garrison, V. H., and Peak, K. K., 2004. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia, 20: 99-110.
Kelly, C., and Pady, S., 1954. Microbiological studies of air masses over Montreal during 1950 and 1951. Canadian Journal of Botany, 32: 591-600.
Klassen, R. D., and Roberge, P. R., 1999. Aerosol transport modeling as an aid to understand atmospheric corrosivity patterns. Material & Design, 20: 159-168.
Lee, C., Choi, B., Yi, S. M., and Ko, G., 2009. Characterization of microbial community during Asian dust events in Korea. Science of the Total Environment, 407: 5308-5314.
Li, D. W., and Kendrick, E. B., 1994. Functional relationships between airborne fungal spores and environmental factors in Kitchener-Waterloo, Ontario, as detected by Canonical correspondence analysis. Grana, 33: 166-176.
Li, M. F., Qi, J. H., Zhang, H. D., Huang, S., Li, L., and Gao, D. M., 2011. Concentration and particle size distribution of bioaerosol in an outdoor environment in the Qingdao coastal region. Science of the Total Environment, 409 (19): 3812-9, DOI: 10.1016/j.scitotenv.2011.06.001.
Li, N. S., 1997. The particle distribution of aerial microbe in Hefei atmosphere. Biological Journal, 14: 36-38 (in Chinese).
Lighthart, B., Shaffer, B., Marthi, B., and Ganio, L., 1993. Artificial wind-gust liberation of microbial bioaerosols previously deposited on plants. Aerobiologia, 9: 189-196.
Lighthart, B., and Shaffer, B. T., 1995. Viable bacterial aerosol particle size distributions in the midsummer atmosphere at an isolated location in the high desert chaparral. Aerobiologia, 11: 19-25.
Lim, N., Munday, C. I., Allison, G. E., O’Loingsigh, T., De Deckker, P., and Tapper, N. J., 2011. Microbiological and meteorological analysis of two Australian dust storms in April 2009. Science of the Total Environment, 412-413: 223-
231.
Lin, Y. C., and Zhou, S. N., 2003. Marine Microorganisms and Its Metabolites. Chemical Industry Press, Beijing, 554pp.
Liu, M. M., Qi, J. H., Gao, D. M., Qiao, J. J., Shi, J. H., and Gao, H. W., 2008. Distribution characteristics of bioaerosol in Qingdao coastal region in Fall 2007. Ecology and Environment, 7 (2): 565-571 (in Chinese).
Maier, R. M., Pepper, L. L., and Gerba, C. P., 2010. Environmental Microbiology. 2nd editon, Science Press, Beijing, 91-92.
Marks, R., and Jankowska, K., 1997. Sea to air bacteria transfer over the Baltic Sea coast and Gulf of Gdansk. Aerosol Science, 28: 593-594.
Matthias-Maser, S., Reichert, K., and Jaenicke, R., 2000. Primary biological aerosol particles at the high alpine site of Jungfraujoch/Switzerland. Journal of Aerosol Science, 31 (Suppl 1): 955-956.
Meklin, T., Reponen, T., Toivola, M., Koponen, V., and Husman, T., 2002. Size distribution of airborne microbes in moisture-damaged and reference school building of two construction types. Atmospheric Environment, 36: 6031-6039.
Moorthy, K. K., Satheesh, S., Krishna, K., and Murthy, B. V., 1998. Characteristics of spectral depths and size distributions of aerosols over tropical oceanic regions. Journal of Atmospheric and Solar-Terrestrial Physics, 60: 981-992.
Ouyang, Y. S., Xie, X. B., Chen, Y. B., Huang, X. M., Peng, H., and Shi, Q. S., 2006. The concentration and variation of airborne microbe in Guangzhou City. Microbiology China, 33: 47-51.
Posfai, M., Li, J., and Anderson, J. R., 2003. Aerosol bacteria over the Southern Ocean during ACE-1. Atmospheric Research, 66: 231-240.
Qi, J. H., and Gao, H. W., 2006. Environment and climate effect of bioaerosol: A review. Ecology and Environment, 15 (4): 854-861 (in Chinese).
Ren, P. T., Jankun, T. M., Belanger, K., Bracken, M. B., and Leaderer, B. P., 2001. The relation between fungal propagules in indoor air and home characteristics. Allergy, 56: 419-424.
Reponen, T., Lehtonen, M., and Raunemaa, R., 1992. Effect of indoor sources on fungal spore concentration and size distribution. Journal of Aerosol Science, 123: 663-666.
Shen, P., Fan, X. R., and Li, G. W., 1999. Microbiology Experiment. 3rd edition, Higher Education Press, Beijing, 234pp.
Shelton, B., Kirkland, K. H., Flanders, W. D., and Morris, G. K., 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Applied and Environmental Microbiology, 68: 1743-1753.
Vlodavets, V., and Mats, L., 1958. The influence of meteorological factors on the microflora of the atmospheric air in Moscow. Journal of Microbiology, 59: 539-544.
Wang, M., and Hu, M., 2001. Major inorganic compositions in fine and coarse particles of ambient aerosol at Qingdao. Chinese Journal of Environmental Science, 22 (5): 35-37 (in Chinese).
Xu, W. B., Qi, J. H., Jin, C., Gao, D. M., Li, M. F., Li, L., Huang, S., and Zhang, H. D., 2011. Concentration distribution of bioaerosol in summer and autumn in the Qingdao coastal region. Environmental Science, 32: 9-17.
Yu, L. M., Qi, J. H., Sun, N. N., Shi, J. H., Gao, H. W., and Guo, X. Y., 2007. Study on inorganic nitrogen faelos in the Qingdao area and Verthe Yellow Sea and the South China Sea. Acta Scientiae Circumstantiae, 27 (2): 319-325 (in Chinese).
Zobell, C. E., 1946. Marine Microbiology. Chronica Botanica Co., Waltham, Mass, 240pp.
(Edited by Xie Jun)
* Corresponding author. Tel: 0086-532-66782356
E-mail: qjianhua@ouc.edu.cn
(Received March 7, 2012; revised April 18, 2012; accepted May 8, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2014
杂志排行
Journal of Ocean University of China的其它文章
- Diagnosis of Physical and Biological Controls on Phytoplankton Distribution in the Sargasso Sea
- QSAR for Photodegradation Activity of Polycyclic Aromatic Hydrocarbons in Aqueous Systems
- Surface Heat Budget and Solar Radiation Allocation at a Melt Pond During Summer in the Central Arctic Ocean
- A Model Study on Dynamical Processes of Phytoplankton in Laizhou Bay
- Properties of Klebsiella Phage P13 and Associated Exopolysaccharide Depolymerase
- Study on Deformation Law of Circular Foundation Under Combined Loading
