Structures and Antiviral Activities of Butyrolactone Derivatives Isolated from Aspergillus terreus MXH-23
2014-04-26MAXinhuaZHUTianjiaoGUQianqunXIRuiWANGWeiandLIDehai
MA Xinhua, ZHU Tianjiao, GU Qianqun, XI Rui, WANG Wei,, and LI Dehai,
1) Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
2) Zhangqiu Maternity and Child Health Care Hospital, Jinan 250200, P. R. China
Structures and Antiviral Activities of Butyrolactone Derivatives Isolated from Aspergillus terreus MXH-23
MA Xinhua1), ZHU Tianjiao1), GU Qianqun1), XI Rui2), WANG Wei1),*, and LI Dehai1),*
1) Key Laboratory of Marine Drugs of Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China
2) Zhangqiu Maternity and Child Health Care Hospital, Jinan 250200, P. R. China
A new butyrolactone derivative, namely butyrolactone VIII (1), and six known butyrolactones (2-7) were separated from the ethyl acetate (EtOAc) extract of the fermentation broth of a fungus, Aspergillus terreus MXH-23. The chemical structures of these metabolites were identified by analyzing their nuclear magnetic resonance (NMR) and mass spectrometry (MS). Known butyrolactone derivatives contain an α,β-unsaturated γ-lactone ring with α-hydroxyl and γ-benzyl, and butyrolactone VIII (1) was the first butyrolactones contains α-benzyl and γ-hydroxyl on α,β-unsaturated lactone ring. All of the butyrolactone derivatives were tested for their anti-influenza (H1N1) effects. Derivatives 4 and 7 showed moderate antiviral activities while the newly-identified, derivative 1, did not.
sponge-derived fungus; secondary metabolite; butyrolactone; anti-influenza activity
1 Introduction
In sponges, the ancient metazoans, many natural products identified showed diverse bioactivities such as antivirus, antitumor, antimicrobe and cytotoxicity (Thomas et al., 2010). Studies showed that their symbiotic organisms (or associated microorganisms) might be the primary producers of these natural products (Thakur and Müller, 2004). In recent years, sponge-derived microorganisms have evolved as a competitive source of structurally novel and biologically potential compounds (Thomas et al., 2010; Thakur and Müller, 2004). Sponge-derived fungi, such as those in genera Aspergillus, Penicillium and Fusarium have been reported to produce a variety of secondary metabolites which were diverse chemically. The reported compounds included polyketides (Varoglu and Crews, 2000), meroterpenoids (Cueto et al., 2006), peptides (Takagi et al., 2010) and alkaloids (Ookura et al., 2008). In our continuous studies on sponge-derived fungi (Xin et al., 2007; Peng et al., 2012; Zhang et al., 2011), we found that the fermented broth of a sponge-derived fungus, Aspergillus terreus MXH-23, collected from Naozhou Sea, Guangdong Province, China, exhibited intriguing UV profiles. The chemical composition analysis of the ethyl acetate (EtOAc) extract of A. terreus fermented broth allowed us to identify a new butyrolactone, namely butyrolactone VIII (1), together with six known analogues, butyrolactones I-III (2-4) (Kiriyama et al., 1977; Nitta et al., 1983; Rao et al., 2000), 3-hydroxy-5-[[4-hydroxy-3-(3-methyl-2-buten-1-yl) phenyl]methyl]-4-(4-hydroxyphenyl)-2(5H)-furanone (5) (Morishima et al., 1994), aspernolide A (6) (Rarvatkar et al., 2009), and 5-[(3,4-dihydro-2,2-dimethyl-2H-1-benzopyran-6-yl)-methyl]-3-hydroxy-4-(4-hydroxyphenyl)-2(5H)-furanone (7) (Kiriyama et al., 1977) (Fig.1). Here we report the structures and activities of these metabolites against influenza (H1N1) virus.

Fig.1 Structures of compounds 1–7.
2 Materials and Methods
2.1 Equipments
In order to decipher the structures of derivatives isolated, NICOLET NEXUS 470 spectrophotometer in KBr discs (IR spectrum), Beckman DU®640 spectrophotometer (UV spectrum), JASCO J-810 circular dichroism spectrometer (CD Spectrum), JEOL Eclipse-600 spectrometer (1H-NMR,13C-NMR, DEPT and 2D-NMR spectra, TMS as internal standard, δ in ppm, J in Hz), Q-TOF ULTIMA GLOBAL GAA076 LC mass spectrometer (ESI-MS) and Semi-preparative HPLC (ODS column, YMC-Pack ODS-A, 10 × 250 mm, 5 μm, 4 mL min-1) were used.
2.2 Fungal Isolation and Culture
Aspergillus terreus MXH-23 was isolated from a sponge (unidentified) collected from Naozhou Sea, Guangdong Province, China, and identified according to its ITS sequence with the voucher specimen conserved at -20℃. The working strain was prepared on potato dextrose agar slants and stored at 4℃. A. terreus MXH-23 was fermented under a static condition at 28℃ in 1000 mL Erlenmeyer flasks containing 300 mL medium (glucose 20 g, peptone 5 g, yeast extract 3 g, and malt extract 3 g, dissolved in 1 L sea water, pH 7.2–7.4).
2.3 Extraction and Isolation
After 30 days of fermentation, 30 L of whole broth was filtered through cheesecloth to separate the broth supernatant and mycelia. The former was extracted with ethyl acetate while the latter was extracted with acetone. The acetone extract was evaporated under a reduced pressure to afford an aqueous solution, and then extracted with ethyl acetate. The two ethyl acetate extracts were combined and concentrated in vacuo to give a crude extract (8.0 g).
The crude extract was loaded onto a silica gel (300–400 mesh) column and separated into six fractions (Fr.1–Fr.6) using a step gradient elution of petroleum ether/acetone. Fr.3, eluted with 9:1 petroleum ether/acetone, was fractionated on a C-18 ODS column using a step gradient elution of MeOH/H2O and separated into seven subfractions (Subfr.3.1–Subfr.3.7). Subfr.3.2 was applied on a semi-preparative HPLC (80:20 MeOH:H2O, 4 mL min-1) to afford compound3(5.0 mg, tR= 15.0 min), compound4(16.0 mg, tR= 17.0 min), and compound5(6.0 mg, tR= 22.0 min). Subfr.3.3 was separated by Sephadex LH-20 using CH2Cl2:MeOH (50:50) and further purified by semi-preparative HPLC (60:40 MeOH:H2O, 4 mL min-1) to give compound2(25 mg, tR= 9.8 min) and compound6(13.0 mg, tR= 14 min). Fr.4 eluted with 2:1 petroleum ether:acetone was fractionated on a C-18 ODS column using a step gradient elution of MeOH/H2O and separated into seven subfractions (Subfr.4.1–Subfr.4.7). The Subfr.4.5 was separated by Sephadex LH-20 using MeOH and further purified by semi-preparative HPLC (60:40 MeOH:H2O, 4 mL min-1) to afford compound1(15.0 mg, tR= 17.5 min) and compound7(2.0 mg, tR= 21.0 min).
2.4 Physico-Chemical Properties
Butyrolactone VIII (1): pale yellow oil (MeOH); [a]D25-11.2˚ (c = 0.1, MeOH); CD (MeOH): 204 (+0.034), 209 (+0.078); UV (MeOH) λmax(logε): 210 (4.45), 307 (4.27);1H-NMR (DMSO-d6, 600 MHz) and13C-NMR (DMSO-d6, 150 MHz), see Table 1;1H-NMR (CD3OD, 600 MHz) and13C-NMR (CD3OD, 150 MHz), see Table 1; HR-ESIMS m/z 447.1413 [M + Na]+(calc. C24H24O7Na: 447.1420).
Butyrolactone I (2): pale yellow oil (MeOH); [a]D25+81.6˚ (c = 0.1, MeOH); CD (MeOH): 204 (-0.151); UV (MeOH) λmax(logε): 225 (4.26), 308 (4.39);1H-NMR (DMSO-d6, 600 MHz) and13C-NMR (DMSO-d6, 150 MHz); ESI-MS m/z 447.1 [M + Na]+.

Table 1 NMR data for compound1(at 600 and 150 MHz, TMS, δ in ppm)
2.5 Antiviral Activity Assay
Antiviral activities of compounds1–7were evaluated by the CPE and MTT methods using influenza (H1N1) virus in MDCK cells. Ribavirin was employed as a positive control (Grassauer et al., 2008, Hung et al., 2009).
3 Results and Discussion
3.1 Structure Determination
Compound1was obtained as light yellow oil. Its mo-lecular formula was determined as C24H24O7on the basis of its high resolution-electrospray ionization-mass spectral (HR-ESI-MS) peak at m/z 447.1413 [M + Na]+ion (calcd. for C24H24O7Na: 447.1420), indicating 13 degrees of unsaturation. Its1H-NMR spectrum (Table 1) showed the presence of a 1,2,4-trisubstituted benzene ring [δH6.86 (1H, d, 2.2), 6.79 (1H, dd, 8.2, 2.2), 6.69 (1H, d, 8.2)], a para-disubstituted phenyl group [δH7.43 (2H, d, 8.8), 6.81 (2H, d, 8.8)], an olefinic triplet [δH5.21 (1H, t, 7.14, 7.68)], two methylene groups [δH3.15 (2H, d, 7.1), 3.72 (1H, s), 3.16 (1H, s)], a methoxy group [δH3.69 (3H, s)] and two methyl groups [δH1.65 (3H, s), 1.61 (3H, s)]. Its13C-NMR spectrum further confirmed the presence of two substituted phenyls. Its13C-NMR spectrum along with1H-NMR spectrum (Table 1) disclosed a prenyl fragment and a fragment of the carboxyl methyl ester. Its13C-NMR spectrum revealed two olefinic carbon atoms [δC155.7 (s), 125.5 (s)], an ester carboxyl [δC172.2 (s)] and an oxygenated quaternary carbon atom [δC102.1 (s)]. HMBC correlations from H-6 to C-2, C-3, and C-4 indicated the existence of α, β-unsaturated lactone ring which was similar to ianthellidones G (Zhang et al., 2012) (Fig.2). HMBC correlations from H-6 to C-8 and C-12, from H-8 to C-6, and from H-12 to C-6 indicated the connection of C-6 to C-7, while correlation from H-2′ to C-4 indicated the connection of C-1′ to C-4 (Fig.2). The constitution of1was proved as 5-hydroxy-3-[4-hydroxy-3-(3-methylbut-2-en-1-yl)benzyl]-4-(4-hydroxyphenyl)-5-methoxycarbox yl-2-furanone, named butyrolactone VIII (Fig.2).

Fig.21H-1H COSY and key HMBC correlations of compound1.
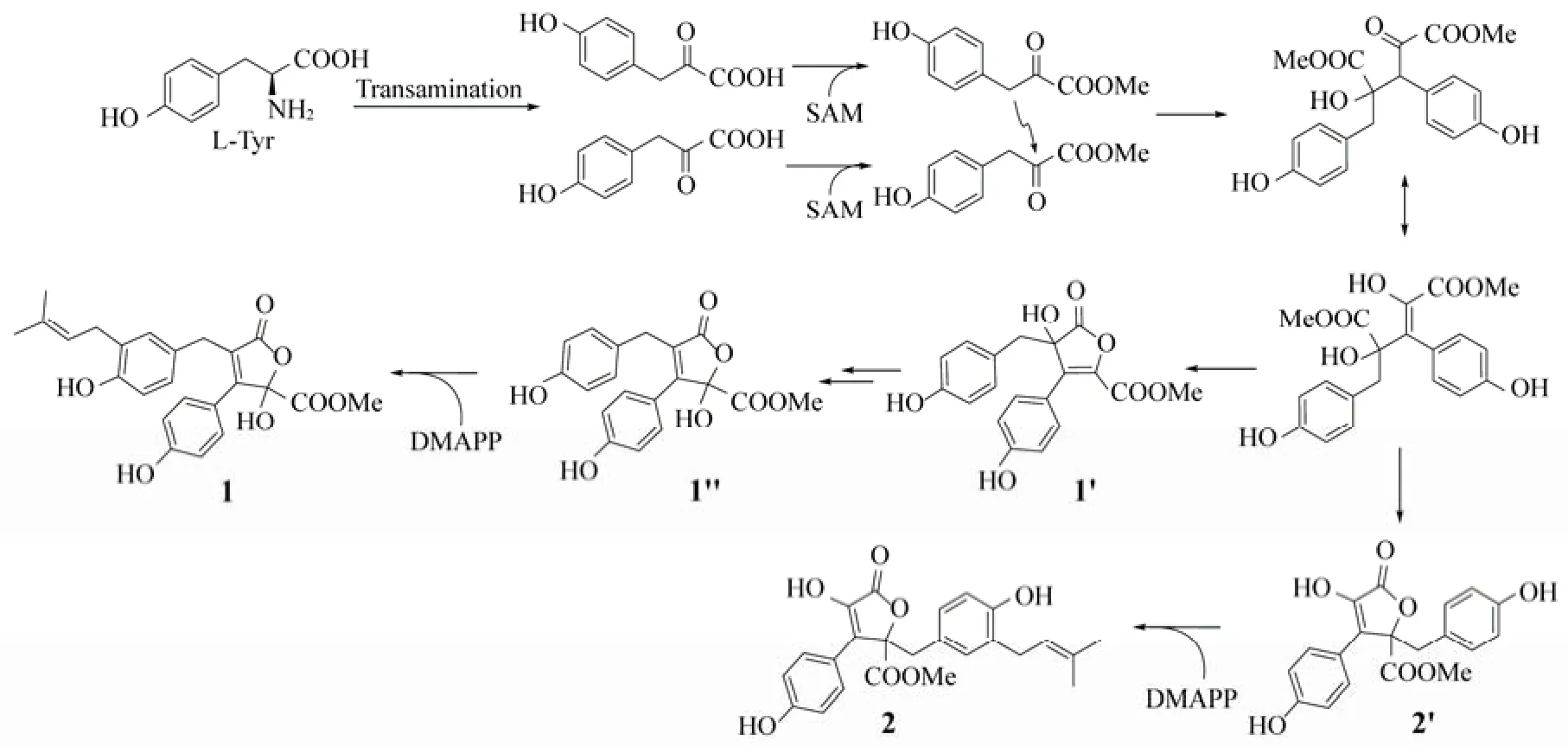
Fig.3 Proposed biosynthetic pathway of compounds1and2.
3.2 Bioactivity
The first butyrolactone I was isolated from A. terreus var. Africans IFO 8355 in 1977 (Kiriyama et al., 1977), which contained one α,β-unsaturated γ-lactone ring with α-hydroxyl and γ-benzyl. It was biosynthesized by prenylation after the condensation of two HPPMe (p-hydroxyphenylpyuvic methyl ester) from phenylalanine in fungus (Nitta et al., 1983). Butyrolactone VIII (1) was the first butyrolactone with α-benzyl and γ-hydroxyl on the unsaturated lactone ring isolated from A. terreus MXH-23 fermented broth. Its biosynthetic pathway was proposed in Fig.3. It might be synthesized from the same precursor as that of butyrolactone I with similar condensation mechanism.
Analogues of butyrolactone I were reported to display several biological activities, such as cytotoxicity (Rao et al., 2000; Rarvatkar et al., 2009; Li et al., 2005; Niu et al.,2008; Haritakun et al., 2010), soybean lipoxygenase inhibitor and DPPH radical scavenger (Yasumasa et al., 2010), and cyclin-dependent kinases (CDKs) inhibitor (Niu et al., 2008; Kitagawa, et al., 1994; Braña et al., 2004). Based on our bioassay results, butyrolactone derivatives 1-7 exhibited very weak or undetectable cytotoxicity (IC50>50 μmol L-1) to P388, K562, A-549 and Hela cell lines. In addition, the antiviral activities of these metabolites were evaluated for the first time with CPE and MTT methods against influenza virus (Table 2). Compounds 4 and 7 were the two most active derivatives while the newly-identified compound 1 was inactive.

Table 2 Inhibition rate of compounds1-7against influenza (H1N1) virus
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 41176120 and 30973627), the National High Technology Research and Development Program of China (No. 2013AA092901), the Program for New Century Excellent Talents in University (No. NCET-12-0499), Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (BS 2010HZ027), the Public Projects of State Oceanic Administration (No. 2010418022-3), and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT0944).
Braña, M. F., García, M. L., López, B., Pascual-Teresa, B., Ramos, A., Pozuelo, J. M., and Domínguez, M. T., 2004. Synthesis and biological evaluation of analogues of butyrolactone I and molecular model of its interaction with CDK2. Organic and Biomolecular Chemistry, 2: 1864-1871.
Cueto, M., MacMillan, J. B., Jensen, P. R., and Fenical, W., 2006. Tropolactones A-D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry, 67: 1826-1831.
Grassauer, A., Weinmuellner, R., Meier, C., Pretsch, A., Prieschl-Grassauer, E., and Unger, H., 2008. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virology Journal, 5: 107.
Haritakun, R., Rachtawee, P., Chanthaket, R., Boonyuen, N., and Isaka, M., 2010. Butyrolactones from the fungus Aspergillus terreus BCC 4651. Chemical and Pharmaceutical Bulletin, 58 (11): 1545-1548.
Hung, H. C., Tseng, C. P., Yang, J. M., Ju, Y. W., Tseng, S. N., Chen, Y. F., Chao, Y. S., Hsieh, H. P., Shih, S. R., and Hsu, J. T., 2009. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antiviral Reseach, 81: 123-131.
Kiriyama, N., Nitta, K., Sakaguchi, Y., Tagushi, Y., and Yamamoto, Y., 1977. Studies on the metabolic products of Aspergillus terreus. Metabolites of the strain IFO 8835 (1). Chemical and Pharmaceutical Bulletin, 25: 2593-2601.
Kitagawa, M., Higashi, H., Takahashi, I., Okabe, T., Ogino, H., Taya, Y., Hishimura, S., and Okuyama, A., 1994. A cyclindependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression. Oncogene, 9: 2549-2557.
Li, G. Y., Li, B. G., Yang, T., Yin, J. H., Qi, H. Y., Liu, G. Y., and Zhang, G. L., 2005, Sesterterpenoids, terretonins A-D, and an alkaloid, asterrelenin, from Aspergillus terreus. The Journal of Natural Products, 68: 1243-1246.
Morishima, H., Fujita, K., Nakano, M., Atsumi, S., Ookubo, M., Kitagawa, M., Matsumoto, H., Okuyama, A., and Okabe, T., 1994. Preparation, antitumor activity, and formulations of dihydrofuran compounds. Japanese Kokai Tokkyo Koho JP 06100445.
Nitta, K., Fujita, N., Yoshimura, T., Arai, K., and Yamamoto, U., 1983. Metabolic products of Aspergillus terreus. IX. Biosynthesis of butyrolactone derivatives isolated from strains IFO 8835 and 4100. Chemical and Pharmaceutical Bulletin, 31: 1528-1533.
Niu, X., Dahse, H., Menzel, K., Lozach, O., Walther, G., Meijer, L., Grabley, S., and Sattler, I., 2008. Butyrolactone I derivatives from Aspergillus terreus carrying an unusual sulfate moiety. The Journal of Natural Products, 71: 689-692.
Ookura, R., Kito, K., Ooi, T., Namikoshi, M., and Kusumi, T., 2008. Structure revision of circumdatins A and B, benzodiazepine alkaloids produced by marine fungus Aspergillus ostianus, by X-ray crystallography. The Journal of Organic Chemistry, 73: 4245-4247.
Parvatkar, R. R., D’Souza, C., Tripathi, A., and Naik, C. G., 2009. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry, 70 (1): 128-132.
Peng, J. X., Jiao, J. Y., Li, J., Wang, W., Gu, Q. Q., Zhu, T. J., and Li, D. H., 2012. Pyronepolyene C-glucosides with NF-κB inhibitory and anti-influenza Aviral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorganic & Medicinal Chemistry Letters, 22: 3188-3190.
Rao, K. V., Sadhhukhan, A. K., Veerender, M., Ravikumar, V., Mohan, E. V. S., Dhanvantri, S. D., Sitaramkumar, M., Moses Babu, J., Vyas, K., and Om Reddy, G., 2000. Butyrolactones from Aspergillus terreus. Chemical and Pharmaceutical Bulletin, 48: 559-562.
Takagi, M., Motohashi, K., and Shin-ya, K., 2010. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, from the sponge-derived Aspergillus sp. fS14. The Journal of Antibiotics, 63: 393-395.
Thakur, N. L., and Müller, W. E. G., 2004. Biotechnological potential of marine sponges. Currret Science, 86: 1506-1512.
Thomas, T. R. A., Kavlekar, D. P., and LokaBharathi, P. A., 2010. Marine drugs from sponge-microbe association–a review. Marine Drugs, 8: 1417-1468.
Varoglu, M., and Crews, P., 2000. Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. The Journal of Natural Products, 63: 41-43.
Xin, Z. H., Fang, Y. C, Du, L., Zhu, T. J., Duan, L., Chen, J., Gu, Q. Q., and Zhu, W. M., 2007. Aurantiomides A-C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. The Journal of Natural Products, 70: 853-855.
Yasumasa, S., Kayo, Y., Naoki, A., and Akira, H., 2010. Soybean lipoxygenase inhibitory and DPPH radical-scavenging activities of aspernolide A and butyrolactones I and II. Bioscience Biotechnology and Biochemistry, 74 (4): 881-883.
Zhang, G. J., Sun, S. W., Zhu, T. J., Lin, Z. J., Gu, J. Y., Li, D. H., and Gu, Q. Q., 2011. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry, 72: 1436-1442.
Zhang, H., Conte, M. M., Huang, X. C., Khalil, Z., and Capon, R. J., 2012. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge, Ianthella sp. Organic Biomolecular Chemistry, 10: 2656-2663.
(Edited by Qiu Yantao)
(Received March 11, 2013; revised May 14, 2013; accepted September 17, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding authors. E-mail: wwwakin@ouc.edu.cn dehaili@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- A Microsatellite Genetic Linkage Map of Black Rockfish (Sebastes schlegeli)
- Ultrastructure Developments During Spermiogenesis in Polydora ciliata (Annelida: Spionidae), a Parasite of Mollusca
- Effects of Pseudoalteromonas sp. BC228 on Digestive Enzyme Activity and Immune Response of Juvenile Sea Cucumber (Apostichopus japonicus)
- Feasibility of Partial Replacement of Fishmeal with Proteins from Different Sources in Diets of Korean Rockfish (Sebastes schlegeli)
- Effect of Spatial and Temporal Scales on Habitat Suitability Modeling: A Case Study of Ommastrephes bartramii in the Northwest Pacific Ocean
- Profiling and Comparison of Color Body Wall Transcriptome of Normal Juvenile Sea Cucumber (Apostichopus japonicus) and Those Produced by Crossing Albino
