Fuel properties and combustion characteristics of some promising bamboo species in India
2014-04-20RiteshKumarChandrashekar
Ritesh Kumar • N. Chandrashekar
Introduction
Rapid industrialization, growing population and changing life styles have increased the demand for energy over recent decades. Global energy consumption in 2011 was 550 exajoules of which around 90% was met by fossil fuels (IEA 2012). This demand is expected to grow an additional 50 percent by 2030, mostly due to increasing demand for energy in the developing countries like China, India and Brazil. The increasing demand for energy, depleting fossil fuel reserves and associated environmental concerns has necessitated production of heat and energy from alternative renewable resources.
Biomass, in its various forms, has been recognized as useful and cost effective alternative renewable energy source. It can be easily converted into high energy content fuels through thermo-chemical and bio-chemical conversion processes (Peter 2002a). Thermo-chemically, biomass can be converted into fuel by combustion, pyrolysis, gasification and liquefaction processes. Bio-chemical methods of converting biomass into fuel include digestion (biogas production) and fermentation (ethanol production). Consequently, biomass is drawing global interest as a renewable feedstock for energy production. Biomass fuels are preferred for reducing CO2emissions and reducing dependency on fossil fuel (Sims 2003; Villeneuve et al. 2012). Presently, biomass is contributing around 14% of total world’s energy supply, second only to coal, oil, and natural gas (Asif and Muneer 2007). Many industrialized nations have already included biomass under competitive energy dynamics. Malaysia has implemented a five fuel policy plan where 5% of the total energy requirement is presently supplied from renewable sources, increasing to 35% by 2030 (Johari et al. 2012). UK plans to generate 10% of national electricity (60 GW) per year from renewable sources, of which biomass will form a significant part (Demirbas et al. 2009).
Several types of biomass are used as energy feedstocks (Sims et al. 2006). Among them, bamboo is known as potential raw material for future bioenergy programs (Chen 2012; Scurlock et al. 2000). Bamboo forests cover approximately 22 ×106ha worldwide of which 13.96 ×106ha are in India (FSI 2011). The total growing stock of bamboo in India is around 180 ×106tons, including bamboo grown in forested and non-forested areas. The estimated annual harvest of the bamboo in the country is around 13.5 ×109tons (FSI 2011). Bamboo produces more biomass per hectare than wood and has potential to be used as substitute for wood in many ways (Singh 2008). Bamboo provides an alternative to wood for energy production, thus reduces pressure on existing forests. Its fast growth, short rotation period and adaptability in varied soil and climatic conditions assure its long-term benefits as sustainable energy feedstock (Kleinhenz and Midmore 2001). Utilization of bamboo for power production is achieving success in many countries (INBAR 2012). Bamboo produces excellent charcoal for domestic purpose as well as fuel for gasifiers (Saikia et al. 2007). In India, many northeastern states like Assam, Manipur and Mizoram are establishing bamboo based power plants for electricity production.
The choice of energy conversion process is significantly affected by the type and quality of biomass feedstock (Peter 2002b). The nature of the biomass resource must meet the technical requirements of energy conversion devices. Therefore, knowledge of physical, chemical and elemental nature of the feedstock is vital. It is equally important to evaluate the thermal behavior of feedstocks in oxidizing atmosphere. Such studies help in effective utilization of raw material for energy production through thermochemical or biochemical conversion processes. In this paper we describe fuel properties (calorific value, ash, volatile and fixed carbon content) and combustion characteristics of five bamboo species, viz. Dendrocalamus strictus, D. brandisii, D. stocksii, Bambusa bambos and B. balcooa. We quantify the physical, chemical and elemental properties of these bamboo species. Burning profiles of the samples were obtained by applying the derivative thermogravimetric technique. Our results have implications for utilizing these bamboo species for energy production.
Materials and methods
Mature culms (3-5 years) of D. strictus and D. brandisii, were obtained from the College of Forestry, Ponampet, Karnataka while the culms of D. stocksii, B.bambos and B. balcooa were obtained from Shimoga Forest Division of Karnataka, India. At least three culms of each species were harvested for experiments. The green bamboo culms were initially converted into thin strips and oven-dried at 102 °C. The oven dried strips were pulverized to fine powder using a Wiley mill. The pulverized material was then placed in a sieve shaker, to pass through 425 μm mesh sieve but retained on 250 μm mesh sieve. The powder thus obtained was again oven dried and used for further analysis. The fuel properties of bamboo were compared with the wood of 20 year old Eucalyptus Hybrid (Eucalyptus tereticornis × Eucalyptus camaldulensis) tree. The values of fuel properties analysis i.e., elemental analysis, proximate analysis, basic density and calorific value of E. hybrid were used from our earlier work (Kumar et al. 2010).
Estimation of moisture content, green density and basic density
The freshly cut water-saturated samples were used for assessment of moisture content, green density and basic density. Sample blocks for study were obtained from the middle portion of the internodes. We recorded initial green (wet) weight (Wg) and final oven-dry weights (Wo) of samples. Moisture content (MC) was determined using Eq. 1 (Chauhan and Walker 2004).

The blocks were measured for volume in green condition using the mercury displacement method (Chauhan and Walker 2004). These blocks were then oven dried at (103±2)°C until they achieved constant mass. Basic density (BD) was determined using Eq. 2.

where, WODis the oven-dry biomass and Vgis the green volume of biomass. For green density the ratio of green weight/green volume was used (Chauhan and Walker 2004).
Estimation of lignin and holocellulose content
The powdered bamboo sample was subjected to soxhlet extraction for 8 hours using a mixture of ethanol-benzene, 1:2 (v/v) (TAPPI, T204 om-88) (Bodirlau et al. 2008). The samples obtained after solvent extraction were further rinsed under hot water to remove water soluble extractives. The extractive-free sample thus obtained was oven dried to constant weight at 80°C and used for chemical analysis. The determination of lignin content was carried out by digesting extractive-free bamboo samples with 72% sulfuric acid for 2 hours (TAPPI, T222 om-88) (Dence 1992). The holocellulose content was determined using a standard method (Timell et al. 1959).
Calorific value (MJ·kg-1) and fuel value index
The powdered bamboo sample was pelleted, oven dried to constant weight at 80 °C and burned in an oxygen bomb calorimeter (LECO AC-350) to estimate the calorific value (CV). Fuel value index (FVI) was calculated as described by Jain (1993).

where, CVis the calorific value (kJ·g-1), ACis ash content (g·g-1), MCis moisture content (g·g-1) and GDis the green density (g·cm-3) of biomass.
Proximate analysis and elemental analysis
Ash and volatile matter were determined according to ASTM D5142, using a proximate analyzer (LECO TGA-701). The elemental parameters (carbon, hydrogen, nitrogen, sulfur) were determined using a CHN analyzer (LECO- CHN-2000). Percent oxygen was calculated by subtracting the sum of percent of C, H, N, S and ash from 100%. The fixed carbon content (FCC) of the sample was estimated using the following equation:

where, ACis ash content and VCis the volatile content of biomass. In order to overcome experimental and instrumental errors, experiments were repeated four times and average values were calculated.
Ash elemental analysis
The elemental composition of ash was determined using Energy Dispersive X-ray Analysis (EDAX) attached to a scanning electron microscope (SEM). The ash samples were finely ground, oven dried and used as pellets for analysis. The results were obtained as elemental oxides. The average of values determined at five different areas of the samples are reported.
Thermogravimetric analysis (TGA)
The combustion characteristic of all five bamboo species and E. hybrid was studied under air atmosphere (21% oxygen and 79% nitrogen). Thermo-gravimetric analysis (TGA) was carried out using TGA Q500 V20.2. A known quantity of powdered sample (10 mg) was placed in a platinum crucible and heated from ambient temperature to 800 °C at a heating rate of 10 °C per min. The air flow rate (60 mL·min-1) was kept uniform during the experiment. General guidelines of ASTM D 3850 were followed. The burning profiles of the samples were derived by applying the derivative thermogravimetry technique.
Results and discussion
Fuel properties
Physical and chemical properties
The basic density of selected bamboo species varied from 0.48 to 0.78 g·cm-3(Table 1). Green density also varied from 0.9 to 1.1 g·cm-3. Highest basic density was recorded for D. stocksii (0.78 g·cm-3), which was even higher then the basic density of 20 year old E. hybrid tree (0.73 g·cm-3). The basic density of D. strictus (0.48 g·cm-3) was lowest of five bamboo species. Biomass having higher density is preferred as fuel due to its high energy content per unit volume.
The chemical composition of bamboo is similar to that of wood. Cellulose, hemicellulose and lignin form most of bamboo biomass, while minor constituents are resins, tannins, waxes and inorganic salts (Fujii et al. 1993). There was marginal variation in holocellulose and lignin content (Table 1). The lignin content (25%) was highest in D. stocksii and lowest in D. brandisii (22%).
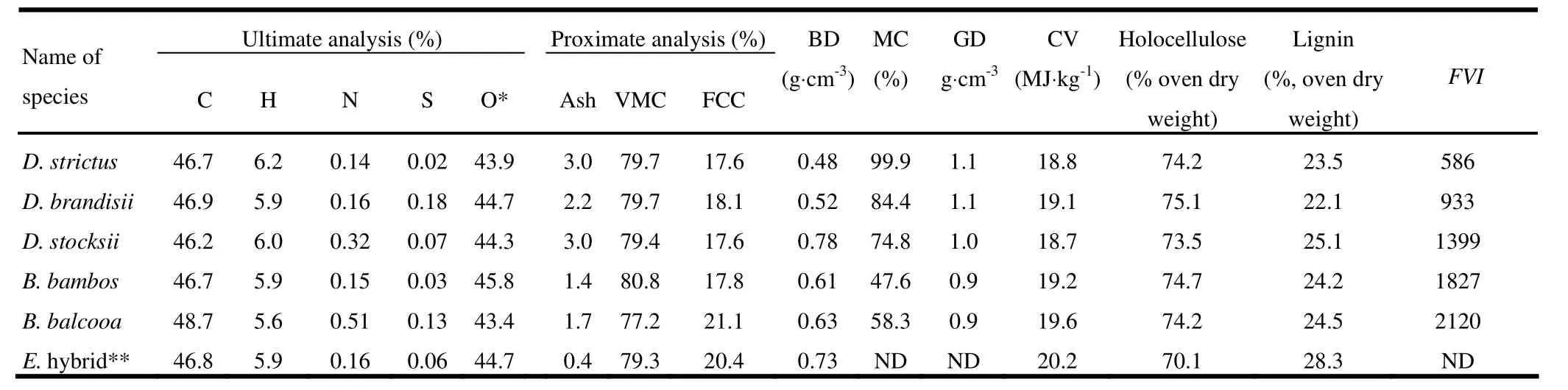
Table 1: Fuelwood characteristics of selected bamboo species
Calorific value and fuel value index
The calorific values of D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa were 18.8, 19.1, 18.7, 19.2 and 19.6 MJ·kg-1, respectively (Table 1). Higher calorific value of B. balcooa (19.6 MJ·kg-1) may be due its high fixed carbon content (21.1 %). Low fixed carbon (17.6 %) and high ash percentage (3.0%) in D. strictus may be the reason for its lower calorific value of 18.8 MJ·kg-1. Compared to bamboo species, the calorific value of E. hybrid (20.2 MJ·kg-1) was marginally higher, possibly due to high lignin content (28%), high fixed carbon content (20%) and very low ash (0.4%) in wood as compared to bamboo (Table 1). The high lignin content contributes to the high heating value of biomass. The holocellulose (cellulose and hemicellulose) is reported to have high heating value of 18.6 MJ·kg-1, whereas, lignin has heating value of 23.3 to 25.6 MJ·kg-1(Demirbas 2001). Fuel value index (FVI) is an important characteristic for screening desirable fuelwood species (Moya and Tenorio 2013). FVI varied widely in bamboo species (Table 1). B. balcooa recorded the highest FVI (2120), followed by B. bambos (1827), D. stocksii (1399), D. brandisii (933) and D. strictus (586). High calorific value and low ash content in B. balcooa and B. bambos explain the high FVI of these species. D. strictus had the lowest FVI (586), possibly due to high ash and moisture content, and comparatively low density.
Proximate analysis and ultimate analysis
The volatile matter contents of D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa were 79.7%, 79.7%, 79.4%, 80.8% and 77.2%, respectively (Table 1). The fixed carbon in bamboo species ranged from 17.6% to 21.1%. The fixed carbon content in B. balcooa was fairly higher (21.1%) and even higher than wood biomass (Table 1). This may be due to presence of little volatile matter (77.2%) and ash content (1.7%) in B. balcooa. The fixed carbon content of E. hybrid wood sample was 20.2%. With higher fixed carbon content and relatively lower ash content, B. balcooa is better suited for thermochemical energy conversions processes.
Proximate analysis results showed wide variation in ash content of bamboo species (Table 1). The ash contents of D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa were 3.0%, 2.2%, 3.0%, 1.4% and 1.7%, respectively (Table 1). Lower ash content (0.4%) was observed in E. hybrid wood (Table 1). The higher ash content in bamboo biomass can be attributed to presence of higher silica content in bamboo as compared to wood (Table 2). High ash content negatively affects the heating value of material.

Table 2: Composition of ash forming minerals in D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E .hybrid (%)
The main chemical elements in biomass (apart from associated mineral matter) are C, O, H, N and S. The results of elemental analysis showed little variation in the elemental composition of bamboo species (Table 1). In a biomass fuel, a higher proportion carbon-carbon bonds is more desirable, whereas, higher proportion of carbon-oxygen and carbon-hydrogen bonds reduces energy value (Peter 2002b). The H/C and O/C ratios obtained from elemental analysis of bamboos were calculated and compared with E. hybrid wood. B. balcooa had an H/C ratio of around 0.11. The remaining bamboo species had similar H/C ratios in the range of 0.13. The O/C ratios recorded for D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E. hybrid were 0.94, 0.95, 0.96, 0.98, 0.89 and 0.95, respectively. The lower percentage of nitrogen and sulfur is important from the environmental point of view.
Ash forming elements in different bamboo species
The principal ash-forming constituents in bamboo are silica (SiO2) and potassium (K2O). It also contains chlorine (Cl), calcium (CaO), magnesium (MgO), etc. The bamboo species in general contained higher concentrations of silica than did wood biomass (Table 2). Among the bamboo species, silica content was lowest in D. brandisii (8.7%) and highest in D. stocksii (49.0%). Higher amounts of chlorine were found in D. brandisii (6.7%) and D. strictus (5.7%). B. balcooa had low chlorine content (0.1%), whereas chlorine concentrations in D. stocksii (1.3 %) and B. bambos (1.4%) were comparable with that in E. hybrid (1.4 %). The higher percentage of chlorine is not desirable in fuel ash as it facilitates salt deposition and also causes corrosive effects on metal surfaces of boilers and furnaces. Potassium (K2O) is the dominant source of alkali in both bamboo and wood biomass fuels. The potassium concentration in bamboo species ranged from 21% to 70%. Highest concentration of potassium was found in D. brandisii and lowest in B. balcooa. E. hybrid contained more calcium (37.5%) than bamboo. During combustion, calcium and magnesium usually help in increasing the melting point of the ash, while the potassium and sodium are responsible for decreasing the ash melting point (Ohman and Nordin 2000). Biomass containing more calcium is preferred to biomass having more alkali metal.
The adverse effects of the alkali and chlorine present in bamboo feedstock can be mitigated. Fouling can be reduced by extracting the inorganic constituents of the biomass feedstock by leaching. Large amount of alkali metals and chlorine can be extracted from the biomass by leaching with water (Ohman and Nordin 2000; Davidsson et al. 2002). The use of mineral additives, such as kaolin, dolomite, calcite, bauxite, emalthite, gibbsite, mullite and oxides of calcium, magnesium, aluminum and iron has also been found effective in alkali sorption or for obstructing reactions with troublesome elements which lead to eutectic mixtures (Davidsson et al. 2002).
Combustion characteristics of bamboo feedstock
The burning profiles of the biomass from D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E. hybrid are given in Table 3 and Fig. 1.
Three major steps of decomposition were observed for all biomass samples under oxidative atmosphere. The initial weight loss between temperatures 40-80 °C was mainly due to the removal of moisture (Fig. 1). The extent of weight loss in this zone was found to be 2.8%, 3.6%, 4.1%, 3.2% and 3.4% for D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa, respectively. When the samples were exposed to higher temperatures (around 180-370 °C), sudden weight loss was observed, mainly due to release of volatiles and their combustion, as described by Munir et al. (2009) and Haykiri-Acma (2003). This zone is called the active pyrolysis zone. Most of the weight loss occurred in this degradation zone; i.e. 68.5%, 66.7%, 68.4%, 66.7% and 68.7% of the total weight loss for D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa, respectively. The third major weight loss occurred at temperatures of 390-490°C, mainly due to combustion of char (Table 3). This zone is called the passive pyrolysis zone. The extent of weight loss in these two combustion steps differed by species. The difference in the profile can be attributed to differences in the physical and chemical properties of biomass. The volatile constituents are CO, CO2, H2, H2O, tar and light hydrocarbons (Kumar et al. 1992). The weight loss in the active pyrolysis zone can be attributed to the evolution of the volatile compounds generated during decomposition of hemicellulose and cellulose. The weight loss in the passive pyrolysis zone is mainly due to lignin conversion (Garcia-Ibanez et al. 2006).

Table 3: Characteristics of thermogravimetric experiment under oxidizing (air) conditions

Fig. 1: Burning profiles of five selected bamboo species
The maximum combustion rates of 0.5833, 0.5215, 0.4962, 0.6067, 0.6708 and 0.3435 mg·min-1were found at peak temperatures of 293, 282, 304, 299, 316 and 335°C for D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E. hybrid, respectively (Table 3). The higher peak temperature may be due to higher volatile matter present in biomass. The presence of more volatile matter in bamboo biomass promotes its suitability for liquid fuel production via pyrolysis (Chutia et al. 2013).
The combustion of char starts at higher temperature, after the removal of initial volatiles and their ignition (Haykiri-Acma 2003). The char combustion zone temperature for all bamboo species ranged from 360 to 480oC (Fig. 1). The maximum combustion rates in the passive zone i.e., 0.2091, 0.5457, 0.3324, 0.2580, 0.2054 and 0.1500 mg·min-1were recorded for D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E. hybrid at peak temperatures of 395, 433, 430, 423, 441 and 471, respectively. The highest char combustion temperature range of 400-500oC was recorded for E. hybrid (Table 3). The difference in the burning profile of the char combustion zone may be due to differences in chemical composition of char produced during the process and also on the mutual interaction of the individual components (Munir et al. 2009). The weight loss percentage for D. strictus, D. brandisii, D. stocksii, B. bambos, B. balcooa and E. hybrid at 800 °C was 97.8%, 97.9%, 97.6%, 98.5%, 97.8% and 98.0%, respectively.
B. balcooa stands out as suitable feedstock compared to other bamboo species based on its high basic density (0.63 g·cm-3), low ash content (1.7%), high fixed carbon content (21.1%) and high calorific value (19.6 MJ·kg-1). Low concentrations of potassium (20.6%) and chlorine (0.1%) in B. balcooa ash give added advantage to this species.
Conclusion
The five bamboo species, viz. D. strictus, D. brandisii, D. stocksii, B. bambos and B. balcooa differed in physical and chemical properties. The calorific value of bamboo was comparable to that of E. hybrid (20.2 MJ·kg-1). B. balcooa, B. bambos and D. stocksii were the species with greatest energetic potential; mainly due to high FVI, calorific value, density and low ash content. The ash content of E. hybrid was lower (0.4%) than bamboo (1.4%-3.0%). Bamboo ash had fairly high percentages of silica and potassium, whereas, a high percentage of calcium was found in E. hybrid ash. The combustion characteristic of investigated biomass shows differences in their burning profile. Ignition temperature in active pyrolysis zone changed marginally, although ash, volatile and fixed carbon content varied considerably among bamboo species.
Asif M, Muneer T. 2007. Energy supply, its demand and security issues for developed and emerging economies. Renewable and Sustainable Energy Review, 11(7): 1388-1413.
Bodirlau R, Teaca CA, Spiridon I. 2008. Chemical modification of beech wood: effect on thermal stability. BioResources, 3(3): 789-800.
Chauhan SS, Walker JCF. 2004. Relationships between longitudinal growth strain and some wood properties in Eucalyptus nitens. Australian Forestry, 67(4):254.
Chen E. 2012. “Growmore Biotech plants up power with bamboo in India”. Available at: http://www.greenprospectsasia.com/ .
Chutia RS, Kataki R, Bhaskar T. 2013. Thermogravimetric and decomposition kinetic studies of Mesua ferrea L. deoiled cake. Bioresource Technology, 139: 66-72.
Davidsson KO, Korsgren JG, Pettersson JBC, Jaglid U. 2002. The effects of fuel washing techniques on alkali release from biomass. Fuel, 81: 137-142.
Demirbas A. 2001. Relationships between lignin contents and heating values of biomass. Energy Conversion and Management, 42: 183-188.
Demirbas MF, Balat M, Balat H. 2009. Potential contribution of biomass to the sustainable energy development. Energy Conversion and Management, 50(7): 1746-1760.
Dence CW. 1992. The determination of lignin. In: S.Y Lin and C.W Dence (eds), Methods of lignin chemistry. Springer-Verlag Berlin Heidelberg, pp. 33-61.
FSI. 2011. India State of Forest Report. Dehradun: Forest Survey of India, p. 57.
Fujii Y, Azuma J, Marchessault RH, Morin FG, Aibara S, Okamura K. 1993. Chemical composition change of bamboo accompanying its growth. Holzforschung, 47(2): 109-115.
Garcia-Ibanez P, Sanchez M, Cabanillas A. 2006. Thermogravimetric analysis of olive-oil residue in air atmosphere. Fuel Processing Technology, 87:103-107.
Haykiri-Acma H. 2003.Combustion characteristics of different biomass materials. Energy Conversion Management, 44: 155-162.
INBAR (International Network for Bamboo and Rattans) 2012. “INBAR and ENEA release the first official study verifying the use of bamboo biomass for energy production”. Available at: http://www.inbar.int/2012/10/ [Accessed May 2013].
IEA 2012. International energy statistics. International Energy Agency, Paris, France. Available at: http://www.iea.org/publications/freepublications/publication/kwes.pdf. [Accessed March 2013].
Jain R. 1993. Fuel characteristics of some tropical trees of India. Biomass and Bioenergy, 4: 454-461.
Johari A, Samseh SH, Ramli M, Hashim H. 2012. Potential use of solar photovoltaic in peninsular Malaysia. International Journal of Renewable Energy Resources, 2:1-5.
Kleinhenz V, Midmore DJ. 2001. Aspects of bamboo agronomy. Advances in Agronomy, 74: 99-153.
Kumar M, Gupta RC, Sharma T. 1992. Effect of carbonization conditions on the yield and chemical composition of Acacia and Eucalyptus wood chars. Biomass and Bioenergy, 3(6): 411-417.
Kumar R, Pandey KK, Chandrashekar N, Mohan S. 2010. Effect of tree-age on calorific value and other fuel properties of Eucalyptus hybrid. Journal of Forestry Research, 21(4): 514 -516.
Moya R, Tenorio C. 2013. Fuelwood characteristics and its relation with extractives and chemical properties of ten fast-growth species in Costa Rica. Biomass and Bioenergy, 56: 14-21.
Munir S, Daood SS, Nimmo W, Cunliffe AM, Gibbs BM. 2009. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresource Technology, 100: 1413–1418.
Ohman M, Nordin A. 2000. The role of kaolin in prevention of bed agglomeration during fluidized bed combustion of biomass fuels. Energy and Fuels, 14: 618.
Peter MK. 2002a. Energy production from biomass (part 2): Conversion Technologies. Bioresource Technology, 83 (1): 47–54.
Peter MK. 2002b. Energy production from biomass (part 1): Overview of biomass. Bioresource Technology, 83 (1): 37–46.
Saikia P, Kataki R, Choudhury PK, Konwer D. 2007. Carbonization of eight bamboo species of northeast India. Energy Sources, 29: 799-805
Scurlock JMO, Dayton DC, Hames B. 2000. Bamboo: an overlooked biomass resource? Biomass and Bioenergy, 19: 229-244.
Sims REH. 2003. Bioenergy to mitigate for climate change and meet the needs of society, the economy and the environment. Mitigation and Adoption Strategies for Global Change, 8: 349-370.
Sims REH, Hastings A, Schlamadinger B, Taylor G, Smith P. 2006. Energy crops: current status and future prospects. Global Change Biology, 12(11): 2054-2076.
Singh O. 2008. Bamboo for sustainable livelihood in India. Indian Forester, 134 (9): 1193-1198.
Timell TE, Glaudemans CPJ, Gillham JK. 1959. TAPPI, 42:623.
Villeneuve J, Palacios JH, Savoie P, Godbout S. 2012. A critical review of emission standards and regulations regarding biomass combustion in small scale units (<3 MW). Bioresource Technology, 111: 1–11.
杂志排行
Journal of Forestry Research的其它文章
- Carbon sequestration in Chir-Pine (Pinus roxburghii Sarg.) forests under various disturbance levels in Kumaun Central Himalaya
- Regional differences of water conservation in Beijing’s forest ecosystem
- Spatial modeling of the carbon stock of forest trees in Heilongjiang Province, China
- Spatial heterogeneity of factors influencing forest fires size in northern Mexico
- Community ecology and spatial distribution of trees in a tropical wet evergreen forest in Kaptai national park in Chittagong Hill Tracts, Bangladesh
- Diversity, regeneration status and population structure of gum- and resin-bearing woody species in south Omo zone, southern Ethiopia
